Back to Journals » Nature and Science of Sleep » Volume 14
The Association of Tonsil Hypertrophy with Pediatric Dentofacial Development: Evidence from a Cross-Sectional Study of Young Children in Shanghai, China
Authors Tong X, Li Y, Yang G, Zhang H, Jiang Y, Yu J, Da D, Zeng X, Liu Y
Received 3 July 2022
Accepted for publication 12 September 2022
Published 19 October 2022 Volume 2022:14 Pages 1867—1875
DOI https://doi.org/10.2147/NSS.S381020
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Xianqin Tong,1,2,* Yuanyuan Li,2,4,* Gang Yang,1,2 Hao Zhang,2,3 Yiwei Jiang,2,3 Jin Yu,2,3 Dongxin Da,2,3 Xiaoli Zeng,2,3 Yuehua Liu1,2
1Department of Orthodontics, Shanghai Stomatological Hospital & School of Stomatology, Fudan University, Shanghai, People’s Republic of China; 2Shanghai Key Laboratory of Craniomaxillofacial Development and Diseases, Fudan University, Shanghai, People’s Republic of China; 3Department of Pediatric Dentistry, Shanghai Stomatological Hospital & School of Stomatology, Fudan University, Shanghai, People’s Republic of China; 4Department of Preventive Dentistry, Shanghai Stomatological Hospital & School of Stomatology, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoli Zeng, Department of Preventive Dentistry, Shanghai Stomatological Hospital & School of Stomatology, Fudan University, Shanghai, People’s Republic of China, Email [email protected] Yuehua Liu, Department of Orthodontics, Shanghai Stomatological Hospital & School of Stomatology, Fudan University, Shanghai, People’s Republic of China, Email [email protected]
Purpose: The prevalence of dentofacial deformity was reportedly higher than decades ago, to which upper airway (UA) obstruction-induced sleep-disordered breathing (SDB) might contribute a lot. Tonsil hypertrophy appears relatively common in the population of young children. Given that the association between tonsil hypertrophy and pediatric dentofacial deformity remained controversial, this cross-sectional research was designed to explore the internal relationship of those among young children in Shanghai, China.
Patients and Methods: A stratified cluster sampling procedure was adopted, and a representative sample of 715 young children (8– 10 years old) was recruited. The OSA-18 quality-of-life questionnaires (OSA-18) were finished by their guardians, and well-trained orthodontists performed the oral examinations. After collecting the valuable information, the descriptions and analyses were run by statistical software (SPSS, version 26.0).
Results: 715 participants (334 boys and 381 girls) were involved in the analyses. As calculated, the current prevalence of malocclusion identified by Angle’s classification was 45.6% in this sample. No evident relation between OSA-18 scores and dentofacial abnormalities (P > 0.05) was found. With the enlargement of tonsil size, the proportion of children with triangular dental arch form (P < 0.05) and high vault palate (P < 0.001) was increasingly higher. More children with protruding profiles and fewer upright profiles were observed as the tonsil size increased, although it did not show a statistical difference (P = 0.103).
Conclusion: Dental and craniofacial growth deficiency has become more prevalent among children, demanding more concerns from health authorities. Tonsil hypertrophy plays an essential role in the direction of dentofacial development. More efforts from local health authorities should be made to enhance public propaganda and education on the prevention and interruption of tonsil hypertrophy and related dentofacial abnormalities.
Keywords: dentofacial growth, malocclusion, tonsil enlargement, sleep-disordered breathing, OSA-18
Introduction
The prevalence of dentofacial deformity among the Chinese population of children and adolescents, as is reported, has increased by 27% from the 1960s to the 21st century, which not only deteriorates oral function but also compromises psychological state.1,2 Among multiple complex factors, upper airway (UA) obstruction is one of the most common pathologies threatening pediatric dentofacial growth,3 attributed to anatomical factors like tonsil enlargement, adenoid hypertrophy, and nasal obstruction.4
Chronic airway obstruction possibly induces long-term sleep-disordered breathing (SDB) behaviors like mouth-breathing, snoring, and the most severe form of obstructive sleep apnea (OSA). If left ignored, long-term SDB might cause morbid behavioral disturbances, neurocognitive dysfunction, and even higher expression of inflammatory mediators throughout the whole system.5,6 Also, alterations in the tongue’s position and posture of the head and neck may break the balance of oral and peri-oral muscles during mouth-breathing, consequently negatively impacting the pattern of morphological growth according to Moss’s theory.7 These children tend to develop a more retrusive mandible, less pronounced nose, integrated with occlusion and dental arch abnormalities.8
Almost 65% of children with habitual mouth-breathing suffered from tonsil and adenoid enlargement, as reported by a cross-sectional study on 114 children.9 The role that tonsil enlargement plays in the process of dentofacial growth has been previously discussed within the orthodontic field and yet remained controversial. In 1987, Behlfelt et al conducted research on a sample of 73 children with enlarged tonsils in Sweden. It was figured out later that these children might tend to be afflicted with functional or morphological disorders such as short dental arches and crossbite, statistically correlated with narrow nasopharyngeal airways.10 The positive relationship between sagittal cephalometric abnormalities and tonsil grades was also confirmed.11,12 A majority of scholars and researchers obtained similar conclusions that tonsil hypertrophy might be one of the risk factors for dentofacial abnormalities.13,14 Different voices, however, also emerged. Deana and his coworkers carried out an observable cross-sectional study among children from 6 to 12 years old and found no correlation between the grades of palatal tonsil and dental arch-related parameters.15
To our best knowledge, related literature on the Chinese population of children was rare. Our cross-sectional study aimed to estimate the prevalence of dentofacial abnormalities and clarify the association of tonsil grades with dentofacial growth characteristics based on a sample of Chinese children aged about 9 years old in Shanghai. Given the frequent occurrence of pediatric dentofacial abnormalities these years, we tried to explore the connection between tonsil hypertrophy and dentofacial abnormalities among Chinese children in Shanghai, hoping to adopt intervention in the earlier stage.
Materials and Methods
Design and Participants
This cross-sectional study was conducted from March to June 2019 in Shanghai, China. A stratified-cluster sampling procedure was adopted, and a representative sample of the primary students of the fourth grade was enrolled. Briefly, two districts in the urban and rural areas in Shanghai were randomly selected. Three primary schools in every district were subsequently obtained, and two classes from the fourth grade were randomly selected. Students (mean age: 9.3 ± 0.4 years) in these classes were identified as participants in the survey. Students with tooth agenesis, any congenital disorders or diseases, and lower behavior management abilities were excluded from the study. Informed consents were obtained from all participants and their guardians, which has been approved by the Ethical Committee of the Shanghai Stomatological Hospital (2016-0007). We conducted this research in compliance with the Declaration of Helsinki.
Methods
OSA-18 Survey
OSA-18 is a specific questionnaire to classify the life quality of children with OSA risk. Compared to polysomnography (PSG), OSA-18 is more comfortable and affordable for families, concise, and suitable for large-scale epidemiological investigations. This questionnaire was chosen in our work to help the orthodontists to evaluate the OSA risk and help guardians to realize the OSA impact more comprehensively. In our research, the OSA-18 questionnaire was modified, consisting of 5 parts and 20 items (sleep disturbance, physical suffering, emotional distress, daytime problems, and caregiver concerns).16 The response categories were set from 1 (rare) to 7 (always) to reflect the frequency and severity of OSA-related symptoms like snoring. The total scores were calculated to be 140. OSA-18 applied in this study was the Mandarin version,17 and all guardians of the children were asked to finish the questionnaires.
Oral Examination
Oral examinations were conducted by three trained and certified orthodontists. Meanwhile, the data was collected and recorded by assistants according to a standard form. The inter-examiner reliability was ensured by Cohen’s kappa coefficient (value > 0.8). The examination generally required lateral profile, intra-oral, and occlusion parts. As follows, some essential items were described in detail.
According to the literature, tonsil size was graded from I to IV,18 implying tonsils were hidden within pillars, extending to pillars, beyond pillars but not to the midline, and extending to the midline.
The dentofacial abnormalities in the present study were qualitatively evaluated, including lateral profile, molar occlusion, dental arch, and palatal depth. The lateral profile was divided into upright, protruding, and concave types. Molar occlusions were collected considering the relationship between the upper and lower first permanent molars; Angle’s classes I (neutral), II (distoclusion), and III (mesioclusion) were recorded. Dental arch forms were recorded as triangular form, U-shape, and square shape. Palatal depth was assessed as a normal or high vault. Within our dentistry examination, overjet, overbite, and crossbite of anterior teeth, maxillary/mandibular crowding, and space were also carefully recorded.
Statistical Analysis
The analyses were performed by commercially available statistical software (SPSS, version 26.0). The significant difference was statistically assumed as both-sided P < 0.05.
Homogeneity and the normality of variances were analyzed by Levene’s test and Shapiro–Wilk test. The distributions of continuous variables were summarized as mean ± standard deviation (M ± SD). The analyses of continuous variables according to gender were carried out by Student’s t-test or Mann–Whitney U-test, including age, height, weight, and scores of OSA-18. Categorical variables were described by absolute frequencies and percentages. The chi-squared test was applied to determine the statistical associations among the dentofacial development variables according to gender, tonsil size, and total OSA-18 scores. Further analyses of the various items in the OSA-18 questionnaire according to tonsil size were performed using the Spearman rank correlation test to determine the strength of association.
Results
Among the 863 students from the 24 classes, 776 families agreed to participate in the survey, and 734 completed the oral examination after the exclusion of samples based on the criteria. After excluding data with apparent errors, 715 participants were involved in the result analyses, including 334 boys and 381 girls aged 8–10 years old (mean age: 9.3 ± 0.4 years). The general physiological features are presented in Table 1. Males were significantly different from females in height and weight (P < 0.05). Body mass index (BMI) was similar regardless of gender (P = 0.118). Besides, males got higher OSA-18 total scores (40.8 ± 11.9) than females (38.8 ± 11.7, P < 0.05).
 |
Table 1 Distributions of General Physical Characterizations Between Genders |
The associations between the scores of the OSA-18 and tonsil status are displayed in Table 2. Tonsil grades were found significantly different in the items of sleep disturbance (P < 0.001), physical suffering (P = 0.003) and total scores (P = 0.002). The children with normal tonsil sizes obtained scores of 38.5 ± 11.8, while children with obstructive tonsil sizes (grade III and IV) got average scores of 41.4 and 42.4, respectively (P = 0.002).
 |
Table 2 Spearman’s Rank Correlation Coefficient Between Tonsil Size and OSA-18 Questionnaire |
The distributions of different dentofacial growth variables were described and analyzed in detail in Table 3. Of those comparisons, mandibular crowding (P = 0.019) and maxillary dentition space (P = 0.045) presented a statistical difference between genders. In the analysis of OSA-18 scores, the average of this sample was calculated to be 39.8 (95% CI, 28.0–51.6). Thus, we roughly classify them into three ranks (<30, 30–45, >45) and explored the associations between OSA-18 scores and dentofacial abnormalities using the chi-square tests (Table 4) and Spearman correlation analyses (data not shown), but no correlations were found.
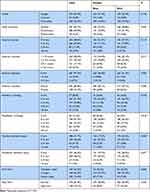 |
Table 3 Distributions of Various Dentofacial Growth Variables Between Genders |
 |
Table 4 Chi-Square Test Between Dentofacial Growth Variables and OSA-18 Grades |
We explored the associations between tonsil status and dentofacial abnormalities (Table 5). With the increase in tonsil size, the proportion of children with triangular dental arch form (P < 0.05) and high vault palate (P < 0.001) was increasingly higher. An increasing number of children with protruding profiles and fewer upright profiles were observed as tonsil size increased, although it did not show a statistical difference (P = 0.103). As for the molar relationship identified by Angle’s classification, no correlations to the tonsil size were discovered. We also explored the associations between tonsil size and anterior overbite, overjet and crossbite, and maxillary/mandibular crowding and spacing using Spearman correlation analyses, but no statistical correlation was detected (data not shown).
 |
Table 5 Chi-Square Test Between Dentofacial Growth Variables and Tonsil Size |
Discussion
Dentofacial development is a complex biological process that could be affected and remodeled by various risk factors breaking the balance. Our team adopted a stringent sampling design and standard protocols for the Chinese version of the OSA-18 questionnaire and oral examination. The cross-sectional research provided an accurate prevalence of dentofacial abnormalities in the young children population of Shanghai and compared several risk factors, including tonsil hypertrophy and SDB issues.
The current prevalence of malocclusion in mixed dentition (Table 3, 45.3%) appeared higher in comparison with the comprehensive investigation of Chinese children (35.42%) in 2002,2 but matched with the estimated overall prevalence of malocclusion in Chinese school children from 1991 to 2018 (47.92%).19 A higher prevalence of overjet, overbite, and crossbite could be observed compared to the literature.19 Occlusion in mixed dentition was not stable due to incomplete eruption, and this malocclusion would be spontaneously corrected with the growth of the mandible.20 Also, it might be attributed to the discrepancy of age, geography, race, time among selected samples, methodological diversity, and publication bias. It has been universally acknowledged that the prevalence of dentofacial deformity increased over the years in children, requiring comprehensive epidemiological researches and proactive interventions.
PSG is a golden standard decisive in the diagnosis of OSA. Speaking of the OSA-18, it was disputable whether OSA-18 could be regarded as one of the reliable predictors for OSA apart from PSG evaluation. Some supported the idea21 but others raised their doubts as well.22 As far as it goes, OSA-18 could be an abbreviated method of predicting pediatric OSA though further evidence was still required. It has been proved that the mandarin version of the OSA-18 was a reliable and valid instrument in an investigation of Chinese children aged 6–12.21 In our study, no evident relationship between dentofacial growth information and OSA-18 scores was observed by our analysis (Table 4). It suggested that OSA-18, reported as an OSA-specific questionnaire, might not be applicable and practicable for predicting dentofacial abnormalities. Most orthodontics believe breathing patterns play an essential role in the process of children’s growth and behaviors.23 The craniofacial disharmony seen in the clinic could be linked to SDB or OSA among children.24 However, the results of OSA-18 relied on subjective guardians’ reports on their children. Compared with the oral quantitative examination, personal bias has been mentioned, such as parental overreporting or ignorance of some symptoms,25 thus possibly leading to a deviated outcome.
Whether pediatric dentofacial development was associated with enlarged tonsils has been contradicted by contemporary evidence. In a cross-sectional investigation, little significant connection between malocclusion and obstructive size of tonsils was observed through analyzing the collected data of 401 children.26 Adenotonsillar surgery, statistically at least, appeared to have no benefits for dentofacial development.27 Mouth-breathing could add an environmental weight to the development of malocclusions. Other possible factors should also be taken into consideration when dentofacial growth was mentioned, like negative maxilla development, narrow airway and heredity. It indicated that elimination of tonsil obstruction would not necessarily lead to normal breathing patterns.26,27 The mainstream viewpoint generally supported that tonsil status was closely linked with dental and craniofacial growth. Chinese experts have reached a consensus that tonsil hypertrophy was defined as one of the risk factors for malocclusion.1 In the assessment of dentofacial parameters (Table 5), participants with grade IV hypertrophy of tonsils were inclined to show triangular dental arch type (P < 0.05) and high vault palates (P < 0.001). In contrast, non-obstructive tonsil enlargement would not affect the dental arch morphology and palatal depth. In addition, we could clearly figure out that the lateral profile intended to be protruding (P = 0.103). The proportion of malocclusion of both II and III types (P = 0.209) went up if tonsil size was added, although it did not show any statistical difference. Among ones with class III malocclusion in this study, children with tonsil grades III and IV (17.7%) were more likely to develop class III malocclusion than those with grades I and II (8.5%). Some researchers believed that independent tonsil hypertrophy correlated with class III malocclusion, while a higher rate of class II would be detected if combined with nasal or adenoid obstruction.9,28 The others shared the opinion that patients with tonsil grade III and IV were more likely to develop class II malocclusion than those with I and II.12 The mixed dentition of young children could naturally result in class II malocclusion in some cases.29 Combining our results with current literature, we believed the severity of obstructive tonsil enlargement could be a crucial indication for SDB and dentofacial growth, requiring preventive and interceptive treatments in the early stage.
Honestly, there were some deficiencies in our research. The first limitation was the absence of cephalometric data like BNS and ANB. The lateral profile, selected as a qualitative representative of facial disharmonies, might not satisfy a more detailed quantified analysis if further studies on tonsil grades were required. Similarly, dental arch morphology was divided into three types, and it would be better if inter-canine width, inter-molar width, arch length, and perimeter were added to the research. Also, it would be better if palatal classification and tonsil grades were combined and considered in our oral examination. Furthermore, there were many other predictive methods for assessing pediatric SDB or OSA when PSG was unavailable.30 We would like to know better whether these convenient tools could help predict the direction of dentofacial growth in the future.
Dentofacial deficiency not only impairs multiple functions but also directly connects with the quality of a child’s whole life. Issues on dentofacial abnormalities induced by adenoid hypertrophy have raised more concerns than decades ago among parents and doctors in communities. However, the tonsil enlargement has not been sufficiently focused. We call for more efforts from local government and health authorities to enhance public propaganda and education on preventive and interceptive measurements for related dentofacial abnormalities. Meanwhile, further studies on tonsil-related dentofacial growth should necessarily be attached with great importance. More resources of versatile and multidisciplinary combinations could be introduced into the clinics mutually. Local joint departments of otorhinolaryngology and orthodontics in communities could be cultured and incubated.
Conclusion
Dental and craniofacial growth deficiency has become more prevalent among Chinese children than before, demanding more concerns from health authorities. The tonsil hypertrophy was significantly related to SDB issues and dentofacial development. More efforts from local health authorities should be made to enhance public propaganda and education on preventing and interrupting of tonsil hypertrophy and related dentofacial abnormalities.
Acknowledgments
We are grateful to the participants for their contributions to the research. This work was supported by Clinical Research Plan of Shanghai Shenkang Hospital Development Center (No. SHDC2020CR2043B) and Shanghai Municipal Health Commission (No. 2019SY041 and No. GWV-10.2-YQ16).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li X-B, Ye Q-F, He H, et al. China experts’ consensus on preventive and interceptive orthodontic treatments of malocclusions of children. West Chin J Stomatol. 2021;39(4):369–376. doi:10.7518/hxkq.2021.04.001
2. Fu M, Zhang D, Wang B, et al. The prevalence of malocclusion in China – an investigation of 25,392 children. Chin J Stomatol. 2002;37(5):371–373.
3. Clark WD. Preventing dentofacial abnormalities with the proper correction of pediatric upper airway obstruction. Arch Otolaryngol. 2005;131(10):916–918. doi:10.1001/archotol.131.10.916
4. Montgomery-Downs HE, Gozal D. Sleep habits and risk factors for sleep-disordered breathing in infants and young toddlers in Louisville, Kentucky. Sleep Med. 2006;7(3):211–219. doi:10.1016/j.sleep.2005.11.003
5. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–2667. doi:10.1161/circulationaha.105.556746
6. Allen A, Jen R, Mazzotti D, et al. Symptom subtypes and risk of incident cardiovascular and cerebrovascular disease in a clinic-based obstructive sleep apnea cohort. J Clin Sleep Med. 2022;18:2093–2102. doi:10.5664/jcsm.9986
7. Moss ML, Salentijn L. The primary role of functional matrices in facial growth. Am J of Orthod. 1969;55(6):566–577. doi:10.1016/0002-9416(69)90034-7
8. Markkanen S, Rautiainen M, Niemi P, et al. Is securing normal dentofacial development an indication for tonsil surgery in children? A systematic review and meta-analysis. Int J Pediatr Otorhi. 2020;133:110006. doi:10.1016/j.ijporl.2020.110006
9. Nunes WR, Di Francesco RC. Variation of patterns of malocclusion by site of pharyngeal obstruction in children. Arch Otolaryngol. 2010;136(11):1116–1120. doi:10.1001/archoto.2010.187
10. Behlfelt K, Linder-Aronson S, Mcwilliam J, et al. Dentition in children with enlarged tonsils compared to control children. Eur J Orthod. 1989;11(4):416–429. doi:10.1093/oxfordjournals.ejo.a036014
11. Diouf JS, Ngom PI, Fadiga MS, et al. Influence of tonsil size on sagittal cephalometric measurements. Int Orthod. 2015;13(2):149–163. doi:10.1016/j.ortho.2015.03.007
12. Diouf JS, Ngom PI, Sonko O, et al. Influence of tonsillar grade on the dental arch measurements. Am J Orthod Dentofacial Orthop. 2015;147(2):214–220. doi:10.1016/j.ajodo.2014.10.028
13. Kim D-K, Rhee CS, Yun P-Y, et al. Adenotonsillar hypertrophy as a risk factor of dentofacial abnormality in Korean children. Eur Arch Oto-Rhino-L. 2015;272(11):3311–3316. doi:10.1007/s00405-014-3407-6
14. Diouf JS, Diallo BK, Diop-Ba K, et al. Relationships between the obstructive character of the tonsils and the type of ventilation and lip posture. Int Orthod. 2018;16(2):349–360. doi:10.1016/j.ortho.2018.03.007
15. Perez I, Alves N, Lizana C, et al. Influence of the palatine tonsil grade on the morphology of the maxillary and mandibular dental arches. Int J Morphol. 2020;38(5):1201–1207. doi:10.4067/S0717-95022020000501201
16. Li Y, Wu J, Guo J, et al. The efficacy of different treatment approaches for pediatric OSAHS patients with mandibular retrognathia: study protocol for a multicenter randomized controlled trial. Trials. 2020;21(1):595. doi:10.1186/s13063-020-04398-9
17. Kang K-T, Weng W-C, Yeh T-H, et al. Validation of the Chinese version OSA-18 quality of life questionnaire in Taiwanese children with obstructive sleep apnea. J Formos Med Assoc. 2014;113(7):454–462. doi:10.1016/j.jfma.2012.10.002
18. Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryng Head Neck. 2002;127(1):13–21. doi:10.1067/mhn.2002.126477
19. Lin M, Xie C, Yang H, et al. Prevalence of malocclusion in Chinese schoolchildren from 1991 to 2018: a systematic review and meta-analysis. Int J Paediatr Dent. 2020;30(2):144–155. doi:10.1111/ipd.12591
20. Birgit T, Lucia P, Clementina I, et al. Prevalence of malocclusion and orthodontic treatment need in children and adolescents in Bogota, Colombia. An epidemiological study related to different stages of dental development. Eur J Orthod. 2001;23(2):153–167. doi:10.1093/ejo/23.2.153
21. Huang Y-S, Hwang F-M, Lin C-H, et al. Clinical manifestations of pediatric obstructive sleep apnea syndrome: clinical utility of the Chinese-version Obstructive Sleep Apnea Questionnaire-18. Psychiat Clin Neurosci. 2015;69(12):752–762. doi:10.1111/pcn.12331
22. Ishman SL, Yang C-J, Cohen AP, et al. Is the OSA-18 predictive of obstructive sleep apnea: comparison to polysomnography. Laryngoscope. 2015;125(6):1491–1495. doi:10.1002/lary.25098
23. Festa P, Mansi N, Varricchio A, et al. Association between upper airway obstruction and malocclusion in mouth-breathing children. Acta Otorhinolaryngo. 2021;41(5):436–442. doi:10.14639/0392-100x-n1225
24. Katyal V, Pamula Y, Martin AJ, et al. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: systematic review and meta-analysis. Am J Orthod Dentofac. 2013;143(1):20–U204. doi:10.1016/j.ajodo.2012.08.021
25. Huynh NT, Morton PD, Rompre PH, et al. Associations between sleep-disordered breathing symptoms and facial and dental morphometry, assessed with screening examinations. Am J Orthod Dentofac. 2011;140(6):762–770. doi:10.1016/j.ajodo.2011.03.023
26. Souki BQ, Pimenta GB, Souki MQ, et al. Prevalence of malocclusion among mouth breathing children: do expectations meet reality? Int J Pediatr Otorhi. 2009;73(5):767–773. doi:10.1016/j.ijporl.2009.02.006
27. Lofstrand-Tidestrom B, Hultcrantz E. Development of craniofacial and dental arch morphology in relation to sleep disordered breathing from 4 to 12 years. Effects of adenotonsillar surgery. Int J Pediatr Otorhi. 2010;74(2):137–143. doi:10.1016/j.ijporl.2009.10.025
28. Iwasaki T, Sato H, Suga H, et al. Relationships among nasal resistance, adenoids, tonsils, and tongue posture and maxillofacial form in Class II and Class III children. Am J Orthod Dentofac. 2017;151(5):929–940. doi:10.1016/j.ajodo.2016.10.027
29. Tinano MM, Godinho J, Becker HMG, et al. Prevalence of malocclusion in children with upper airway obstruction. Rev Port Estomatol M. 2017;58(4):199–204. doi:10.24873/j.rpemd.2017.12.209
30. Wu C-R, Tu Y-K, Chuang L-P, et al. Diagnostic meta-analysis of the Pediatric Sleep Questionnaire, OSA-18, and pulse oximetry in detecting pediatric obstructive sleep apnea syndrome. Sleep Med Rev. 2020;54:101355. doi:10.1016/j.smrv.2020.101355
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
