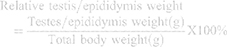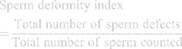Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
The Beneficial Role of Anchomanes difformis in STZ-Induced Reproductive Dysfunction in Male Wistar Rats
Authors Alabi TD , de Villiers C , du Plessis SS , Monsees TK, Brooks NL, Oguntibeju OO
Received 5 July 2020
Accepted for publication 16 September 2020
Published 23 November 2020 Volume 2020:13 Pages 4543—4560
DOI https://doi.org/10.2147/DMSO.S270783
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Toyin Dorcas Alabi,1 Charon de Villiers,2 Stephan S du Plessis,3 Thomas K Monsees,4 Nicole L Brooks,5 Oluwafemi Omoniyi Oguntibeju1
1Phytomedicine & Phytochemistry Group, Oxidative Stress Research Centre, Department of Biomedical Sciences, Faculty of Health and Wellness Sciences, Cape Peninsula University of Technology, Bellville, South Africa; 2PUDAC-Delft Animal Facility, South African Medical Research Council, Cape Town 7505, South Africa; 3Division of Medical Physiology, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg 7505, South Africa; 4Department of Medical Biosciences, University of the Western Cape, Bellville 7535, South Africa; 5Faculty of Health and Wellness Sciences, Cape Peninsula University of Technology, Cape Town, South Africa
Correspondence: Oluwafemi Omoniyi Oguntibeju Tel +27 219538495
Email [email protected]
Background: Progression of diabetes mellitus has increasingly led to several diabetic complications. Diabetes is one of the major factors implicated in male reproductive system damage. Recent approaches such as the use of medicinal plants have been explored in the management of diabetes and associated complications. Anchomanes difformis (common name: children’s umbrella) has been shown to possess anti-diabetic ability in animal model. Therefore, this study seeks to investigate the potency of Achomanes difformis in ameliorating diabetes-induced reproductive dysfunction.
Methods: Type 2 diabetes was induced in male Wistar rats with 10% fructose administration for 2 weeks and an intraperitoneal injection of 40mg/kgBW of streptozotocin. Aqueous extract (200mg and 400mg/kgBW) of Anchomanes difformis leaves was administered daily for 6 weeks. The rats were randomly divided into 7 groups with a minimum of eight rats in each (8 rats in normal groups and 10 in diabetic groups). The impact of diabetes and treatment was investigated by estimating sperm concentration, motility indices, viability and morphological parameters in the normal, treatment controls and diabetic rats using CASA-SCA system. Histological examination of the testes and epididymis was performed.
Results: Diabetes induction resulted in significant decrease in sperm concentration, viability and some motility parameters with 40% abnormalities in sperm morphology. The administration of Anchomanes difformis significantly increased sperm concentration and sperm viability, while it significantly improved the percentage of morphologically normal sperm in diabetic rats. Anchomanes difformis ameliorated testicular damage such as vacuolization and loss of germinal epithelium in the diabetic-treated rats when compared to the diabetic controls.
Conclusion: The potency Anchomanes difformis displayed against diabetic-induced damage in the reproductive system might be a new and promising tool in the management of male reproductive dysfunctions and associated complications in diabetes mellitus.
Keywords: Anchomanes difformis, diabetes mellitus, male fertility, sperm function
Introduction
Diabetes mellitus (DM) is one of the many diseases that contribute to male infertility in the present age, as an estimated 51% of diabetic subjects are suffering from different reproductive dysfunctions.1,2 DM impacts the reproductive system at all functional levels which are the pre-testicular (hormonal regulation), the testicular (spermatogenesis) and the post testicular (ejaculation) stages.3 Oxidative stress, especially hyperglycaemia-induced, has been implicated in the pathogenesis of reproductive complications arising from diabetes.4 Some of the pathways by which oxidative stress results in reproductive dysfunction include increased lipid peroxidation of polyunsaturated fatty acids (PUFA), damaging the sperm membrane which are prone to reactive oxygen species (ROS) attack.5 Thereafter, increased lipid peroxidation further degenerate to DNA damage in the spermatozoa.6 In addition, oxidative stress accelerates the increased production of advanced glycation end-products (AGEs) which are key players in the process of erectile dysfunction.7 AGEs cause vascular thickening, endothelial dysfunction, decreased elasticity and atherosclerosis by forming covalent bonds with vascular collagen.7 Furthermore, overproduction of AGEs results in impaired relaxation of the cavernosal smooth muscle via the increased generation of ROS which in turn quench nitric oxide leading to reduced cyclic guanosine monophosphate (cGMP) levels. Reduced cGMP through cascades of reactions further leads to impaired relaxation of the cavernosal muscle.7,8 Other pathways implicated in the pathogenesis of male infertility in DM include; the progression of diabetic neuropathy and endocrine disorder which affects reproductive hormones.1 Diabetes is known to cause microvascular complications including that of the nervous system especially autonomy neuropathy and peripheral neuropathy9 which causes erectile and ejaculating dysfunctions.8,10 Figure 1 summarizes the different mechanisms and factors implicated in the progression of diabetes to reproductive dysfunctions. Endocrine disorder results in loss of hormonal control which is an important factor in male fertility as spermatogenesis; the processes involved in the production and development of spermatozoa from germ cells11 are controlled and regulated by the hypothalamic-pituitary-gonadal hormones. The hypothalamus produces gonadotropin-releasing hormone (GnRH) which in turn triggers the secretion of follicle-stimulating hormone (FSH) and luteinising hormone (LH) from the anterior pituitary lobe.12 The release of LH stimulates the production of testosterone from the Leydig cells, while FSH prompts the Sertoli cells to supply nutrients to the germ cells and activate spermatogenesis.13 Any imbalance or impairment in this array of hormones can cause dysfunction in the male reproductive system. Subsequently, decreased LH and FSH levels have been reported in diabetic conditions investigated in rats.14,15
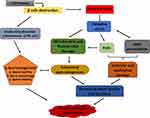 |
Figure 1 Mechanisms involved in the pathogenesis of diabetes-induced reproductive dysfunctions. |
The use of streptozotocin (STZ) to induce hyperglycaemia-mediated reproductive toxicity has been documented,1,16 and has been known to affect the antioxidant status, the spermatogenic processes, sperm functions and the reproductive organs such as testes.13,17 The contributory role of medicinal plants in combating diabetic-induced reproductive impairment has been emphasized in recent times. Anchomanes difformis (AD) has a strong ethnopharmacological relevance and it is known for its numerous biological activities such as its potency against diabetes, nephropathy, inflammation, microbial activities, and gastrointestinal pathologies. These folkloric claims have been scientifically established and more investigations to explore its other potentials are ongoing.18,19 Agyare and colleagues20 investigated the potentials of leaves and rhizome extracts of AD on histamine and serotonin; mediators of acute inflammation. AD demonstrated more anti-inflammatory ability when compared with aspirin as reference drug.20 Adebayo also reported the anti-inflammatory potentials of AD leaves against raw-egg albumin-induced inflammation in chicks.21 An ethanolic extract of AD rhizome displayed hypoglycemic effect in alloxan-induced diabetes using Wistar rats.22 Egwurugwu and co-workers proposed the effect of AD rhizome against uterine fibroid showing its ability to modulate female sex hormones implicated in uterine fibroid.23 We carried out previous studies on the antioxidant capacities and phytochemical analysis of six different extracts of AD, which revealed that AD is a natural source of antioxidant with aqueous leaves extract exhibiting the highest antioxidant ability and had the highest concentrations of polyphenols.24 Phytochemical characterisation of the leaves aqueous extract was also performed using ultra performance liquid chromatography mass spectrometry (UPLC-MS) and high-performance liquid chromatography (HPLC). The results showed the presence of bioactive compounds including phloridzin, quercetin, rutin, and kaempferol which are antioxidants and have therapeutic effects against diabetes, inflammation and apoptosis.24 The antioxidant, hypoglycaemic, anti-inflammatory activities of AD and its impact in modulating female sex hormone previously stated indicate its potentials in attenuating reproductive dysfunctions associated with diabetes. However, no study has investigated the possible effects of AD leaves extract on sperm functional parameters as well as testicular and epididymal damage in diabetes. This paucity in the literature was a major drive that led to this study which focuses on assessing the ameliorative effect of Anchomanes difformis (AD) on reproductive dysfunction mediated by diabetes in male Wistar rats.
Methodology
Reagents and Chemicals
n-Hexane, fructose, streptozotocin, glibenclamide, isoflurane, Ham’s F10, SpermBlue® fixative, BrightVit stain, buffered formalin (10%), DPX (distyrene, plasticizer and xylene).
Plant Collection and Extraction
The fresh leaves of AD were from the south-western part of Nigeria and authenticated at the University of Lagos Herbarium, Nigeria. A specimen was deposited in the herbarium; LUH6623. The harvested leaves were dried under shade, ground into fine particles and defatted by soaking in n-hexane (10%W/V) for 48 hours. Following defatting, the leaves were subjected to a cold-stirred extraction in water (5% W/V) for 48 hours at 2–8°C which gave an extraction yield of 25%. The extract was pulverised and stored at −20°C. A stock solution (200mg/mL) of the extract was freshly prepared daily before treatment.
Ethical Consideration
Ethical approval for this study was granted by the Research Ethics Committee (REC) of the Faculty of Health and Wellness Sciences, Cape Peninsula University of Technology, Bellville, South Africa (CPUT/HW-REC 2016/A4) and the Ethics Committee for Research on Animals at the South African Medical Research Council (SAMRC/ECRA), South Africa (REF.04/17), where the animal experiment was conducted.
Animal Care
Male Wistar rats (n=64) with approximate weights of 180 ± 10g were secured from the Animal facility in Stellenbosch University, South Africa. The animals were accommodated at the Primate Unit & Delft Animal Centre (PUDAC), SAMRC and acclimatized for 3 weeks before the commencement of the study. Housing conditions were controlled: humidity – 45% to 55%, temperature – 22°C to 26°C. They were exposed to normal photo period (12hour dark/12hour light) and fed with standard rat chow (SRC). Animal handling, care and other procedures were done in accordance with the standard operating procedure of SAMRC PUDAC (SOP No: 2016-R01) which conforms to the revised South African National Standard for the Care and Use of Animals for Scientific Purposes (South African Bureau of Standards, SANS 10386, 2008).
Induction of Diabetes
Type 2 diabetes (T2D) was induced with 10% fructose and STZ (BIOCOM Africa, South Africa) according to the method of Wilson and Islam.25 The diabetic model rats were placed on 10% fructose water ad libitum for two weeks. A single dose of 40mg/kg body weight (BW) STZ was administered intraperitoneally. After 5 days, animals with fasting blood glucose level above 15mmol/l were considered diabetic. Oral glucose tolerance test (OGTT) was performed to confirm insulin resistance.
Experimental Design
Wistar rats with weights ranging from 270–300g were used for this study. The rats were randomly grouped into seven with a minimum of eight rats in each (8 rats in normal groups and 10 in diabetic groups) as summarised in Figure 2. Water served as the vehicle for fructose and AD administration, while citrate buffer was the vehicle for STZ. Animals in group 1 are normal rats and served as the negative controls (NC) and received vehicle only. Animals in group 2 and 3 are normal rats who received 200 and 400mg/kgBW of AD aqueous extract only (N+AD 200 and N+AD 400) and served as the treatment controls. Group 4–7 consist of animals that were placed on 10% fructose for 2 weeks followed by STZ. Group 4 received vehicle only and served as the diabetic controls (DC), group 5 and 6 were given 200 and 400mg/kgBW of AD aqueous extract (D+AD 200 and D+AD 400) respectively, while group 7 received 5mg/kgBW of glibenclamide; an antidiabetic drug (D+G). The animal study was carried out over a period of 12 weeks; 3 weeks of acclimatization, 3 weeks for diabetes induction and 6 weeks of treatment.
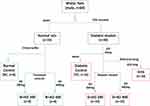 |
Figure 2 Experimental design. Water and citrate buffer served as vehicles of administration. |
Sample Collection
Rats were anaesthetized by inhalation of 2% isoflurane (Biofarm, Cape Town, South Africa) 1L/min flow rate, thereafter, terminated via exsanguination. Complete anaesthesia was confirmed through ‘pedal withdrawal reflex’ by toe-pinching using mosquito forceps to check loss of pedal reflex. In addition, loss of whiskers’ movement in response to stimulus was checked to confirm complete anaesthesia. Both testes, epididymis, and vas deferens were excised. The dissected male reproductive tract was cleaned by carefully trimming off all unnecessary blood vessels and fat tissue until all parts of the reproductive tract could be clearly seen and differentiated. The cauda epididymis, comprising the larger convoluted tubes, was removed and placed in a pre-warmed petri dish containing 200µL Ham’s F10 (Invitrogen, Cape Town, South Africa). The testes and epididymis were washed and weighed accordingly. The relative weights of the testes and epididymis were calculated using the body weight of the same rats against the weight of the testes or epididymis.
Sperm Isolation (Swim Out Method)
The swim-out technique was used to select the motile spermatozoa. Small incisions were made at the distal end of the cauda epididymis and the motile spermatozoa could swim out of the ducts into the surrounding medium (Ham’s F10 supplemented with 3% bovine serum albumin), forming a cloud of spermatozoa.
Measurement of Sperm Concentration and Motility
Semen samples were taken from the edge of the spermatozoan cloud to measure sperm concentration, motility and other related parameters. The semen (5 µL) was pipetted into a 20µm deep chamber Leja slide; SC 20–01-04B (Leja Products B.V., Nieuw-Vennep, The Netherlands). The slide was pre-heated on the microscope’s warming stage at 37°C. For assessment of sperm concentration, the semen sample was diluted with Ham’s F10 in the ratio 1:20. To measure sperm motility, 2–10 fields were captured until a total number of 200 spermatozoa were analyzed. Other motility parameters evaluated include curved-linear velocity (VCL), straight-line velocity (VSL), average-path velocity (VAP), beat-cross frequency (BCF), linearity (LIN), straightness (STR), oscillation index; also known as wobble (WOB), and amplitude of lateral head displacement (ALH). These measurements are presented as an average percentage of all the captured fields. The sperm concentration and motility were determined with the Rodent SCA® CASA (sperm class analyser, computed assisted sperm analysis) system version 6.2.0.15 (Microptic S.L, Barcelona, Spain). Photomicrographs were captured with a Basler A602fc digital camera (Microptic S.L, Barcelona, Spain) that was mounted (C-mount) onto a Nikon Eclipse 50i microscope (IMP, Cape Town, South Africa), equipped with phase contrast optics and a heated stage.
Evaluation of Sperm Morphology and Viability
Sperm smears for morphology and viability were prepared separately from the swim-out sperm preparation after 10 minutes incubation, allowed to confirm the motility of the selected spermatozoa to be smeared.
Morphology
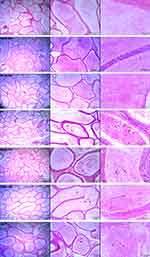
10µL of the swim-up sperm were pipetted to make duplicate smears, making it about two to ten sperm cells per field when viewed at a 103x magnification. Smears were allowed to air dry and stained with Rapid SpermBlue® fixative (Microptic S.L. Barcelona, Spain) following the method described by van der Horst and Maree.26 Stained smears were dried at room temperature for 24 hours and mounted with DPX (Merck, South Africa). Sperm morphology was assessed using SCA® on a Nikon E200 microscope, with a blue filter and phase “A” setting, and 60x magnification. Spermatozoa with background staining or that overlap another were excluded and a total of fifty spermatozoa were analyzed per slide. Other indices associated with sperm morphology were estimated which include area, width, angle of the head and midpiece, while perimeter, chord and roughness were assessed in the head only. Examples of stained spermatozoa with normal and abnormal morphology are displayed in Figure 3.
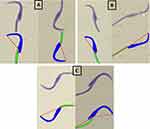 |
Figure 3 Examples of normal and abnormal rat spermatozoa stained with SpermBlue®. (A) Normal sperm, (B) abnormal (with head defects) and (C) abnormal spermatozoa with midpiece defects. |
Viability
BrightVit; a nigrosin-eosin-based solution (Sigma Aldrich, Cape Town, South Africa) was used to stain the sperm cells for the analysis of sperm viability. Briefly, 40µL of BrightVit was pre-heated to 37°C and mixed with the 10µL of semen for 5 minutes at 37°C. The stained smears were left to dry in the dark room at room temperature and mounted with DPX. Spermatozoa were viewed at 20x magnification under a light microscope. Percentage viability was calculated as the percentage of live spermatozoa from a total of 100 spermatozoa analyzed per slide. Live spermatozoa appear white (unstained) due to the ability of the intact membranes to exclude the dye, while the dead sperm cells appear pink/red due to eosin stain.
Sperm Deformity Index (SDI)
Sperm deformity index was estimated following the method described by Ahmad and Tariq.27 SDI was calculated using the sum of sperm morphological deformities observed and the total numbers of sperm randomly selected and counted in a sperm population.
Histological Analysis of the Gonadal Tissues
The testes and epididymis were excised, washed, weighed and fixed in 10% buffered formalin. After fixation, the tissues were passed through ascending series of alcohol for dehydration, cleared in xylene and embedded in paraffin. Sections (5 µm) of each tissue were deparaffinized, rehydrated and stained with hematoxylin and eosin. The stained tissues were mounted and examined under a light microscope.
Statistical Analysis
Values are expressed as mean ± standard error of mean (SEM). Data were analysed using GraphPad Prism Version 5.00 for Windows, GraphPad Inc., San Diego, California USA. Differences between means of groups were determined using one-way analysis of variance (ANOVA). The Bonferroni test was used for all pair-wise comparisons. Differences (F values) were considered statistically significant at p values less than 0.05.
Results
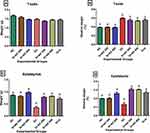
The Effect of Treatment with AD on Organ Toxicity in the Testis and Epididymis
Figure 4 presents the weights of the testis, epididymis and their relative weights. Induction of diabetes with STZ and fructose resulted in significant decrease in the weight of the epididymis in the diabetic controls (Figure 4C), while no significant changes were observed in the weight of the testes in normal, diabetic and treated rats (Figure 4A). The relative testicular weight of the diabetic controls was significantly increased (54.1%) when compared with the relative testicular weight of the normal rats. Treatment with 200 and 400mg/kgBW of AD reduced relative testis weight by 7.8% and 10.4%, respectively, while 5mg/kgBW of glibenclamide reduced relative testis weight by 4.2% (Figure 4B) when compared with the diabetic control. Inversely, induction of diabetes led to a significant decrease in the relative weight of the epididymis when compared with the epididymis of the normal rats. However, the administration of 200 and 400mg/kgBW of AD and glibenclamide significantly increased the relative epididymal weights to be comparable to normal (Figure 4D).
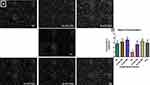
The Impact of AD on Sperm Function in Normal and Diabetic Rats
Sperm concentration was significantly reduced (70%) in diabetic control rats when compared with the normal rats. Supplementation with 200 and 400mg/kgBW of AD significantly restored sperm concentration back to normal in treated diabetic rats (Figure 5B). Administration of 200 and 400mg/kgBW triggered a 10% and 28.5% increase, respectively, in the sperm concentration of normal rats serving as treatment controls. Figure 5A is a representative micrograph showing the sperm density in the normal, diabetic and treated rats. The sperm population in the normal (NC) and treated normal rats were densely packed, while it was sparsely populated in the untreated diabetic rats (DC). This is the same trend observed in the quantitative measurement of the sperm concentration (Figure 5B), where treatment with AD increased sperm concentration in the non-diabetic-treated rats.
The effect of diabetes, treatment with AD and glibenclamide on indices of sperm function and quality is presented in Figure 6. Sperm morphology was significantly distorted in the untreated diabetic rats with 62.8% normal morphology when compared to the normal rats with 80% normal morphology (Figure 6A). A population of rats’ spermatozoa is considered to have normal morphology if the percentage normal morphology is ≥67%.28 Sperm morphology of diabetic rats that received 200 and 400mg/kgBW of AD were restored to normal (76% and 82%, respectively). In addition, there was a significant increase in the sperm deformity index (SDI) in the diabetic controls when compared with normal rats (Figure 6B). SDI was significantly abated to normal with 200 and 400mg/kgBW AD treatment in treated diabetic rats as well as the standard drug. Induction of diabetes also caused a significant decrease in sperm viability of diabetic controls (Figure 6C). This was significantly enhanced in diabetic rats treated with both concentrations of AD; however, 400mg/kgBW increased sperm viability, as comparable to normal. There was no significant difference in the total sperm motility in normal, diabetic and treated rats (Figure 6D).
The Effect of AD Administration on Sperm Morphometry of Normal and Diabetic Rats
To further investigate the effect of diabetes, and treatment with AD and glibenclamide, certain parameters relating to morphology of the sperm cell were measured in the normal, diabetic and treated rats. The arc, width, area, perimeter, angle, roughness and chord of the sperm head, together with the area, width and angle of the sperm midpiece were measured (Table 1). Result showed that there were observable trends (increase or decrease) in the sperm morphometry of diabetic rats when compared to normal and treated rats, though not statistically significant. It is noteworthy that the CASA system used has been programmed to analyse sperm morphometry and classify spermatozoa with normal and abnormal morphology based on the established cut-off values.28
 |
Table 1 Morphology Parameters of Spermatozoa from Normal and Diabetic Rats |
AD Enhanced Sperm Velocities and Kinematics in STZ-Induced Diabetes
Other parameters associated with sperm motility such as velocities, linearity, straightness and oscillation index were measured in the semen of normal, treated and untreated diabetic rats. The curved linear velocity (VCL) observed in the semen of diabetic rats was not significantly different from the normal controls and the treated rats (Figure 7A). The straight-line velocity (VSL) was significantly reduced in the diabetic controls when compared with the normal controls (Figure 7B) while VSL was enhanced in the rats treated with 200 and 400mg/kgBW of AD. Spermatozoa of diabetic controls had a 28.5% reduced average path velocity (VAP) when compared with sperm cells from normal rats. An observable increase of 15.7% and 23.7% in the VAP was noticed in the sperm of rats treated with 200 and 400mg/kgBW (Figure 7C). Linearity (LIN), straightness and oscillation index (WOB) of the spermatozoa were significantly affected and decreased in diabetic control. Administration of both concentrations of AD improved the LIN, WOB and straightness of the spermatozoa of treated diabetic rats (Figure 8A–C).
The impact of STZ-induction of diabetes and treatment on the factors affecting WOB is presented in Figure 9. ALH was increased by 20% in the diabetic controls and diabetic rats treated with 200mg/kgBW when compared with normal rats. The administration of 400mg/kgBW led to a 25% reduction in the abnormal ALH values in treated diabetic rats comparable to normal and standard drug (Figure 9A). There were no significant changes in the sperm BCF values in the normal, diabetic and treated rats (Figure 9B).
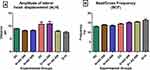 |
Figure 9 Effect of AD administration on indices of oscillation index; (A) ALH and (B) BCF of spermatozoa in normal and diabetic rats. Bars are indicative of mean values ± SEM of group values. |
The Effect of Intervention with AD on Gonadal Tissues; Testis and Epididymis in STZ-Induced Diabetes
Figures 10 and 11 present the findings from the microscopic examination of the testes and epididymal tissues. The normal and treatment controls showed normal architecture of the testes and epididymis, while the morphology of the germinal epithelium and spermatozoa were intact. Autolytic changes characterized with vacuolization within the germinal epithelium and loss of the adluminal part of Sertoli cells together with attached germ cells were observed in the testes of diabetic control rats (Figure 10). These pathological changes led to a severe disruption in spermatogenesis depicted by degeneration of mainly spermatocytes, spermatids and some spermatogonia. The testes of the rats administered 200mg/kgBW of AD revealed moderate vacuolization while the testes of rats treated with 400mg/kgBW had very mild vacuolization. Treatment with AD repaired the germinal epithelium of the treated diabetic rats to near normal when compared with the diabetic control. The administration of 5mg/kgBW mildly reduced germinal epithelial loss in the testes of treated diabetic rats with moderate vacuolization occurring in the germinal epithelium.
The structures of the epithelia lining the tubules of the epididymis were clearly seen with neatly arranged cilia and high intraluminal sperm density in the normal and treated controls. In diabetic controls, several sloughed germ cells were observed in the lumen of the epididymis, and a reduced sperm density when compared with the normal epididymis. Also, the brush border; ciliated epithelium was disrupted (Figure 11). Treatment with 200mg and 400mg/kgBW of AD markedly impede sloughing of germ cells and restored the epithelium of the epididymis with the cilia; visible and well arranged. Administration of AD further increased the intraluminal sperm density when compared with diabetic control. Treatment with glibenclamide (5mg/kgBW) also increased the intraluminal sperm density of the epididymis of treated diabetic rats as comparable to normal rats. However, sloughed germinal cells from the testes were observed in the lumen of the epididymis.
Discussion
DM is known to adversely affect male reproductive functions which ultimately leads to a reduced fertility.2 Male reproductive irregularities associated with diabetes and the ability of various medicinal plants to ameliorate diabetes and testicular dysfunction in diabetic rats have been explored.4,29,30 The findings from this study established the potency of AD in ameliorating diabetes-induced reproductive dysfunction. Persistent hyperglycaemia resulted in significant increase in the relative testicular weight and a significant decrease in the weight and relative weight of the epididymis. Similar findings have been reported in diabetic studies involving the use of STZ.15,31 Progression of diabetic condition is known to cause body weight loss and significant changes in the weight of internal organs including the gonads in Wistar rats and mice.2,32 Disparity in relative organ weights serves as one of the crucial factors in assessing the presence of toxicity and pathology in an organ.33,34 In addition, reduced reproductive organ weight as observed in the diabetic epididymis alongside higher sperm deformity index is indicative of spermatogenic dysfunction.2 The ability of AD to increase the total and relative epididymal weight, and also reduce the relative testicular weight in treated diabetic rats comparable to standard drug displays its potency against diabetes-induced testicular and epididymal toxicity. Nelli and Kilari35 obtained similar results in his study which investigated the protective role of α-mangostin against STZ-induced reproductive damage, the report showed significant changes in the weight of the testis and epididymis, and this was improved by treatment with α-mangostin.
There was no significant difference in the total sperm motility of the normal, diabetic and treated rats. This was also reported in a study conducted by Arokoyo and colleagues15 on diabetic-induced reproductive dysfunction. Interestingly, in our study, a significant decrease was observed in the sperm velocities and kinematics of untreated diabetic rats and this was significantly increased with AD supplementation in treated diabetic rats (Figures 7 and 8). A very similar trend on decreased sperm velocities and kinematics was documented by Mukhopadhyay and colleagues,36 where routine semen analyses of patients exposed to heavy metals and cigarette smoke showed normal motility. However, further probing by Mukhopadhyay on the same semen samples using the CASA system, showed that VCL, STR and ALH which are sperm velocity parameters were significantly reduced. This highlights the possibilities of impaired sperm motion in sub-populations within the group which may not reflect in the overall motility of the group but displayed in the significant differences in sperm velocities and kinematics as observed in the diabetic control rats. Furthermore, STZ administration can affect sperm velocities and kinematics.31 However, AD seems to prevent these anomalies in a similar manner to the standard drug. The reported VAP values were twice the VSL values (Figure 7B and C), and these are observed in cases of irregular sperm motion path which have significant effects on the WOB, STR, ALH and BCF of sperms.37 Sperm velocities directly correlate to LIN, STR, WOB, ALH and BCF,38 which further strengthens our findings in this study.
Sperm concentration, motility, morphology and viability are important factors to consider when assessing the sperm or semen quality.39,40 There was a significant reduction in the sperm concentration, viability, and morphology of diabetic controls as observed in comparable studies involving diabetes-induced reproductive dysfunctions.35,41,42 The reduced sperm quality parameters may be linked to impaired spermatogenesis observed in the seminiferous tubules as it affects the quantity and quality of spermatozoa that is produced.43 Spermatogenesis is usually disrupted in diabetes through hyperglycaemia-induced oxidative stress,44 and antioxidants have shown to increase sperm viability and reduce sperm defects by alleviating oxidative stress.29 Phytochemical characterisation carried out on the aqueous extract of AD leaves established the presence of bioactive compounds that have high antioxidant and antidiabetic properties such as quercetin, kaempferol, rutin, and phloridzin amongst others.24 The significant reduction in sperm concentration corresponds with the significantly reduced weight of the epididymis and the relative epididymal weight as the epididymis stores spermatozoa until maturation. The ability of AD to improve hyperglycaemia-induced sperm functions is most likely due to its possession of these bioactive compounds.
Male fertility largely depends on the complete development of spermatozoa in the testis and their maturation in the epididymis,45 inferentially, sperm quality is compromised in the case of pathological alterations in the testis or epididymis. The severe pathological features observed in the testis and epididymis of diabetic controls can be associated with the decreased sperm function parameters such as concentration, viability and morphology in the same animals. The germinal cells observed in the epididymal lumen of diabetic rats were immature germ cells that were sloughed from the germinal epithelium of the testis and were transported with the spermatozoa into the epididymis. It is evident from the findings that the level of damage in the germinal epithelium of the testes was commensurate to the proportion of sloughed germ cells observed in the epididymal lumen which were noticeably reduced in the diabetic rats treated with AD. Sertoli cells are potential targets for toxicants,46 severe damage and loss of some of these cells as noticed in our study is due to the administration of STZ as previously established.47,48 The loss of Sertoli and germ cells in the diabetic controls was accountable for the reduced sperm production, as the number of Sertoli cells are strongly related to sperm concentration. Sertoli cells play a key role in the attachment, development and sustenance of germ cells, they supply the nutrients and hormones required by the germ cells.46 Hence, damage or the loss of Sertoli cells results in disrupted spermatogenesis, loss of germ cells, decreased sperm production and reproductive dysfunction in the diabetic control which was repaired with treatment with AD. The ability of AD to induce repair and recovery of the germinal epithelium can be associated to its phytochemical content especially polyphenols, as other medicinal plants containing polyphenols have demonstrated ameliorative potentials on toxicity-induced damage in the testes.49–51 This is also supported by the report of Khaki and colleagues41 on the ability of quercetin to attenuate loss of germinal epithelium and vacuolization of germ cells. The potentials of AD to prevent germinal epithelial loss and improve sperm quality and functions may provide a natural alternative and therapy in attenuating diabetes-related reproductive dysfunctions in males. These findings, however, provide the basis for further studies that will aim at investigating the underlying mechanisms by which AD exerts its pro-fertility abilities in diabetic and non-diabetic conditions.
Conclusion
Treatment with 400mg of A difformis was more effective than 200mg A difformis and 5mg glibenclamide in repairing the germinal epithelium and overall damage in the testis and epididymis of diabetic rats. However, 200mg/kg BW exhibited more potency in ameliorating testicular and epididymal damage in the diabetic rats than 5mg glibenclamide. Both doses of AD were able to effectively improve sperm quality, though 400mg A difformis showed a non-significant increase in sperm concentration, morphology, and a significant increase in sperm viability in diabetic rats when compared to 200mg/kg BW. The potency of Anchomanes difformis displayed against diabetic-induced damage in the male reproductive system might be a new and promising tool in the management of reproductive dysfunctions and associated complications that arise in DM.
Acknowledgment
We would like to thank Temidayo Omolaoye, Division of Medical Physiology, Faculty of Medicine and Health Sciences, Stellenbosch University, for her technical support and assistance. We would also like to appreciate Mrs Joritha van Heerden, SAMRC, Cape Town, for her constant support and advises during the animal experiment.
Funding
This research was supported by National Research Foundation, South Africa [NRF 105249] and Cape Peninsula University of Technology, South Africa [CPUT-RJ23 and CPUT-NRF RO22] granted to Professor OO Oguntibeju. The first author also received financial assistance in the form of a bursary from DST-NRF [107580 and 116563].
Disclosure
The authors do not have any conflicts of interest.
References
1. Omolaoye Temidayo S, Du Plessis Stefan S. Diabetes mellitus and male infertility. Asian Pacific J Reprod. 2018;7(1):6–14. doi:10.4103/2305-0500.220978
2. Han XX, Jiang YP, Liu N, et al. Protective effects of Astragalin on spermatogenesis in streptozotocin-induced diabetes in male mice by improving antioxidant activity and inhibiting inflammation. Biomed Pharmacother. 2019;110:561–570. doi:10.1016/j.biopha.2018.12.012
3. La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl. 2012;33(2):145–153. doi:10.2164/jandrol.111.013193
4. Shoorei H, Khaki A, Khaki AA, Hemmati AA, Moghimian M, Shokoohi M. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed Pharmacother. 2019;111:568–578. doi:10.1016/j.biopha.2018.12.054
5. Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017;84(10):1039–1052. doi:10.1002/mrd.22871
6. Makker K, Agarwal A, Sharma R. Oxidative stress and male infertility. Indian J Med Res. 2009;4:357–367. doi:10.1007/978-981-10-4017-7_10
7. Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25(2):129–136. doi:10.1016/j.jdiacomp.2010.03.003
8. Barkabi-Zanjani S, Ghorbanzadeh V, Aslani M, Ghalibafsabbaghi A, Chodari L. Diabetes mellitus and the impairment of male reproductive function: possible signaling pathways. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):1307–1314. doi:10.1016/j.dsx.2020.07.031
9. Brzóska MM, Moniuszko-Jakoniuk J, Piłat-Marcinkiewicz B, Sawicki B. Liver and kidney function and histology in rats exposed to cadmium and ethanol. Alcohol Alcohol. 2003;38(1):2–10. doi:10.1093/alcalc/agg006
10. Salter CA, Mulhall JP. Diabetes and men’s health. In: Yafi FA, Yafi NR, editors. Effects of Lifestyle on Men’s Health. Academic Press; 2019:121–147. doi:10.1016/B978-0-12-816665-9.00006-8
11. Alves MG, Martins AD, Rato L, Moreira PI, Socorro S, Oliveira PF. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta - Mol Basis Dis. 2013;1832(5):626–635. doi:10.1016/J.BBADIS.2013.01.011
12. Manocha A, Kankra M, Singla P, Sharma A, Ahirwar AK, Bhargava S. Clinical significance of reproductive hormones. Curr Med Res Pract. 2018;8(3):100–108. doi:10.1016/J.CMRP.2018.05.006
13. Johnson A, Cheng S-C, Tsou D, Kong Z-L. Attenuation of reproductive dysfunction in diabetic male rats with timber cultured Antrodia cinnamomea ethanol extract. Biomed Pharmacother. 2019;112:108684. doi:10.1016/J.BIOPHA.2019.108684
14. Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004;25(5):706–719. doi:10.1002/j.1939-4640.2004.tb02845.x
15. Arokoyo DS, Oyeyipo IP, Du Plessis SS, Chegou NN, Aboua YG. Reproductive parameters in streptozotocin-induced diabetic male wistar rats: beneficial role of basella alba aqueous leave extract. J Kerman Univ Med Sci. 2017;24(6):467–479.
16. Navarro-Casado L, Juncos-Tobarra MA, Chafer-Rudilla M, de Onzono LI, Blazquez-Cabrera JA, Miralles-Garcia JM. Effect of experimental diabetes and stz on male fertility capacity. Study in rats. J Androl. 2010;31(6):584–592. doi:10.2164/jandrol.108.007260
17. Arokoyo DS, Oyeyipo IP, Du Plessis SS, Aboua YG. Antioxidant activities of basella alba aqueous leave extract in blood, pancreas, and gonadal tissues of diabetic male wistar rats. Pharmacognosy Res. 2018;10(1):31–36. doi:10.4103/pr.pr_84_17
18. Ahmed HA. Anchomanes difformis: a multipurpose phytomedicine. IOSR J Pharm Biol Sci. 2018;13(2):62–65. doi:10.9790/3008-1302036265
19. Udje TD, Brooks NL, Oguntibeju Oluwafemi O. Medicinal activities of anchomanes difformis and its potential in the treatment of diabetes mellitus and other disease conditions.pdf. In: Goyal MR, Ayeleso AO, editors. Bioactive Compounds of Medicinal Plants: Properties and Potential for Human Health.
20. Agyare C, Boakye YD, Apenteng JA, Dapaah SO, Appiah T, Adow A. Antimicrobial and anti-inflammatory properties of anchomanes difformis (Bl.) Engl. and Colocasia esculenta (L.) Schott. Biochem Pharmacol Open Access. 2015;05(01). doi:10.4172/2167-0501.1000201
21. Adebayo AH, Binda John-Africa L, Grace Agbafor A, Elizabeth Omotosho O, Olusoji Mosaku T. Anti-nociceptive and anti-inflammatory activities of extract of Anchomanes difformis in rats. Pak J Pharm Sci. 2014;27(2):265–270.
22. Adeyemi O, Makinwa TT, Uadia RN. Ethanol extracts of roots of anchomanes difformis ENGL roots as an antihyperglycemic agent in diabetic rats. Chem J. 2015;1(3):68–73.
23. Egwurugwu J, Nwafor A, Chinko B, et al. Effects of extracts of anchomanes difformis on female sex hormones: preliminary results. Asian J Med Heal. 2016;1(6):1–9. doi:10.9734/AJMAH/2016/30286
24. Alabi TD, Brooks NL, Oguntibeju OO. Antioxidant capacity, phytochemical analysis and identification of active compounds in anchomanes difformis. Nat Prod J. 2019;09:1. doi:10.2174/2210315509666190422155347
25. Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Reports. 2012;64(1):129–139. doi:10.1016/S1734-1140(12)70739-9
26. Van Der Horst G, Maree L. SpermBlue®: a new universal stain for human and animal sperm which is also amenable to automated sperm morphology analysis. Biotech Histochemistry. 2010;84(6):299–308. doi:10.3109/10520290902984274
27. Ahmad MO, Tariq M. A study of sperm deformity index at Islamabad. Pakistan J Physiol. 2011;7(1):1–3.
28. van der Horst G, Skosana B, Legendre A, Oyeyipo P, Du Plessis S. Cut-off values for normal sperm morphology and toxicology for automated analysis of rat sperm morphology and morphometry. Biotech Histochem. 2018;1–10. doi:10.1080/10520295.2017.1380842
29. Suresh S, Prithiviraj E, Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl. 2010;33(1):22–32. doi:10.1111/j.1365-2605.2008.00949.x
30. Aboua YG, Brooks N, Mahfouz RZ, Agarwal A, Du Plessis SS. A red palm oil diet can reduce the effects of oxidative stress on rat spermatozoa. Andrologia. 2012;44:
31. Omolaoye TS, Skosana BT, Du Plessis SS. Diabetes mellitus induction: effect of different streptozotocin doses on male reproductive parameters. Acta Histochem. 2018;120(2):103–109. doi:10.1016/J.ACTHIS.2017.12.005
32. Cintra LTA, Samuel RO, Prieto AKC, Sumida DH, Dezan-Júnior E, Gomes-Filho JE. Oral health, diabetes, and body weight. Arch Oral Biol. 2017;73:94–99. doi:10.1016/j.archoralbio.2016.10.002
33. Akhigbe RE. Discordant results in plant toxicity studies in Africa: attempt of standardization. Toxicol Surv African Med Plants. 2014;53–61. doi:10.1016/B978-0-12-800018-2.00004-2
34. Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of moringa oleifera lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Biomed Res Int. 2015;2015:1–13. doi:10.1155/2015/381040
35. Nelli GB, K AS KEK. Antidiabetic effect of α-mangostin and its protective role in sexual dysfunction of streptozotocin induced diabetic male rats. Syst Biol Reprod Med. 2013;59(6):319–328. doi:10.3109/19396368.2013.820369
36. Mukhopadhyay D, Varghese AC, Nandi P, Banerjee SK, Bhattacharyya AK. CASA-based sperm kinematics of environmental risk factor-exposed human semen samples designated as normozoospermic in conventional analysis. Andrologia. 2010;42(4):242–246. doi:10.1111/j.1439-0272.2009.00984.x
37. Lu JC, Huang YF, Lü NQ. Computer-aided sperm analysis: past, present and future. Andrologia. 2014;46(4):329–338. doi:10.1111/and.12093
38. Farooq U, Malecki IA, Mahmood M, Martin GB. Correlation between objective semen analysis and fertility in Japanese quail. Theriogenology. 2018;115:23–29. doi:10.1016/J.THERIOGENOLOGY.2018.04.012
39. Khalil WA, El-Harairy MA, Zeidan AEB, Hassan MAE. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology. 2019;126:121–127. doi:10.1016/j.theriogenology.2018.12.017
40. Zhou Y, Meng T, Wu L, et al. Association between ambient temperature and semen quality: a longitudinal study of 10 802 men in China. Environ Int. 2020;135:105364. doi:10.1016/j.envint.2019.105364
41. Khaki A, Fathiazad F, Nouri M, et al. Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother Res. 2010;24(9):1285–1291. doi:10.1002/ptr.3100
42. Akomolafe SF, Odeniyi IA, Oyetayo FL, Ajayi OB. African star apple fruit pulp‐supplemented diet modulates fertility‐related biomolecules in the testis and epididymis of high‐fat diet/streptozotocin‐induced diabetic rats. J Food Biochem. 2019;43:9. doi:10.1111/jfbc.12969
43. Bo C, Zhao W, Jia Q, et al. Effects of α-zearalanol on spermatogenesis and sex hormone of male mice. Int J Clin Exp Med. 2015;8(11):1–12.
44. Armstrong JS, Rajasekaran M, Chamulitrat W, Gatti P, Hellstrom W, Sikka SC. Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radic Biol Med. 1999;26(7–8):869–880. doi:10.1016/S0891-5849(98)00275-5
45. Yeung C-H, Cooper TG. Acquisition and development of sperm motility upon maturation in the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. Boston, MA: Springer US;2002:417–434. doi:10.1007/978-1-4615-0679-9_24
46. Monsees TK, Franz M, Gebhardt S, Winterstein U, Schill WB, Hayatpour J. Sertoli cells as a target for reproductive hazards. Andrologia. 2000;32(4–5):239–246. doi:10.1046/j.1439-0272.2000.00391.x
47. Altay B, Çetinkalp Ş, Doǧanavşargil B, Hekimgil M, Semerci B. Streptozotocin-induced diabetic effects on spermatogenesis with proliferative cell nuclear antigen immunostaining of adult rat testis. Fertil Steril. 2003;80(SUPPL. 2):828–831. doi:10.1016/S0015-0282(03)00984-1
48. Kianifard D, Sadrkhanlou RA, Hasanzadeh S. The ultrastructural changes of the sertoli and leydig cells following streptozotocin induced diabetes. Iran J Basic Med Sci. 2012;15(1):623–635. doi:10.22038/ijbms.2012.4831
49. Nahid Z, Tavakol HS, Abolfazl GK, et al. Protective role of green tea on malathion-induced testicular oxidative damage in rats. Asian Pacific J Reprod. 2016;5(1):42–45. doi:10.1016/j.apjr.2015.12.007
50. Sakhaee E, Emadi L, Siahkouhi H. Histopathological evaluation of supportive effects of Rosa damascene on mice testes, following long term administration of copper sulfate _ Elsevier Enhanced Reader.pdf. Asian Pacific J Reprod. 2016;5(5):46–50. doi:10.1016/j.apjr.2015.12.008
51. Davoodi F, Taheri S, Raisi A, et al. Investigating the sperm parameters, oxidative stress and histopathological effects of salvia miltiorrhiza hydroalcoholic extract in the prevention of testicular ischemia reperfusion damage in rats. Theriogenology. 2020;144:98–106. doi:10.1016/j.theriogenology.2020.01.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.