Back to Journals » Patient Preference and Adherence » Volume 16
The Benefits of Safely Achieving Near Normoglycemia from the Perspective of People with Type 2 Diabetes: A Quantitative Survey Study
Authors Gelhorn HL, Ross MM , Shinde S, Thieu VT, Boye KS
Received 16 April 2022
Accepted for publication 16 July 2022
Published 3 August 2022 Volume 2022:16 Pages 1897—1906
DOI https://doi.org/10.2147/PPA.S366966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Heather L Gelhorn,1 Melissa M Ross,1 Shraddha Shinde,2 Vivian Thuyanh Thieu,3 Kristina S Boye2
1Patient-Centered Research, Evidera, Inc, Bethesda, MD, USA; 2Global Patient Outcomes and Real World Evidence, Eli Lilly and Company, Indianapolis, IN, USA; 3Global Medical Affairs, Eli Lilly and Company, Indianapolis, IN, USA
Correspondence: Heather L Gelhorn, Patient-Centered Research, Evidera, Inc, 7101 Wisconsin Ave, Suite 1400, Bethesda, MD, 20814, USA, Tel +1-970-363-7333, Email [email protected]
Purpose: To understand the perspectives of people with type 2 diabetes (T2D) on safely reaching near normoglycemia, defined as a glycated hemoglobin A1c (HbA1c) value of < 6%. HbA1c indicates the average blood sugar level over the past few months.
Patients and Methods: This cross-sectional online quantitative survey of people with T2D asked about the current impacts of T2D, the anticipated benefits of safely achieving near normoglycemia among participants with a current HbA1c ≥ 6%, or the actual benefits of safely achieving near normoglycemia among participants who had an HbA1c < 6%. Participants reported on specific areas of psychological/emotional impact of T2D and the psychological/emotional benefits of achieving near normoglycemia.
Results: Participants (N = 1000; United States = 500 and United Kingdom = 500) were 53.1% male and had a mean age of 62.9 years (SD = 13.3). The majority reported that the current HbA1c ≥ 6% (81.2%) and 49.2% had been diagnosed more than 10 years ago. The vast majority of participants (> 90%) indicated that achieving near normoglycemia was meaningful, with 95% of the participants indicating that achieving near normoglycemia would be of somewhat or extreme importance to them. In total, 93.8% of participants with a current HbA1c ≥ 6% and 80.3% of those with a current HbA1c < 6% anticipated/reported having experienced improvements as a result of achieving near normoglycemia. Among those who experienced or anticipated positive psychological/emotional impacts (n = 247), the most commonly reported impacts included less worry about future diabetes-related complications (79.8%), feeling in control over one’s life (72.9%), and overall health-related quality of life (59.9%).
Conclusion: Achieving near normoglycemia is both meaningful and important to the majority of people with T2D, including both those who have and those who have not experienced reaching near normoglycemia. A wide range of specific impacts, including psychological/emotional concepts, are experienced by people with T2D, many of which may be improved through achieving near normoglycemia.
Keywords: burden of disease, emotional impact, patient perspective, psychological impact, quality of life
Introduction
Diabetes is a serious chronic condition, affecting approximately 7% of the United Kingdom (UK) population1 and 10% of the United States (US) population, and 90% to 95% of cases are type 2 diabetes (T2D).2 T2D is typically diagnosed by testing glycated hemoglobin A1c (HbA1c), which indicates the average blood sugar level over the past few months; HbA1c levels of less than 5.7% are normal, 5.7% to 6.4% reflects prediabetes, and 6.5% or greater indicates T2D.2,3
Despite the breadth of available treatments, people with T2D struggle with glycemic management, with nearly half of those with T2D having an HbA1c value of 7.0% or higher, in addition to having a variety of unmet needs while experiencing a wide range of impacts.4–6 Previous research has found that poor glycemic management among people with T2D is associated with feelings of self-blame, poor psychological well-being, and diabetes-related worries.7,8 A recent qualitative concept elicitation study was conducted to understand and document what achieving near normoglycemia (HbA1c levels near 5.7% or those observed among individuals without T2D) would mean to people with T2D. This study demonstrated that people on currently available treatments for T2D have a high disease burden and a broad range of unmet needs and that a treatment that safely achieves near normoglycemia would be a paradigm shift for many people with T2D and a highly valued option with many anticipated benefits. Many participants indicated that achieving near normoglycemia would substantially change their lives in terms of both their physical and psychological/emotional health.5 Emergent treatment options for T2D, such as the weekly GIP/GLP-1 receptor agonist tirzepatide, may make safely reaching near normoglycemia achievable for many people with T2D.9–13
The current cross-sectional survey study aimed to build on the prior qualitative study to quantify patients’ perspectives on how their lives would be, or had been, impacted by achieving near normoglycemia and what humanistic gains they expect to experience from safely lowering their HbA1c level. Additionally, this study was conducted to better understand the potential value of safely achieving near normoglycemia from the patient perspective. Achieving near normoglycemia is a relatively new concept among patients with T2D that has not been studied beyond the previously mentioned qualitative work. This information may be helpful to the wider scientific community, particularly physicians, who may not be aware of patients’ perspectives or the specific humanistic benefits of safely achieving near normoglycemia.
Materials and Methods
Overall Design and Sample
This was a cross-sectional study conducted among 1010 people with T2D in the US and UK, including 505 participants from the US and 505 participants from the UK. Participants completed a web-based survey that elicited perspectives on safely achieving near normoglycemia. The study consisted of two phases: a qualitative phase and a quantitative phase. In the first phase of this study, 10 participants took part in qualitative pilot interviews to ensure the clarity and understandability of the web survey for the quantitative phase. The subsequent quantitative phase began with a quantitative pilot (ie, a soft launch of the survey) to confirm the functionality of the web survey, and the main quantitative data collection was subsequently implemented.
Participants were residents of the US or the UK, were at least 18 years old, reported that they had been diagnosed with T2D by a medical doctor, and knew of their current HbA1c level (taken within the past 6 months). Individuals were excluded from the study if they had type 1 diabetes or gestational diabetes, or if they were employed in the pharmaceutical industry or in a position with a direct role in treating people with diabetes. In the qualitative pilot, individuals were also excluded if they had a cognitive disability, visual impairment, or hearing difficulty.
Invitations to participate were sent out to online patient panels via email and advertisements accessible only to panelists. Participants completed a series of online screening questions based on the inclusion/exclusion criteria to confirm eligibility. Eligible participants provided electronic informed consent before data collection commenced. All participants were recruited from March through June 2021. Participants were remunerated for their participation.
Survey
The web-based survey used in the current study was informed by a prior qualitative concept elicitation study5 on achieving near normoglycemia. This prior study included 50 patients from the US and the UK and used open-ended interview questions to explore the impacts and relevant outcomes associated with HbA1c among people with T2D. The interviews explored patients’ treatment journey, perceptions of their future with T2D, and the value of achieving normoglycemia through presentation of two vignettes. The study specifically focused on anticipated changes relevant to and associated with reduced HbA1c and was designed to ensure that the survey in the current study comprehensively included all relevant impacts. All questions were multiple choice, and the response options were reflective of the most commonly endorsed themes from the prior qualitative concept elicitation study. The questionnaires administered in the US and the UK were identical except for parts of the sociodemographic and clinical form where country-specific adjustments were made (eg, spelling and units for reporting demographic and clinical information such as income ranges, height, and weight). Additionally, HbA1c was reported only as a percent for US participants, but as a percent and as mmol/mol for UK participants.
The survey began with general questions about participants’ experiences with diabetes. Next, participants were asked about the ways in which T2D currently impacted their lives and which of these impacts were most important. Among those for whom it was relevant, participants were probed on which impacts of T2D affected them emotionally or psychologically.
The next section of the survey provided participants with an overview of what HbA1c is and what levels of HbA1c are associated with diabetes, prediabetes, and individuals without T2D. For the purposes of this survey, near normoglycemia was defined as HbA1c <6%. Participants were asked to imagine a medication that could safely lower their HbA1c to <6% (ie, near normoglycemia) and how it would impact their lives. Participants were probed on how they thought these impacts would affect them emotionally/psychologically. For participants who had already achieved near normoglycemia (ie, reported their most recent HbA1c as <6%), the questions in this section were worded to ask them to report on how they were actually impacted when they lowered their HbA1c to near normoglycemia. Finally, participants completed a brief questionnaire on sociodemographic and clinical characteristics.
Pilot Study
A small qualitative pilot testing study was conducted in which five participants with T2D each from the US and the UK completed a virtual web-assisted interview. During the virtual pilot interviews, participants shared their screen and completed the full survey. The interviewer probed participants carefully during the interview to assess whether they understood the survey instructions and questions. Additionally, interviewers queried participants on the clarity, relevance, and completeness of the response options to the survey questions. Following the qualitative pilot, the response options were updated to reflect the findings from the qualitative pilot interviews. The data collected from these 10 qualitative pilot participants were not included in the final quantitative analysis.
The web survey was then quantitatively pilot tested in the first 93 participants (53 in the US and 40 in the UK). During the quantitative pilot, participants were permitted to enter free-text response options if they felt that a response option was missing from a multiple-choice question; this information was used to update the survey and ensure that all relevant response options were included for the main phase of data collection. Because no significant changes were made to the survey during the quantitative pilot phase, the data collected from these 93 pilot participants were combined with those collected during the main survey administration and included in the final analysis. All multiple-choice questions in the final survey were closed (ie, no free-text responses were permitted).
Statistical Analyses
Descriptive statistics (eg, mean, median, standard deviation [SD], range for continuous variables, frequency and percentages for categorical variables, and 95% confidence intervals for the main outcomes) were used to summarize participants’ survey responses as well as characterize the sample in terms of sociodemographic and clinical characteristics. For all endpoints, the numbers for missing data are reported.
The results were weighted by age group and gender to match the population, based on recent Centers for Disease Control and Prevention data for the T2D population.6 The weighting was applied iteratively for these two characteristics (ie, gender and age) until the distribution of participants was aligned with the distribution reported in the T2D population. For example, the weights were calculated as the proportion of males in the T2D population divided by the proportion of males in the sample and the same formula was used to calculate weights for females and age categories. The weights were applied to one characteristic at a time, so first gender and then age. Then, the marginal totals in each category were compared to the T2D population and the weights were iteratively applied until the marginal totals were aligned with the distribution observed in the T2D population. The weighted and unweighted characteristics of the sample are presented in Tables 1 and 2; however, the weighted data are the focus of this manuscript.
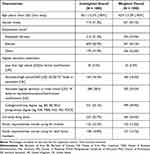 |
Table 1 Sample Sociodemographic Characteristics |
 |
Table 2 Sample Self-Reported Clinical Characteristics |
Results
Sample
The unweighted and weighted (balanced in terms of age group and gender) sociodemographic and clinical characteristics of the study sample (N = 1000) are presented in in Tables 1 and 2; all subsequent text descriptions of the results summarize the weighted results. The mean age of the sample was 62.9 years (SD = 13.3). The majority of the sample was male (n = 531, 53.1%), was retired (n = 541, 54.1%), and 49.9% had a college/university or postgraduate degree (n = 499).
The mean participant body mass index (BMI) was 31.1 (SD = 6.95). The majority (n = 610, 61.0%) had an HbA1c of 6.00% to 7.99% (42–63 mmol/mol). Most participants currently saw a primary care provider or general practitioner (n = 681, 68.1%) for T2D treatment, and 49.2% (n = 492) had been diagnosed with T2D more than 10 years ago.
A large proportion were treating their T2D with diet and exercise (n = 715, 71.5%) and were currently taking an oral T2D medication (n = 804, 80.4%). Some participants reported experiencing severe hypoglycemia that required the help of another person in the past year (n = 101, 10.1%). Over half of the participants reported experiencing no T2D-related complications (n = 577, 57.7%). The majority of the participants reported their overall health as either “good” (n = 384, 38.4%) or “fair” (n = 316, 31.6%).
Perspectives on Achieving Near Normoglycemia
Participants were asked whether safely achieving near normoglycemia would be meaningful and important. The vast majority (n = 919, 91.9%) of participants indicated that achieving near normoglycemia was meaningful; in addition, 95.0% (n = 950) of participants indicated that achieving near normoglycemia would be of either somewhat or extreme importance to them. Participants who had currently had an HbA1c <6% tended to report that their experience with achieving near normoglycemia was more important than what those with an HbA1c ≥6% anticipated (Figure 1).
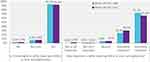 |
Figure 1 How meaningful and important is achieving near normoglycemia? (by current HbA1c). Abbreviation: HbA1c, glycated hemoglobin A1C. Note: All figures present weighted results. |
When asked how achieving near normoglycemia would positively impact them, 93.8% of participants with a current HbA1c ≥6% anticipated improvements in at least one area, and 80.3% of those with a current HbA1c <6% reported having experienced improvements in at least one area. Participants were asked which specific benefits would be, or were, associated with achieving near normoglycemia. Among those with a current HbA1c ≥6%, the most commonly anticipated impacts of achieving near normoglycemia were diet (n = 405, 49.9%), medical complications/health (n = 359, 44.2%), weight changes or control (n = 356, 43.9%), monitoring of glucose levels (n = 315, 38.7%), and general inconvenience (n = 292, 35.9%; Figure 2). Among those with a current HbA1c <6%, the most commonly experienced impacts of achieving near normoglycemia were diet (n = 66, 35.3%), weight changes or control (n = 52, 27.5%), monitoring of glucose levels (n = 48, 25.8%), psychological or emotional impacts (n = 45, 23.8%), and daily activities (n = 43, 22.9%). The anticipated and experienced impacts between those who had and those who had not achieved near normoglycemia were most closely aligned for daily activities and psychological/emotional domains.
 |
Figure 2 Perceived benefits of achieving near normoglycemia by current HbA1c. Abbreviation: HbA1c, glycated hemoglobin A1C. Note: All figures present weighted results. |
As the nature and frequency of the psychological and emotional benefits of near normoglycemia are less well understood, participants were asked to report what specific psychological and emotional impacts they anticipated or had experienced. Among those who experienced or anticipated positive psychological/emotional impacts (n = 247, 24.7%), the most commonly reported impacts included less worry about future diabetes-related complications (n = 197, 79.8%), feeling in control over one’s life (n = 180, 72.9%), and overall health-related quality of life (n = 148, 59.9%; Figure 3).
 |
Figure 3 Anticipated/experienced positive psychological or emotional impacts of near normoglycemia (N = 247). Note: All figures present weighted results. |
Discussion
Overall, safely achieving near normoglycemia (defined in this study as HbA1c <6%) is both meaningful and important to the vast majority (>90%) of people with T2D. This was true both for those who currently have and those who were not currently at near normoglycemia.
Diabetes impacts people with T2D in many ways, even those who were diagnosed less recently. Specifically, participants reported being most impacted with respect to diet, weight changes or control, monitoring glucose levels, medical complications, and general inconvenience (eg, planning: meals, travel, medications, etc.). Additionally, a wide range of specific psychological/emotional impacts are experienced by people with T2D, including maintaining diet and exercise discipline, worrying about future diabetes-related complications, feeling depressed, and dealing with medications. Many of these impacts may be improved by achieving near normoglycemia.
Those with a current HbA1c ≥6% anticipated that near normoglycemia would have a positive effect on all five of the most commonly reported impacts of T2D: diet, medical complications/health, weight changes or control, monitoring of glucose levels, and general inconvenience. Those who had achieved near normoglycemia (ie, current HbA1c <6%) most commonly reported positive impacts with respect to three of these: diet, weight changes or control, and monitoring of glucose levels. However, they also noted positive impacts on their psychological or emotional wellbeing and daily activities.
For most of the impact areas, the proportion of patients who anticipated benefits was notably greater than the proportion of patients who had experienced near normoglycemia. However, two exceptions to this pattern were impacts on psychological/emotional and daily activities, where anticipated and experienced benefits were similar between those who had and those who had not achieved near normoglycemia. Interestingly, the disparities between the experienced and anticipated benefits of achieving near normoglycemia highlight the potentially more immediate and tangible benefits of near normoglycemia (eg, improvements in daily activities and psychological/emotional domains) in contrast to the potentially longer-term benefits such as improvements in health/reductions in medical complications and weight changes. This discrepancy may also indicate that patients may not realize the full extent of the psychological/emotional and daily activities impacts until they experience some relief from them by achieving near normoglycemia. Many patients with T2D have been living and coping with this chronic condition for a long time. These patients may have unconsciously adapted to the impacts of dealing with the condition in ways that they do not recognize until they experience noticeable improvements in their health status, and at that point, they may realize the extent of these burdens in hindsight.
The findings of this study may be useful to clinicians who treat people with T2D using motivational interview approaches. This study highlights the specific benefits of low HbA1c that matter most to patients, and this information might help focus discussions between clinicians and their patients with T2D.
Future research may be useful to explore the nature of the psychological/emotional transition that people with T2D experience as they achieve near normoglycemia. This could be explored through a longitudinal research design that allows for the timing and specific experiences of each individual to be characterized more precisely.
The results of the current study should be interpreted while considering the following limitations. First, all data, including clinical and sociodemographic data, were self-reported. Second, participants in this study were mostly retired, an HbA1c between 6.00% and 7.99%, and half had been diagnosed with diabetes for 10 years or longer. The findings may not be generalizable to all people with T2D, for example, patients who are younger, who have less well-controlled HbA1c, and who were diagnosed more recently. Third, all questions were close ended, so no additional data were collected regarding participants’ perspectives when selecting “other.” However, the response options included in the multiple-choice survey questions were informed by a prior qualitative concept elicitation study.5 Furthermore, the survey was qualitatively and quantitatively pilot tested. During pilot testing, additional data were collected about participants’ perspectives when selecting “other”, and after pilot testing, the survey response options were revised to include any newly arising responses.
Conclusion
Although studies examining patients’ perspectives on how diabetes impacts their daily activities and how it impacts them psychologically/emotionally were found in the literature, this is the first-known study to quantify the anticipated and experienced impacts of achieving near normoglycemia directly from the perspective of people with T2D. The results of the present study highlight the potential value of achieving near normoglycemia in people with T2D, from both the perspectives of those who have achieved near normoglycemia and those who have not. The findings of this study imply that achieving near normoglycemia is likely both meaningful and important to the vast majority of people with T2D. The potential benefits of achieving these blood glucose targets encompass both immediate and longer-term impacts and have the potential to meaningfully improve the lives of people with T2D.
Abbreviations
BMI, body mass index; HbA1c, glycated hemoglobin A1c; T2D, type 2 diabetes; UK, United Kingdom; US, United States.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study received approval from Ethical & Independent Review Services [EVA-28953] on February 17th, 2021. The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Compliance with Ethics Guidelines
This study received approval from Ethical & Independent Review Services [EVA-28953] on February 17th, 2021. E&I is a central independent review board (IRB) that was used because there are no internal IRBs at the authors’ institutions. The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Acknowledgments
Editorial assistance for manuscript preparation was provided by Karen Trewick.
Funding
This study was funded by Eli Lilly and Company, Indianapolis, USA.
Disclosure
Heather L Gelhorn is an employee of Evidera, a consultancy that provides scientific consulting services to pharmaceutical companies. Melissa Ross is an employee of Evidera, a consultancy that provides scientific consulting services to pharmaceutical companies. Shraddha Shinde is an employee and shareholder of Eli Lilly and Company. Vivian Thuyanh Thieu is an employee and shareholder of Eli Lilly and Company. Kristina S Boye is an employee and shareholder of Eli Lilly and Company. The authors report no other conflicts of interest in this work. Parts of this research were presented at the International Society for Pharmacoeconomic and Outcomes Research (ISPOR) European Conference in December 2021 as a poster presentation. The abstract was cited in: Value in Health, Volume 24, Issue 12, S2 (December 2021).
References
1. Whicher CA, O’Neill S, Holt RIG. Diabetes in the UK: 2019. Diabet Med. 2020;37(2):242–247. doi:10.1111/dme.14225
2. Centers for Disease Control and Prevention. Type 2 diabetes; 2021. Available from: https://www.cdc.gov/diabetes/basics/type2.html.
3. American Diabetes Association. Understanding A1C: diagnosis; 2021. Available from: https://www.diabetes.org/diabetes/a1c.
4. Gelhorn H, Balantac Z, Shinde S, Thieu VT, Boye KS. The burden of type 2 diabetes and the value of achieving near normoglycemia from the patient perspective. Diabetes Ther. 2021;12(7):1821–1837. doi:10.1007/s13300-021-01054-6
5. Paczkowski R, Poon JL, Cutts K, et al. PDB46 A best-worst scaling preference study: the mealtime insulin experience for people with diabetes in US and UK. Value Health. 2021;24(Suppl 1):S85–S86. doi:10.1016/j.jval.2021.04.443
6. Centers for Disease Control and Prevention. National diabetes statistics report, 2020; 2020. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html.
7. Beverly EA, Ritholz MD, Brooks KM, et al. A qualitative study of perceived responsibility and self-blame in type 2 diabetes: reflections of physicians and patients. J Gen Intern Med. 2012;27(9):1180–1187. doi:10.1007/s11606-012-2070-0
8. Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (Dawn) Study. Diabet Med. 2005;22(10):1379–1385. doi:10.1111/j.1464-5491.2005.01644.x
9. Rosenstock J, Wysham C, Frias JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, Phase 3 trial. Lancet. 2021;398(10295):143–155. doi:10.1016/S0140-6736(21)01324-6
10. Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–515. doi:10.1056/NEJMoa2107519
11. Ludvik B, Giorgino F, Jodar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583–598. doi:10.1016/S0140-6736(21)01443-4
12. Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811–1824. doi:10.1016/S0140-6736(21)02188-7
13. Dahl D, Onishi Y, Norwood P, Huh R, Patel H, Rodriguez A. 80-LB: tirzepatide, a dual GIP/GLP-1 receptor agonist, is effective and safe when added to basal insulin for treatment of type 2 diabetes (SURPASS-5). Diabetes. 2021;70(Suppl):1. doi:10.2337/db21-80-LB
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
