Back to Journals » Drug Design, Development and Therapy » Volume 16
The Astragaloside IV Derivative LS-102 Ameliorates Obesity-Related Nephropathy
Authors Li Z , Yang W, Yang Y, Wu J, Luo P, Liu Y
Received 6 November 2021
Accepted for publication 20 January 2022
Published 14 March 2022 Volume 2022:16 Pages 647—664
DOI https://doi.org/10.2147/DDDT.S346546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Ziyu Li,1 Wei Yang,1 Yong Yang,1 Jianbo Wu,2 Pei Luo,3 Yong Liu1,4,5
1Department of Pathology, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 2Drug Discovery Research Center, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 3State Key Laboratories for Quality Research in Chinese Medicines, Macau University of Science and Technology, Macau, People’s Republic of China; 4Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Luzhou, People’s Republic of China; 5Medical Equipment Department, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China
Correspondence: Yong Liu, Email [email protected]
Background: Astragaloside IV is the most important bioactive component of Radix Astragali. Previous studies have shown that astragaloside IV plays an important role in the control of early- and mid-stage diabetes and late diabetic nephropathy. However, it is disappointing that the in vivo solubility of astragaloside IV and its bioavailability after oral administration are very low. We recently obtained a new water-soluble derivative of astragaloside IV-astragaloside formic acid (LS-102), which has higher bioavailability than the parent compound. In our previous study, we found that there was a significant inflammatory response in the perirenal adipose tissue of mice with obesity-related nephropathy induced by a high-fat diet (HFD), which was related to macrophage infiltration. We hypothesized that in model mice with obesity-related nephropathy, LS-102 effectively regulated the inflammatory response and pathological changes in obesity-related nephropathy through macrophages in perirenal adipose tissue. If this hypothesis is true, the effects of LS-102 and astragaloside IV on TGF-β 1/Smad signal transduction will be further investigated.
Methods: In this study, adipose stem cells and an HFD-induced obesity-related nephropathy mouse model were used to observe the regulatory effect of LS-102 on perirenal fat inflammation and the mechanism. Adipose mesenchymal stem cells were extracted from mice that were fed a normal diet and those with obesity-related nephropathy. The effects of LS-102 on the proliferation of two kinds of cells were measured by the CCK-8 method. The levels of tumor necrosis factor-α (TNF-a) and plasminogen activator inhibitor-1 (PAI-1) were measured by ELISA. Obesity-related nephropathy mice were randomly divided into five groups: the HFD group, the LAS group (HFD+low concentration of astragaloside IV [10 mg/kg], intragastrically [ig]), the HAS group (HFD+high concentration of astragaloside IV [40 mg/kg], ig), the L102 group (HFD+low concentration of LS-102 [10 mg/kg], ig) and the H102 group (HFD+high concentration of LS-102 [40 mg/kg], ig). Body weight was measured, and the levels of serum glucose, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), total cholesterol (TC), serum creatinine (Crea) and blood urea were measured. The kidneys were stained with HE, PAS and Masson’s trichrome. Perirenal adipose tissue was harvested to examine the expression of CD68, LCA, CD11C, TNF-a, TGF-β 1, Fn1, Smad2, Smad3, Smad4, and Smad7 by immunohistochemical staining, and F4/80 was examined by immunofluorescence staining.
Results: LS-102 significantly inhibited the in vitro secretion of TNF-a and PAI-1 by adipose stem cells in a concentration-dependent manner (P < 0.05). In vivo, the body weights in the LAS group, HAS group, L102 group and H102 group were significantly lower than those in the HFD group (P < 0.05). Except for that in the HFD group, the volume of perirenal adipocytes in the other groups was small and uniform (P < 0.05). Compared with the LAS, HAS, L102 and H102 groups, the HFD group had a larger glomerular cross-sectional area, proliferation of mesangial cells and the mesangial matrix, and increased matrix area/glomerular area (P < 0.05). The effect of LS-102 was better than that of astragaloside IV at the same concentration (P < 0.05). Compared with those in the HFD group, glucose, HDL-C, LDL-C and urea levels in the LAS group, HAS group, L102 group and H102 group were significantly decreased (P < 0.05). The expression of F4/80, CD68, LCA, TNF-a, CD11C, and PAI-1 in perirenal adipose tissue in the HFD group was significantly higher than that in the LAS group, HAS group, L102 group and H102 group (P < 0.05). Compared with those in the HFD group, the expression levels of TGF-β 1 and Fn1 in the HAS group, L102 group and H102 group were significantly increased (P < 0.05). Compared with the HFD group, the HAS group, L102 group and H102 group had decreased immunopositive rates of Smad2, Smad3 and Smad4 (P < 0.05). At the same concentration, the effect of LS-102 was better than that of astragaloside IV (P < 0.05). There was no significant difference in the expression of Smad7 among the different experimental groups (P > 0.05).
Conclusion: Astragaloside IV and LS-102 improved the inflammatory reaction in perirenal adipose tissue and renal pathological changes in obesity-related nephropathy model mice and inhibited the TGF-β 1/Smad signaling cascade. At the same concentration, the effect of LS-102 was better than that of astragaloside IV. These results suggest that LS-102 has a better protective effect against obesity-related nephropathy. LS-102 may be a new type of traditional Chinese medicine for the clinical treatment of obesity and its related metabolic diseases.
Keywords: LS-102, obesity-related nephropathy, inflammation, macrophages, PAI-1, TGF-β 1, Smads
Introduction
Obesity is a worldwide problem that affects human health. With improvements in the standard of living and changes in lifestyle and eating habits, the harm caused by obesity is becoming increasingly prominent. Obesity can affect the function of many systems and cause pathological changes in organs and tissues, among which obesity-related diabetic nephropathy is common. A series of cytokines produced by adipocytes can affect the kidney, and the important endocrine function of this organ may be related to obesity-related diabetic nephropathy. Obesity and inflammation have often been associated. It is believed that the accumulation of adipose tissue macrophages (ATMs) is the main cause of chronic inflammation-related obesity and metabolic syndrome.1 ATMs show at least two immunophenotypes: proinflammatory (M1) and anti-inflammatory (M2). The relative number of M1 and M2 macrophages in adipose tissue seems to be the main factor leading to chronic inflammation associated with obesity and metabolic syndrome.2 The perirenal adipose tissue of obesity-related nephropathy model mice showed obvious inflammatory reactions compared with renal tissue. Further study showed that the perirenal ATMs of obesity-related nephropathy mice were significantly polarized, mainly in the M1 proinflammatory direction. Although our obesity-related nephropathy mouse model does not reach the pathological degree of the obesity-related early diabetic nephropathy model, recent literature has reported that ectopic renal lipid accumulation is related to kidney disease, especially the development of diabetic nephropathy.3 Thus, we know that obesity-related nephropathy is very likely to develop into diabetic nephropathy with the progression of the disease, and there is a significant correlation between the two diseases. Therefore, our model and follow-up research have certain practical importance for obesity-related early diabetic nephropathy.
Plasminogen activator inhibitor-1 (PAI-1) is an important that controls fibrinolytic activity centers and can be synthesized and secreted by adipose tissue. Therefore, PAI-1 is often closely related to obesity and its associated diseases, such as type 2 diabetes, cardiovascular disease and insulin resistance.4 Recently, PAI-1 was shown to be increased in the renal tissues of humans and animals with diabetic nephropathy and is associated with an increase in interstitial macrophage recruitment.5 In contrast, PAI-1 gene knockout or the use of PAI-1 inhibitors can reduce the pathological changes in diabetic nephropathy.6,7 PAI-1 is generally not expressed in normal kidneys. However, PAI-1 is overexpressed in diabetic nephropathy. These results show that PAI-1 is produced in adipose tissue and plays an important role in the progression of diabetic nephropathy. We also studied the relationship between the inflammatory response and PAI-1 in obesity-related nephropathy. We found that PAI-1 gene knockout or the use of PAI-1 inhibitors not only reduced the pathological changes in obesity-related nephropathy but also reduced inflammation in perirenal adipose tissue by significantly reducing the number of macrophages in perirenal adipose tissue induced by a high-fat diet (HFD) and changing the direction of macrophage polarization.8 Deletion or pharmacological inhibition of PAI-1 shows a significant protective effect against obesity-related nephropathy. PAI-1 is effectively induced by the TGF-β1/Smad pathway through a Smad binding element (SBE) on the PA-1 promoter.9,10 Therefore, we further hypothesized that drugs that inhibit the TGF-β1/Smad signaling cascade may improve obesity-related nephropathy by regulating PAI-1 expression.
In the treatment of diabetes, Astragalus membranaceus, a traditional Chinese medicine with few side effects and mild pharmacological effects, is often used due to the different side effects of Western medicines. Astragalus membranaceus is a bioactive natural flavonoid that has been used medicinally for more than two thousand years. Some studies have shown that astragaloside can regulate liver glucose enzymes in type 2 diabetic rats induced by a HFD. The results showed that different doses of astragaloside not only reduced blood glucose and TGs (TGs) in rats but also inhibited lipolysis in 3T3-L1 adipocytes induced by the inflammatory factor tumor necrosis factor-α (TNF-a), reduced free fatty acids and increased insulin sensitivity. Thus, astragaloside plays a role in reducing blood sugar and blood lipids. Radix Astragali, a traditional Chinese medicine, has a variety of pharmacological properties, such as anti-inflammatory, antioxidative, cardioprotective and antidiabetic properties. However, the solubility and bioavailability after oral administration of astragaloside IV are very low, which greatly limits its clinical application. We recently obtained a new water-soluble derivative of astragaloside IV-astragaloside formic acid (LS-102). LS-102 was rapidly absorbed, attaining a maximum concentration of 248.7±22.0 ng/mL at 1.0±0.5 h after oral administration. The relative bioavailability of LS-102 was twice that of astragaloside IV. LS-102 had a Papp (mean) of 15.72–25.50×10−6 cm/s, which was almost 500-fold higher than that of astragaloside IV. An acute toxicity study showed no abnormal changes or mortality in mice treated with LS-102, even at a single high dose of 5000 mg/kg body weight.11 This result shows that LS-102 has higher absorptivity and bioavailability than astragaloside, mainly because it has higher water solubility. Furthermore, its safety is extremely high. Astragaloside IV has a wide range of therapeutic effects on diabetes and its complications, and its pharmacological action has multitarget and multisystem characteristics; therefore, astragaloside IV may become the main traditional Chinese medicine for the treatment of diabetes and its complications in the future. LS-102 makes the use of the Chinese medicine astragaloside more effective. Previous studies only discussed the regulatory effect of astragaloside IV on the kidney in diabetic nephropathy and did not study the relationship between astragaloside IV and perirenal fat inflammation in diabetic nephropathy. In this study, we used in vitro and in vivo experiments to observe inflammatory changes in perirenal adipose tissue and renal pathomorphology and the effects on obesity-related nephropathy after LS-102 treatment and then examined the molecular mechanism of its pharmacological effects.
Materials and Methods
Sample Preparation
Both astragaloside IV and LS-102 are from State Key Laboratories for Quality Research in Chinese Medicines, Macau University of Science and Technology. Astragaloside IV (>98.0%) was isolated from Radix Astragali, and LS-102 (>98.0%) was synthesized from astragaloside IV in laboratory. They were the first to describe a one-step direct reaction of TEMPO-mediated oxidation to efficiently obtain a new water-soluble derivative of astragaloside IV (astragalosidic acid, LS-102).12
Animals
Male C57BL/6J healthy SPF mice aged 8–10 weeks were used in this study. C57BL/6J mice were purchased from Beijing Huafukang Biotechnology Co., Ltd. All protocols for the use of experimental animals were reviewed and approved by the Animal Ethics Committee of Southwest Medical University and were performed in accordance with the guidelines issued by the Institutional Animal Care and Use Committee.
HFD-Fed Mouse Model
Male C57BL/6J mice aged 8–10 weeks were fed a HFD (45% fat, kcal) (D12451) for 14 weeks. Age-matched male C57BL/6J mice were fed a standard laboratory ND (14% fat, kcal) as a control group. Body weight was measured every 3 days. Blood glucose in samples from the femoral artery was measured by a glucometer. A biochemical analyzer was used to analyze the levels of blood cholesterol and TGs. Three mice were randomly selected, and were anesthetized by peritoneal injection of pentobarbital sodium. Then the mice’s kidney and perirenal adipose tissue were isolated. Immunohistochemistry was used to examine macrophages and inflammatory cells in perirenal adipose tissue. We also used RT–PCR to examine the polarization of macrophages in perirenal adipose tissue. The perirenal adipose tissue were harvested and kept in liquid nitrogen for RT–PCR analysis.Total RNA was extracted from the perirenal adipose tissue and reverse transcribed to cDNA according to the manufacturer’s protocol. Then, real-time PCR was proceeded with SYBR™ Select Master Mix. GAPDH was employed as the internal standard in our study. The ratio of CD11C, IL-6, IL-10 and CD206 was normalized with GAPDH. We examined all the results to confirm whether the model was successful.
Isolation, Culture and Treatment of Cells
Adipose tissue was subcutaneously extracted from ND C57BL/6J mice and HFD C57BL/6J mice and was transferred to petri dishes (labeled ND-ASCs and HFD-ASCs, respectively) and weighed. MSC isolation medium (1 mL/g) was added to the ND-ASC and HFD-ASC petri dishes. The adipose tissue was cut and placed into a centrifuge tube, and MSC separation medium (2.5 mL/g) was added to the centrifuge tube. Collagenase solution (0.5 mL/g) was added, digested, and centrifuged, and the supernatant was discarded. MSC separation medium (1 mL) was added to resuspend the cells. Then, 9 mL of MSC separation medium was added, mixed, and centrifuged for 10 minutes, and this process was repeated once. The cells were resuspended in 1 mL of MSC growth medium. Finally, ND-ASCs and HFD-ASCs were inoculated in culture medium. The cells were cultured in an incubator containing 5% CO2 at 37°C, and the medium was replaced every three days. The cells were subcultured twice per week, and 0.05% trypsin was used to digest the cells and carry out the subculture procedure.
Cell Proliferation Measurement
The CCK- 8 method was used to examine the effects of different concentrations of LS-102 (10 µg/mL, 20 µg/mL, 30 µg/mL, 40 µg/mL) for different times (6 hours, 12 hours, 24 hours, 48 hours) on the proliferation of ND-ASCs and HFD-ASCs. CCK-8 reagents were bought from Beyotime Biotechnology (Jiangsu, China).
Measurement of Total Cell Protein Concentration
According to the instructions, the levels of TNF-a and PAI-1 secreted by ND-ASCs in the blank control group and the DMSO control group were measured by ELISA (CUSABIO, USA). The corresponding ND-ASCs were washed with phosphate-buffered saline (PBS), lysis solution was added, and the cells were centrifuged. The total protein concentration of ND- ASCs in the experimental group and the control group was measured by the BCA method. Then, this procedure was repeated on P2 HFD-ASCs.
Measurement of TNF-α and PAI-1 Secretion
The culture supernatants of the ND-ASC experimental groups, the solvent control DMSO group and the blank control group were collected and measured by ELISA (CUSABIO, USA). Then, each experimental group of HFD-ASCs, the solvent control DMSO group and the blank control group were examined in the same way.
Administration of Astragaloside IV and LS-102
After being fed a HFD for 14 weeks, mice of similar weights were randomly divided into five groups. There were 24 mice. The HFD, LAS and HAS groups had 4 mice in each group, and the L102 and H102 groups had 6 mice in each group. The HFD group of mice was fed a HFD (45% fat, kcal) for 4 weeks. The mice in the LAS group were treated with a low concentration of astragaloside IV (10 mg/kg) by intragastric administration and were fed a HFD (45% fat, kcal) for 4 weeks. The mice in the HAS group were treated with a high concentration of astragaloside IV (40 mg/kg) by intragastric administration and continued to be fed a HFD (45% fat, kcal) for 4 weeks. The mice in the L102 group were treated with a low concentration of LS-102 (10 mg/kg) by intragastric administration and continued to be fed a HFD (45% fat, kcal) for 4 weeks. The mice in the H102 group were treated with a high concentration of LS-102 (40 mg/kg) by intragastric administration and were fed a HFD (45% fat, kcal) for 4 weeks.
Weight Measurement
Body weight was measured once per week, and changes in body weight were recorded. Body weight was measured at 9:00 a.m. each time.
Blood Analysis
Under fasting conditions, whole blood was collected from the femoral artery of mice, transferred to a centrifuge tube and centrifuged at a low speed for 10 minutes (1500 ×g). A glucometer (Accu-Check; Roche Diagnostics, Mannheim) was used to measure blood glucose levels. The serum levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), TG and total cholesterol (TC) were analyzed by an AU680 biochemical analyzer (Beckman Coulter, Indianapolis, IN). The levels of serum creatinine (Crea) and blood urea were analyzed by SRL, Inc. (Tokyo, Japan).
Histological Assessment
Perirenal adipose tissue and kidney tissue were fixed with a 4% neutral formaldehyde buffer, dehydrated and embedded in paraffin. Four-micrometer-thick sections were stained with HE, PAS and Masson trichrome. An optical microscope was used to take pictures of tissues that were stained with HE, PAS and Masson’s trichrome. To measure the size of adipocytes, adipocytes in a nonrepetitive visual field were randomly selected from each section and measured by software (Image-ProPlus 6.0). The length and width of the adipocytes were measured. Different slices were selected for quantitative measurement in each group, and finally, the average adipocyte area was calculated. PAS- and Masson-stained sections were used to analyze the extracellular matrix in glomeruli and collagen deposition in the renal interstitium. Nonoverlapping visual fields were selected in each section, and different sections in each group of mice were selected for quantitative measurement.
Immunohistochemistry and Immunofluorescence Analysis
For immunohistochemistry and immunofluorescence analysis, 5 μm frozen sections were prepared. The section was fixed in acetone at 4°C for 5 minutes. To block the binding of nonspecific antibodies, 1% BSA in 10 mmol/L LPBS was applied for 20 minutes at room temperature. The tissue sections were incubated with each antibody overnight at 4°C and washed with PBS 3 times for 5 minutes each. The secondary antibody was added and incubated for 30 minutes at 37°C. White blood cells were labeled with the LCA antibody, which is a common marker of inflammatory cells. Macrophages were labeled with the CD68 antibody. M1 macrophages were labeled with CD11C (Proteintech, Wuhan, China) and TNF-a (Bioworld Technology, USA) antibodies. M2 macrophages were labeled with TGF-β1 (Bioworld Technology, USA) and Fn1 (Bioworld Technology, USA) antibodies. The expression level of PAI-1 was examined with a PAI-1 antibody (Abcam, 168 Cambridge, UK). The expression levels of Smad2, Smad3, Smad4 and Smad7 were determined by Smad2 (Bioworld Technology, USA), Smad3 (Bioworld Technology, USA), Smad4 (Bioworld Technology, USA), and Smad7 (Proteintech, Wuhan, China) antibodies, respectively. The F4/80 antibody (Proteintech, Wuhan, China) was used to label macrophages. Goat anti-rabbit IgG labeled with Cy3 (Beyotime, Shanghai, China) was used as the secondary antibody and was incubated at room temperature for 1 hour. Images were taken by microscopy (Leica, Germany) and fluorescence microscopy (Leica, Germany). For tissue slices from the mice in each group, three different high-power fields from each of five different sections were analyzed. The total number of nuclei and the number of positive cells in each visual field were calculated. The proportion of positive cells in each sample indicated the number of nuclei of positive cells divided by the total number of nuclei in the sample section.
Statistical Analysis
The data are presented as the mean ± SEM. Differences between the two groups were compared by group t-tests, and differences between multiple groups were compared by single factor analysis of variance (one-way ANOVA). A p value <0.05 was considered to represent a statistically significant difference.
Results
Establishment of an Obesity-Related Nephropathy Model in Mice
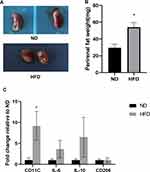
After HFD feeding for two weeks, body weights increased significantly. The mean body weight measured every other week was significantly higher than that of ND mice. Randomly selected mice were euthanized at 14 weeks. Blood glucose, cholesterol and TG levels in the HFD group were significantly higher than those in the ND group (P<0.05). The kidneys in the HFD group were significantly larger than those in the ND group. The thickness of yellowish adipose tissue on the kidney surface led to an uneven kidney surface, and the weight of the kidney and perirenal adipose tissue increased significantly in the HFD group. The expression of F4/80, CD68 and LCA in perirenal adipose tissue in the HFD group was significantly higher than that in the ND group (P<0.05). RT–PCR was used to examine the mRNA expression of inflammatory factors (IL-6, CD11C, IL-10, and CD206) in perirenal adipose tissue in the two groups. The mRNA level of CD11C was significantly increased (P<0.05). However, there were no significant differences in the mRNA expression of IL-6, IL-10, CD206 or other genes between the two groups (P>0.05). This finding suggested that the inflammatory factors secreted by M1 macrophages were the main inflammatory factors in perirenal adipose tissue. These results are consistent with our previous studies on obesity-related nephropathy model mice. The obesity-related nephropathy model was established by continuously feeding C57BL/6J mice a HFD, and the indices were consistent with the changes in obesity-related nephropathy (Figure 1). Thus, the model was successful.
Effect of LS-102 on the Proliferation of ASCs (HFD-ASCs, ND-ASCs) in vitro
The proliferation of ASCs was examined by the CCK-8 method. The results showed that different concentrations of LS-102 had no significant effect on HFD-ASCs or ND-ASCs, and there was no significant difference in proliferation in the different LS-102 treatment groups, the control group and the DMSO group (P>0.05). After HFD-ASC treatment, the cell density was highest at 6 hours. Over time, cell proliferation was inhibited to varying degrees, especially in the 30 µg/mg LS-102 group. Cell proliferation was most significantly inhibited at 12 hours and 24 hours and tended to slow down by 24 hours. Cell proliferation in the other groups decreased to varying degrees from 6 hours to 12 hours but tended to slow down by 12 hours. There was no significant difference between the control group and DMSO group that were treated with LS-102 (P>0.05). There was no significant difference in proliferation between ND-ASCs treated with different concentrations of LS-102 and cells in the control and DMSO groups. There were no obvious time- or concentration-dependent effects, indicating that LS-102 may have little effect on the proliferation of ND-ASCs (Figure 2A and B).
The Effect of LS-102 on the Secretion of PAI-1 and TNF-a by ASCs (HFD-ASCs, ND-ASCs) in vitro
ELISA was used to examine the effects of four different concentrations of LS-102 on the secretion of PAI-1 and TNF-a by ND-ASCs and HFD-ASCs. The results showed that different concentrations of LS-102 significantly inhibited the secretion of PAI-1 by ND-ASCs and HFD-ASCs compared with cells in the control group and DMSO group, and secretion was significantly decreased (P<0.05). With increasing LS-102 concentrations, the inhibitory effect on PAI-1 secretion was enhanced in a concentration-dependent manner. Similarly, compared with the control and DMSO groups, different concentrations of LS-102 had different inhibitory effects on TNF-a secretion by ND-ASCs and HFD-ASCs. However, unlike that of ND-ASCs, the secretion of TNF-a by HFD-ASCs was significantly inhibited when the concentration of LS-102 was greater than 20 µg/mL. However, there was no significant inhibition in response to 10 µg/mL, and there was no significant difference between the control group and the LS-102 group. In ND-ASCs, 10 µg/mL showed an obvious inhibitory effect, and the inhibition of TNF-a secretion was enhanced with increasing LS-102 concentrations. LS-102 also inhibited the secretion of TNF-a in ND-ASCs and HFD-ASCs fed a ND in a concentration-dependent manner (Figure 2C–F).
Astragaloside IV and LS-102 Regulate Mouse Body Weight
After successful modeling, the body weight of each experimental mouse was similar, and the difference was not statistically significant (P>0.05). A HFD was administered to each group, and the body weights in the LAS group, HAS group, L102 group and H102 group were significantly lower than those in the HFD group at the 15th week (P<0.05). Then, the body weights in the LAS group, HAS group, L102 group and H102 group decreased compared with those in the HFD group each week, indicating that astragaloside IV and LS-102 inhibited body weight gain in obese mice. At the 17th week, weight loss in the H102 group was more significant than that in the L102 group (P<0.05) (Table 1). This finding suggested that with increasing drug concentrations, the inhibitory effect on body weight gain in obese mice was stronger. The inhibitory effect of LS-102 on body weight gain in obese mice may be concentration-dependent. The results showed that LS-102 had a higher absorption rate and bioavailability than astragaloside IV, which was not reflected in the change in body weight inhibition in this experiment, and there was no significant difference between astragaloside IV and LS-102 at the same concentration (P>0.05) (Figure 3A).
 |
Table 1 Changes of Body Weight in Mice |
Astragaloside IV and LS-102 Improve Renal Pathological Changes
In this study, we used several specific staining methods to evaluate the morphology of renal pathology in the different experimental groups.
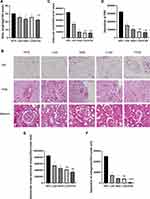
HE staining and PAS staining were performed, and the HFD group, the perirenal fat was thicker and larger, and the adipocytes were larger, uneven in size and irregular in shape. In the other experimental groups, the perirenal fat was thinner and smaller, and the adipocytes were smaller, uniform in size and regular in shape. In the HFD group, glomeruli were enlarged to varying degrees. Some glomeruli showed balloon adhesion. Glomerular capillary endothelial cells proliferated irregularly. Glomerular mesangial cells and mesangial matrix showed slight to moderate proliferation. In addition, the capillary basement membrane thickened slightly. The kidneys in the other four experimental groups were normal under a microscope. Quantitative analysis showed that the volume of perirenal adipocytes in the HFD group was larger than that in the LAS group, HAS group, L102 group and H102 group (P<0.05). Except for those in the HFD group, perirenal adipocytes in the other groups were smaller and more uniform in size. The volume of perirenal adipocytes in the LS-102 group at the same concentration was smaller than that in the astragaloside IV group (P<0.05). In the HFD group, glomeruli were enlarged, mesangial cells and mesangial matrix had proliferated, and the matrix area/glomerular area increased, which was significantly higher than that in the LAS group, HAS group, L102 group and H102 group (P<0.05). There were significant differences in renal parenchymal pathological features between the astragaloside IV group and LS-102 group at the same concentration (P<0.05). Glomeruli in the LS-102 group were smaller and more uniform, and mesangial cells and mesangial matrix hyperplasia were more improved. These results are consistent with LS-102 having a higher absorption rate and bioavailability than astragaloside (Figure 3B–E).
Masson staining was performed to observe the formation and deposition of collagen fibers in glomeruli and renal interstitium; the positive signal was blue, which reflected the degree of renal interstitial fibrosis. Collagen deposition was examined by Masson staining, and the results showed that collagen deposition in glomeruli and the interstitium increased significantly in the HFD group, and there was partial collagen deposition in glomeruli and the interstitium in the LAS group. However, in the HAS group, L102 group and H102 group, collagen deposition induced by a HFD was partially prevented, resulting in a significant decrease in collagen deposition. Collagen deposition in the H102 group was significantly lower than that in the L102 group (P<0.05). Compared with the same concentration of astragaloside IV, LS-102 prevented collagen deposition more effectively and decreased collagen significantly, especially in the high concentration group (P<0.05) (Figure 3B and F).
Astragaloside IV and LS-102 Improve Metabolic Disturbances and Renal Function
At the end of the 18th week, blood samples were taken from the femoral artery. During blood collection, it was observed that the blood in the HFD group was viscous and difficult to collect. The blood in the other groups was normal, and the blood collection process was smooth.
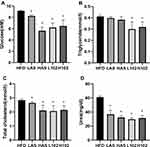
Each group showed different characteristics of blood and renal function (Figure 4). The levels of blood glucose, HDL-C, LDL-C and urea in the LAS group, HAS group, L102 group and H102 group were significantly lower than those in the HFD group (P<0.05). This finding suggested that different concentrations of astragaloside IV and LS-102 may improve renal metabolism and renal function. TC levels in the LAS group, HAS group, L102 group and H102 group decreased significantly (P<0.05). The level of TGs decreased significantly in the L102 group and H102 group (P<0.05), but there was no significant change in the LAS group and HAS group compared with the HFD group (P>0.05). Compared with those in the HFD group, Crea levels in the HAS group, L102 group and H102 group decreased significantly (P<0.05). The concentration-dependent effect of LS-102 on improving renal metabolism and renal function was not significant between the L102 group and H102 group (P>0.05) (Table 2).
 |
Table 2 Plasma Lipid and Kidney Function Characteristics |
Astragaloside IV and LS-102 Attenuate Perirenal Adipose Inflammation Induced by a HFD
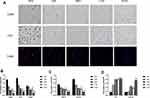
A large number of studies have shown that a HFD or high-fat intake can lead to the accumulation of a large number of macrophages in adipose tissue, thus inducing inflammation. In our previous studies, we also confirmed that the number of macrophages in the perirenal adipose tissue of obesity-related nephropathy mice was significantly greater than that in ND mice and induced inflammation in perirenal adipose tissue. F4/80 and CD68 mainly label macrophages, while LCA is a broad-spectrum marker of leukocytes. We found that the expression of F4/80, CD68 and LCA in perirenal adipose tissue in the HFD group was significantly higher than that in the LAS group, HAS group, L102 group and H102 group. The expression of F4/80, CD68 and LCA in perirenal adipose tissue in the H102 group was significantly lower than that in the L102 group (P<0.05). In groups with the same concentration of astragaloside IV group and LS-102, the expression of F4/80, CD68 and LCA in the LS-102 group was lower than that in the astragaloside IV group (P<0.05) (Figure 5A–B).
Astragaloside IV and LS-102 Alter the Polarization of Perirenal Adipose Macrophages
We quantitatively evaluated the phenotypic markers of M1 and M2 macrophages by immunohistochemistry. The results showed that the levels of proinflammatory M1 macrophage markers (TNF-a, CD11C) in the LAS group, HAS group, L102 group and H102 group were significantly lower than those in the HFD group (P<0.05). Compared with those in the astragaloside IV group, the markers of proinflammatory M1 macrophages in the LS-102 group at the same concentration decreased significantly (P<0.05). The expression levels of proinflammatory M1 macrophage markers in the H102 group were lower than those in the L102 group (P<0.05). The expression of anti-inflammatory M2 macrophage markers (TGF-β1 and Fn1) in the HAS group, L102 group and H102 group was significantly higher than that in the HFD group (P<0.05). The expression of Fn1 in the LAS group was higher than that in the HFD group (P<0.05). At the same concentration, the expression of anti-inflammatory M2 macrophage markers increased more significantly in the LS-102 group than in the astragaloside IV group (P<0.05). The expression level of anti-inflammatory M2 macrophage markers in the H102 group was higher than that in the L102 group (P<0.05) (Figure 5C and D).
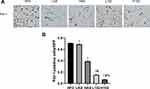
Astragaloside IV and LS-102 Reduce the Expression of PAI-1 in Perirenal Fat
Compared with that in the HFD group, the expression of PAI-1 in the perirenal adipose tissue of the LAS group, HAS group, L102 group and H102 group decreased significantly (P<0.05). Compared with that in the astragaloside IV group, the expression of PAI-1 in LS-102 group at the same concentration decreased significantly (P<0.05). The expression of PAI-1 in the H102 group decreased more significantly than that in the L102 group (P<0.05) (Figure 6).
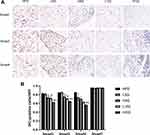
Astragaloside IV and LS-102 Inhibit the TGF-β1/Smad Signaling Cascade
To investigate the effects of astragaloside IV and LS-102 on TGF-β1/Smad signal transduction in perirenal fat in obesity-related nephropathy, immunohistochemical staining was performed. Compared with the HFD group, the HAS group, the L102 group and H102 group had decreased immunopositive rates of Smad2, Smad3 and Smad4 (P<0.05). However, the differences in Smad2, Smad3 and Smad4 immunopositivity in the LAS group were not significant (P>0.05). Compared with that in the astragaloside IV group, the immunopositivity of Smad2 and Smad3 decreased significantly in the LS-102 group at the same concentration. Compared with the HAS group, the H102 group exhibited decreased Smad4 immunopositivity (P<0.05). The expression of Smad2, Smad3 and Smad4 in the H102 group was lower than that in the L102 group (P<0.05). There was no significant difference in the expression of Smad7 among the different experimental groups (P>0.05) (Figure 7).
Discussion
Obesity is closely related to the incidence of a variety of diseases and can not only significantly increase common risk factors for a variety of diseases but also increase the prevalence of less common risk factors. Fat deposition is caused by obesity, and adipocytes in adipose tissue secrete a variety of cellular factors, inflammatory factors and bioactive mediators, including IL-6, TNF-a and PAI-1, through endocrine and paracrine pathways. These factors affect not only body weight but also insulin resistance, diabetes, blood lipid levels, blood coagulation, and fibrinolysis processes.13 Adipose tissue inflammation is clearly observed in diet-induced human and sawtooth obesity models and is considered to be a key link between obesity and type 2 diabetes with insulin resistance.14 Obesity is typically associated with subacute or chronic inflammation in adipose tissue. Although the effect of this inflammation is mild, it can also damage various internal organs, including the kidneys. In obesity, there are many kinds of inflammatory cells in adipose tissue, but the highest proportion is made up of ATMs. ATMs are the main inflammatory cells involved in adipose tissue inflammation induced by obesity. Xu et al15 first examined the increase in macrophages in the white adipose tissue of obese mice induced by a HFD. In our previous study, we found that macrophages in the perirenal adipose tissue of obesity-related nephropathy mice were significantly polarized, mainly toward the M1 phenotype, and promoted inflammation.
TNF-a and PAI-1 are two important inflammatory factors secreted by adipocytes in diabetes mellitus and their complications. TNF-a is a physiological factors that is a strong agonist of PAI-1 expression, playing an important role in the regulation of PAI-1 in many diseases. The increased level of PAI-1 is associated with obesity, and a large amount of evidence also shows that TNF-a is an important regulator of PAI-1 expression in adipose tissue. In addition, TNF-a may lead to an increase in plasma PAI-1 in obese patients.16 When some researchers examined the relationship between obesity and cardiovascular disease, it was suggested that there was a close direct relationship between TNF-a and PAI-1. There was a significant positive correlation between PAI-1 mRNA expression and serum TNF-a expression levels.17
For many years, medicinal plants have often been the main source of clinical therapeutic drugs. Astragalus membranaceus is a bioactive natural plant. Plant extracts that are rich in flavonoids have significant anti-inflammatory activity.18 These extracts relieve inflammation in mouse models of mastitis and lung injury induced by lipopolysaccharide (LPS) by reducing myeloperoxidase activity and the expression of IL-1β, IL-6 and TNF-a. Astragaloside IV is the most important bioactive component in Radix Astragali, and its level is often used as an indicator to evaluate the quality of Radix Astragali.
However, astragaloside IV has very low solubility in vivo and poor bioavailability after oral administration, which greatly limits its clinical application. In fact, previous studies have reported that the high molecular weight and low lipophilicity of astragaloside IV may limit its passive transport in the intestinal tract, which directly leads to low permeability and low bioavailability (absolute bioavailability in rats: only 2.2%).19,20 Recently, we obtained a new water-soluble derivative of astragaloside-astragaloside formic acid (LS-102), which has higher absorptivity and bioavailability than the parent compound, mainly because of its increased water solubility. Furthermore, the safety of LS-102 was extremely high, and there was no death or toxicity in the experimental animals. LS-102 is an ideal and promising astragaloside derivative oral drug candidate. An increasing number of studies have focused on Astragalus membranaceus and its active components as potential treatments for diabetes and its complications. The results suggest that astragaloside IV plays an important role in the control of early- and mid-stage diabetes and late diabetic nephropathy. In this study, we used different concentrations of LS-102 to interfere with ASCs in vitro and observe its effect on ASC proliferation and secretion of TNF-a and PAI-1 in the presence of a HFD and a ND. We intragastrically administered low-concentration (10 mg/kg) and high-concentration (40 mg/kg) astragaloside IV and LS-102 to HFD-induced obesity-related nephropathy mice to conduct drug control trials. The drug concentration was determined according to the results of the in vitro experiments.
According to our results, they showed that the secretion of TNF-a and PAI-1 by HFD-ASCs was significantly increased compared with that of ND-ASCs. In response to different concentrations of LS-102, the secretion of TNF-a and PAI-1 decreased significantly in a concentration-dependent manner. It has been suggested that obesity caused by a HFD leads to chronic inflammation of adipose tissue, and adipocytes secrete increased levels of inflammatory factors (TNF-a and PAI-1). However, whether the increase in PAI-1 was due to direct secretion by inflamed adipose tissue or was indirectly caused by the increase in TNF-a stimulating the production of PAI-1, as reported in most studies, needs to be confirmed by further research. LS-102 can significantly inhibit the secretion of TNF-a and PAI-1 by ASCs in vitro, thus reducing the degree of adipose tissue inflammation, which provides a new and important option for the treatment of obesity and its related diseases. On the other hand, we used CCK-8 assays to examine the effects of different concentrations of LS-102 on the proliferation of ASCs in vitro. The results showed that the proliferation of HFD-ASCs and ND-ASCs were not affected by different concentrations of LS-102, and there was no time- or concentration-dependent effect, which was contrary to most research results.21,22 Most previous studies have shown that astragaloside IV can promote cell proliferation. A possible reason for this effect is the different cell types. Different stem cells have different reactions and sensitivities to drugs. ASCs have the potential for multidirectional differentiation, and different differentiation directions establish different properties in cells. This effect may lead to the outcome that although most cell types are stimulated by astragaloside IV to proliferate, ASCs are inhibited. Second, HFD-ASCs were suppressed. As mentioned earlier, compared with ND-ASCs, HFD-ASCs secrete large amounts of PAI-1 and TNF-a. High concentrations of PAI-1 and TNF-a can inhibit cell proliferation.23,24 Treatment with LS-102 to reduce the secretion of PAI-1 and TNF-a only partially counteracted this inhibitory effect. Finally, cell proliferation was inhibited to varying degrees, which may explain why LS-102 slightly inhibited the proliferation of HFD-ASCs but not ND-ASCs.
In vivo, the mouse model showed renal morphological changes related to a HFD. These pathomorphological structural changes were accompanied by a significant increase in perirenal fat weight and varying degrees of renal dysfunction in the HFD group. The thickness of perirenal fat is an independent predictor of renal insufficiency in human type 2 diabetes mellitus.25 Using this model, We concluded that both astragaloside IV and LS-102 could improve perirenal adipose tissue inflammation and renal pathological changes in mice with obesity-related nephropathy, and the effect of LS-102 was better than that of the same concentration of astragaloside IV.
This study demonstrated for the first time that LS-102 effectively acted on macrophages and perirenal adipocytes to regulate the inflammatory response and pathological changes in obesity-related nephropathy. In recent years, macrophages, as important immune cells, have become a new research hotspot in the prevention and treatment of obesity. Obesity not only increases the number of macrophages but also affects the polarization of macrophages in adipose tissue. M1 macrophages mainly play a role in inflammation and immunity after infection with bacteria, viruses and other microorganisms. M2 macrophages play an important role in anti-inflammatory reactions, tissue repair and remodeling, lipid metabolism and allergic reactions. Some studies suggest that during the process of obesity induced by a HFD, the relative number of M1 and M2 macrophages in adipose tissue seems to be the main factor leading to chronic inflammation associated with obesity and metabolic syndrome.
In the early modeling process, mice fed a HFD were compared with mice fed a ND, and the perirenal fat became thicker and larger. The infiltration of macrophages and the expression of M1 macrophage markers in perirenal adipose tissue were significantly increased. However, after treatment with astragaloside IV and LS-102, the perirenal adipose tissue of mice became thinner. The infiltration of macrophages in perirenal adipose tissue was significantly decreased, and the increase in M1 macrophage markers was inhibited. M2 macrophage markers increased. The infiltration of macrophages is related to the recruitment and in situ proliferation and accumulation. Macrophage chemotactic factors play a key role in macrophage recruitment, and the most common chemotactic factor is MCP-1. Most studies have shown that the expression of MCP-1 in obese adipose tissue is significantly higher than that in the control group. Local hypoxia has an important effect on the accumulation of macrophages. The rapid expansion of adipose tissue in the development of obesity leads to insufficient local blood flow. Blood circulation cannot support tissue oxygen consumption and causes local hypoxia in adipose tissue. Eventually, ATMs accumulate. A large number of studies have reported that astragaloside IV decreases the expression of MCP-1 in a variety of cells. Astragaloside IV treatment could significantly inhibit the increase in serum levels of MCP-1 induced by LPS.26 After drug treatment, the perirenal fat of mice became thinner, adipocytes were smaller and uniform, and the shape was more regular, which alleviated the effects of local hypoxia. Therefore, we hypothesize that LS-102, as a derivative of astragaloside, can also reduce the infiltration of macrophages in perirenal adipose tissue by reducing the expression of MCP-1 in adipocytes and alleviating local hypoxia.
Studies have shown that the M1 polarization of macrophages in obesity is mainly related to the initiation of JNK, NF-κB, TLR-4 and other signaling pathways. The JNK signaling pathway is an important pathway in obesity-related metabolic responses. Knockout of the JNK gene can significantly reduce the number of macrophages in adipose tissue and significantly inhibit the polarization of ATMs to the M1 phenotype.27 Both the NF-κB and TLR-4 signaling pathways have been shown mediate the polarization of M1 macrophages. A large number of studies have examined the relationship between astragaloside IV and these pathways. This evidence suggests that astragaloside IV can improve hyperglycemia and inhibit NOD2-related NF-κB signal transduction. Astragaloside IV interacts with the MAPK signaling pathway to reduce inflammation in diabetic nephropathy rats.28 Astragaloside IV can inhibit apoptosis and inflammation induced by high glucose in human umbilical vein endothelial cells (HUVECs) by inhibiting the JNK signaling pathway.29 In other cells, such as human bronchial epithelial cells30 and endometrial cells,31 astragaloside IV can inhibit inflammation by inhibiting the NF-κB and MAPK signaling pathways. Therefore, we can infer that astragaloside IV inhibits M1 macrophage polarization mainly by inhibiting the JNK and NF-κB signaling pathways. Similarly, LS-102 may also inhibit M1 polarization in macrophages by inhibiting signaling pathways such as JNK and NF-κB. According to our results, the expression of TGF-β1 and Fn1 increased significantly after treatment with astragaloside IV and LS-102. Activation of PPAR-γ signaling, adiponectin, IL-4 and unsaturated fatty acids can significantly increase the expression of related genes in mouse M2 macrophages. However, there is currently no research on the relationship between astragaloside IV and these factors.
In this study, the expression of PAI-1 in perirenal adipose tissue decreased significantly after treatment with astragaloside IV and LS-102. This outcome may be related to the inhibitory effect of astragaloside IV on the TGF-β/Smad signaling pathway in adipocytes. Astragaloside IV has been shown to inhibit the TGF-β/Smad signaling pathway in renal tubular epithelial cells32 and HSCs (one of the key cells in hepatic fibrosis). Smads are not only signal transducers but are also transcriptional effectors that determine the fate of TGF-β-specific transcription factors. Some studies have shown that astragaloside IV has a robust activating effect on Smad7 expression.33 Smad7, which is a negative factor, can downregulate the signal transduction of TGF-β1/Smads, which leads to a decrease in PAI-1 expression. Therefore, we hypothesize that LS-102 can also decrease the expression of PAI-1 by inhibiting the TGF-β/Smad signaling pathway in perirenal adipocytes. In our previous study, we showed that PAI-1 had an obvious inflammatory effect on perirenal adipose tissue in obesity-related nephropathy, which was mainly characterized by a significant increase in the number of macrophages in perirenal adipose tissue. Macrophage markers were examined and showed that the M1 proinflammatory macrophages were predominant. However, PAI-1 gene knockout or PAI-1-039 inhibitor treatment significantly reduced the number of macrophages that infiltrated perirenal adipose tissue, accompanied by an increase in M2 anti-inflammatory macrophages, thus reducing inflammation in perirenal adipose tissue. It has been shown that the deletion or inhibition of PAI-1 can significantly improve obesity-related nephropathy. The decrease in perirenal adipocyte macrophage infiltration, decrease in M1 proinflammatory macrophages and increase in M2 anti-inflammatory macrophages may be related to the decreased expression of PAI-1. However, more evidence is needed to explain the molecular and cellular mechanisms by which PAI-1 regulates renal structural and functional changes caused by perirenal adipose tissue inflammation during metabolic disorders. According to our experimental results, TGF-β1, Smad2, Smad3 and Smad4 were downregulated in the perirenal fat of obese mice treated with astragaloside IV and LS-102, and the H102 group had the most significant downregulation of expression. The expression of Smad7 in all experimental groups showed no significant difference and was high, which may be related to the strong nonspecific staining of the antibodies. These results indicated that LS-102 had a certain inhibitory effect on the TGF-β/Smad signaling pathway in perirenal fat in obese mice, and the effect was superior to that of astragaloside IV.
Conclusion
In conclusion, our study suggests that LS-102 can significantly inhibit the secretion of TNF-a and PAI-1 by ASCs. In an obesity-related nephropathy mouse model, the weight of perirenal fat and the volume of adipocytes decreased, and renal structure and corresponding functions were significantly improved after treatment with LS-102. By significantly reducing the number of macrophages in perirenal adipose tissue induced by a HFD, changing macrophage polarization and inhibiting the TGF-β/Smad signaling cascade, perirenal adipose tissue inflammation was ultimately reduced, showing a better protective effect against obesity-related nephropathy. The therapeutic effect of LS-102 on obesity-related nephropathy was better than that of the astragaloside IV prototype. These findings suggest that LS-102 may be a new Chinese medicine preparation for the clinical treatment of obesity and its related diseases.
Data Sharing Statement
All authors have confirmed that all data and materials support their published claims.
Ethics Approval
All plans for the use of experimental animals were reviewed and approved by the Animal Ethics Committee of Southwest Medical University in accordance with the guidelines issued by the Institutional Animal Care and Use Committee.
Acknowledgments
The authors thank Professor Wu Jianbo from Southwest Medical University and Professor Luo Pei from Macau University of Science and Technology for their help.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by The Open Project Program of Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province[HYX20009]; Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University[20012]; The Science and Technology Strategic Cooperation Programs of Luzhou Municipal People’s Government and Southwest Medical University [2021LZXNYDJ11].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu U, Cha Y, Huang X, et al. Protective effects of berberine on high fat-induced kidney damage by increasing serum adiponectin and promoting insulin sensitivity. Int J Clin Exp Pathol. 2015;8(11):14486.
2. Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–894. doi:10.1007/s00125-016-3904-9
3. Opazo-Ríos L, Sebastián M, Marín-Royo G, et al. Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int J Mol Sci. 2020;21(7):2632. doi:10.3390/ijms21072632
4. Serrano R, Barrenetxe J, Orbe J, et al. Tissue-specific PAI-1 gene expression and glycosylation pattern in insulin-resistant old rats. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1563. doi:10.1152/ajpregu.00093.2009
5. Matsuo S, J M L-G, Cai X, et al. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1–overexpressing mice. Kidney Int. 2005;67(6):2221–2238. doi:10.1111/j.1523-1755.2005.00327.x
6. Jeong BY, Uddin MJ, Park JH, et al. Novel plasminogen activator inhibitor-1 inhibitors prevent diabetic kidney injury in a mouse model. PLoS One. 2016;11(6):e0157012. doi:10.1371/journal.pone.0157012
7. Nicholas SB, Eisa A, Yuelan R, et al. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 2005;67(4):1297–1307. doi:10.1111/j.1523-1755.2005.00207.x
8. Liu Y, Wang L, Luo M, et al. Inhibition of PAI-1 attenuates perirenal fat inflammation and the associated nephropathy in high-fat diet-induced obese mice. Am J Physiol Endocrinol Metab. 2019;316(2):E260–E267. doi:10.1152/ajpendo.00387.2018
9. Chen C, Sun MZ, Liu S, et al. Smad4 mediates malignant behaviors of human ovarian carcinoma cell through the effect on expressions of E-cadherin, plasminogen activator inhibitor-1 and VEGF. BMB Rep. 2010;43:554–560. doi:10.5483/bmbrep.2010.43.8.554
10. Zhu Y, Yin WL, Ba YF, et al. Transforming growth factor-1 promotes the transcriptional activation of plasminogen activator inhibitor type 1 in carcinoma-associated fibroblasts. Mol Med Rep. 2012;6:1001–1005. doi:10.3892/mmr.2012.1020
11. Qing LS, Chen TB, Sun WX, et al. Pharmacokinetics comparison, intestinal absorption and acute toxicity assessment of a novel water-soluble astragaloside IV derivative (astragalosidic acid, LS-102). Eur J Drug Metabol Pharmacokinet. 2019;44:251–259. doi:10.1007/s13318-018-0515-5
12. Qing L-S, Peng S-L, Liang J, et al. Astragalosidic acid: a new water-soluble derivative of astragaloside IV prepared using remarkably simple TEMPO-mediated oxidation. Molecules. 2017;22(8):1275. doi:10.3390/molecules22081275
13. Noriyuki O, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi:10.1038/nri2921
14. Jeffery E, Wing A, Holtrup B, et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016;24(1):142–150. doi:10.1016/j.cmet.2016.05.012
15. Xu X, Grijalva A,Skowronski A,et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activatio Cell Metab. 2013,18(6):816-830. doi:10.1016/j.cmet.2013.11.001
16. Baidong H, Mesut E, Painter CA, et al. Tumor necrosis factor alpha activates the human plasminogen activator inhibitor-1 gene through a distal nuclear factor kappaB site. J Biol Chem. 2004;279(18):18127–18136. doi:10.1074/jbc.M310438200
17. Bilgic GS, Akan G, Atalar F, et al. PAI-1 and TNF-α profiles of adipose tissue in obese cardiovascular disease patients. Int J Clin Exp Pathol. 2015;8(12):15919.
18. Walker J, Reichelt KV, Obst K, et al. Identification of an anti-inflammatory potential of Eriodictyon angustifolium compounds in human gingival fibroblasts. Food Funct. 2016;7(7):3046–3055. doi:10.1039/C6FO00482B
19. Gu Y, Wang G, Pan GY, Fawcett JP, A. J, Sun J. Transport and bioavailability studies of Astragaloside IV, an active ingredient in Radix astragali. Basic Clin Pharmacol. 2004;95(6):295–298. doi:10.1111/j.1742-7843.2004.t01-1-pto950508.x
20. Huang CR, Wang GJ, Wu XL, et al. Absorption enhancement study of astragaloside IV based on its transport mechanism in caco-2 cells. Eur J Drug Metab Pharmacokinet. 2006;31:5–10. doi:10.1007/BF03190635
21. Wang W, Jiang Y, Wang W, et al. Effect of astragaloside on biological behavior of adipose-derived stem cells in vitro. Chin Pharm J. 2013;29(2):220–224.
22. Riaz A, Rasul A, Hussain G, et al. Astragalin: a bioactive phytochemical with potential therapeutic activities. Adv Pharmacol Sci. 2018;2018(32):1–15. doi:10.1155/2018/9794625
23. Huang J, Qin S, Zhang D. Effects of simvastatin on proliferation, migration and fibrinolytic activity of vascular smooth muscle cells regulated by RhoA. J North Sichuan Med Coll. 2017;32(6):816–820.
24. Liang W, Shuyang Z, Chenxi Y, et al. Effect of PAI-1 on proliferation and ERK expression of airway smooth muscle. Chin J Mod Med. 2017;27(26):18–24.
25. Hunley TE, Li-Jun M, Valentina K. Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens. 2010;19(3):227–234. doi:10.1097/MNH.0b013e3283374c09
26. Zhang W-J, Frei B. Astragaloside IV inhibits NF-κB activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm. 2015;2015:274314. doi:10.1155/2015/274314
27. Solinas G, Vilcu C, Neels JG, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6(5):386–397. doi:10.1016/j.cmet.2007.09.011
28. Zhang Y, Tao C, Xuan C, Jiang J, Cao W. Transcriptomic analysis reveals the protection of astragaloside IV against diabetic nephropathy by modulating. Oxid Med Cell Longev. 2020; 2020:9542165.
29. Mukherjee T, Hovingh ES, Foerster EG, et al. “NOD1 and NOD2 in inflammation, immunity and disease”. Arc. Biochem Biophys. 2009;670:69–81. doi:10.1016/j.abb.2018.12.022
30. Hsieh H-L, Liu S-H, Chen Y-L, et al. Astragaloside IV suppresses inflammatory response via suppression of NF-κB, and MAPK signalling in human bronchial epithelial cells. Arch Physiol Biochem. 2020;14:1–10. doi:10.1080/13813455.2020.1727525
31. You L, Fang Z, Shen G, et al. Astragaloside IV prevents high glucose-induced cell apoptosis and inflammatory reactions through inhibition of the JNK pathway in human umbilical vein endothelial cells. Mol Med Rep. 2019;19(3):1603–1612. doi:10.3892/mmr.2019.9812
32. Wang Y-N, Zhao S-L, Su YY, et al. Astragaloside IV attenuates high glucose-induced EMT by inhibiting the TGF-β/Smad pathway in renal proximal tubular epithelial cells. Biosci Rep. 2020;40(6):BSR20190987. doi:10.1042/BSR20190987
33. Du N, Xu Z, Gao M, et al. Combination of Ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des Devel Ther. 2018;12:3517–3524. doi:10.2147/DDDT.S171286
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.