Back to Journals » International Journal of General Medicine » Volume 14
The Association of Serum Lactate Level with the Occurrence of Contrast-Induced Acute Kidney Injury and Long-Term Prognosis in Patients Undergoing Emergency Percutaneous Coronary Intervention
Authors Yan G, Wang D, Tang C , Ma G
Received 16 April 2021
Accepted for publication 18 June 2021
Published 1 July 2021 Volume 2021:14 Pages 3087—3097
DOI https://doi.org/10.2147/IJGM.S316036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Gaoliang Yan, Dong Wang, Chengchun Tang, Genshan Ma
Department of Cardiology, Zhongda Hospital of Southeast University Medical School, Nanjing, Jiangsu, People’s Republic of China
Correspondence: Genshan Ma
Department of Cardiology, Zhongda Hospital of Southeast University Medical School, No. 87 Dingjiaqiao, Gulou District, Nanjing, Jiangsu, 210009, People’s Republic of China
Tel/Fax +86 25 83262410
Email [email protected]
Objective: The association of lactate and contrast-induced acute kidney injury (CI-AKI) has not been well established. This prospective study was planned to identify the effects of lactate level on the occurrence of CI-AKI and long-term prognosis with acute myocardial infarction (AMI) patients undergoing emergency percutaneous coronary intervention (PCI).
Methods: A total of 280 patients with AMI who underwent emergency PCI were selected from March 2018 to March 2019. A receiver operating characteristic (ROC) curve was used to analyze the optimal cut-off value of lactate on predicting CI-AKI after PCI. A multivariable logistic regression model was used to explore the significant predictors that might affect the occurrence of CI-AKI after univariate analysis. The primary endpoints were clinical outcomes including events: a combined endpoint of major adverse cardiovascular events, re-hospitalization due to heart failure, and worsening renal function. The Cox regression model was further used to analyze the predictors of the long-term prognosis after PCI.
Results: Among the 280 patients, 64 patients (22.9%) developed CI-AKI after emergency PCI procedure. Multivariable logistic regression analysis revealed that baseline lactate level was the independent risk factor for the development of CI-AKI (OR, 3.657; 95% CI, 2.237– 5.978; p< 0.001). The area under the ROC curve for predicting CI-AKI of lactate was 0.786, and the optimum cut-off point of lactate was 3.02 mmol/L, with sensitivity of 65.6% and specificity of 85.2%. The incidence of primary endpoints in the high lactate group (lactate ≥ 3.02mmol/L) was significantly increased compared with the control group [26.3% (42/160) vs 15.8% (19/120), χ2=4.430, p=0.035]. Cox regression analysis also confirmed high lactate was an independent predictor for primary endpoint outcomes at 1-year follow-up (HR, 1.916; 95% CI, 1.118– 3.285; p=0.018).
Conclusion: Our study demonstrates that baseline high lactate levels may be associated with an increased risk of CI-AKI and are the important predictors of long-term poor cardiorenal outcomes in AMI patients undergoing emergency PCI.
Keywords: myocardial infarction, contrast media, acute kidney injury, lactate, percutaneous coronary intervention, prognosis
Background
Iatrogenic renal impairment suffered from contrast media is the third leading cause of hospital-acquired acute renal failure.1,2 Contrast-induced acute kidney injury (CI-AKI) is closely related to hospital mortality, 1- and 2-year mortality.3,4 Continuous elevation of lactate indicators indicates adverse clinical consequences.5 Lactate is closely related to capillary perfusion and is a good microcirculation biomarker.6–9 Blood lactate can reflect the oxygen supply and metabolism of tissues and insufficient perfusion,10 which may be related to the occurrence of CI-AKI. For acute myocardial infarction (AMI), acute changes in hemodynamics may cause changes in blood lactate due to impaired cardiac function. This study was planned to identify the effects of lactate level on the occurrence of CI-AKI and long-term prognosis with AMI patients undergoing emergency percutaneous coronary intervention (PCI).
Materials and Methods
Study Design
This project was a prospective and registration study. Of AMI participants who underwent emergency PCI in Zhongda Hospital Affiliated to Southeast University from March 2018 to March 2019, 280 were selected. The criteria for admission were: (1) the diagnosis of ST-segment elevation myocardial infarction(STEMI) was in accordance with the 2015 guidelines for the diagnosis and treatment of acute ST-segment elevation myocardial infarction of the Cardiovascular Diseases Branch of the Chinese Medical Association; (2) the diagnosis of non-acute ST-segment elevation myocardial infarction (NSTEMI) was in accordance with the guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndrome formulated by the Cardiovascular Diseases Branch of the Chinese Medical Association. Exclusion criteria were: (1) hemodynamic instability before emergency PCI; (2) long-term renal replacement therapy (including hemodialysis and peritoneal dialysis); renal transplantation; (3) asthma attack, chronic obstructive pulmonary disease, pulmonary fibrosis and pulmonary heart disease; (4) diabetic ketoacidosis; (5) malignant tumors; (6) CT, MRI and other contrast agent examinations within 14 days before admission; (7) death or emergency coronary artery bypass grafting during emergency PCI; (8) contrast agent allergy; (9) the use of nephrotoxic drugs (including large doses of loop diuretics, non-steroidal anti-inflammatory drugs other than aspirin, aminoglycosides, amphotericin B and traditional Chinese medicine containing aristolochic acid, etc.) in the past two weeks. This study was approved by the Ethics Committee of our hospital, and all selected patients provided informed consent.
CI-AKI Criteria and Lactate Assay
According to the criteria formulated by the European Association for Urogenital Radiation published in 2011,11 CI-AKI refers to the exclusion of renal function damage caused by other reasons. Between 48 and 72 hours after the application of contrast medium, the serum creatinine (SCr) increased by more than 44.2 umol/L or 25% compared with the basic SCr. SCr levels were measured before and after emergency PCI for 2–3 days. The blood samples for lactate assay were collected at baseline coronary angiography by the sheath of the radial/femoral artery, and then measured by ABL720 blood gas analyzer (Danish Reddo Company). The estimated glomerular filtration rate (eGFR) was calculated according to the modified MDRD formula by Chinese chronic kidney disease patients’ data (eGFR = 175 × SCr−1.234 × age−0.179 × [0.79 (if female)]), where the unit of SCr is mg/dl.
Emergency PCI and Clinical Medication
Emergency PCI is performed in the following STEMI and NSTEMI patients. (1) STEMI patients with chest pain within 12 hours of onset or with new left bundle branch block; and also emergency PCI should be considered in patients with clinical and/or electrocardiographic evidence of progressive ischemia within 12 to 24 hours after onset. (2) NSTEMI patients with an extremely high risk of myocardial ischemia, and NSTEMI patients with a GRACE score of >140 and many other high-risk factors.
All patients were operated on by interventional physicians in the catheter room of our hospital. Seldinger puncture was used to puncture the radial/femoral artery. Coronary angiography was performed by JudKins method. In principle, according to the specific results of coronary angiography, emergency PCI only deals with criminal vessels. The operation method and operation time were not limited. All emergency PCI patients were treated with a minimum required amount of iodixanol contrast agent. The type of stent used in emergency PCI was determined by the interventional physician according to the clinical condition of patients. All patients were treated with load-dose double antiplatelet aggregation drugs (aspirin 300mg and clopidogrel 300 mg, or ticagrelor 180 mg) before operation. Antiplatelet drugs and statins were used after operation; vasoactive drugs (such as dopamine, sodium nitroprusside, etc.), intra-aortic balloon pump, platelet membrane glycoprotein II b/IIIa receptor antagonist, and the use of β-receptor blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, nitrates and calcium antagonists were determined by physicians based on respective clinical conditions. During the study period, nephrotoxic drugs (including large doses of loop diuretics, non-steroidal anti-inflammatory drugs other than aspirin, aminoglycosides, amphotericin B and traditional Chinese medicine containing aristolochic acid, etc.) were avoided.
Follow-Up and Endpoints
All patients had a follow-up evaluation at a clinic visit or via telephone contact at 1 year. The primary endpoints were clinical cardiorenal outcomes including events: a combined endpoint of major adverse cardiovascular events (MACE, defined as: all-cause death, non-fatal myocardial infarction, target vascular revascularization), re-hospitalization due to heart failure, and worsening renal function (defined as: chronic kidney disease classification worsening, dialysis or hemofiltration due to symptoms or signs of uremic syndrome or management of refractory hypervolemia, hyperkalemia or acidosis).
Statistical Analysis
SPSS 19.0 statistical software was used for statistical analysis. The measurement data were expressed by mean + standard deviation or median and interquartile range, and the comparison between groups was expressed by independent sample t-test or Mann–Whitney U-test, where appropriate. The counting data were expressed by percentage and the comparison between groups was tested by chi-square analysis or Fisher’s exact test. A multivariable logistic regression model was subsequently used to explore the risk factors that might affect the occurrence of CI-AKI after univariate analysis. To determine the accuracy and respective best cut-off values of lactate for predicting CI-AKI, the receiver operating characteristic (ROC) curve and the corresponding area under curve (AUC) were used. The survival analysis was performed by Kaplan–Meier method, and the comparison between the two groups was performed by log rank test. Multivariate Cox regression analysis combined with the backward stepwise method was used to analyze the predictive factors of clinical outcomes in AMI patients during 1-year follow-up after PCI. Two-sided p-values of <0.05 were considered statistically significant.
Results
Baseline Characteristics
In our study, 280 patients were consecutively enrolled (Figure 1). The mean age of them was 63 ± 13 years (range: 26–93), and 218 patients (77.9%) were male. Of these patients, 179 (63.9%) had hypertension, 85 (30.4%) were previously diagnosed with diabetes mellitus, and 27 (9.6%) received previous percutaneous coronary intervention (pre-PCI). Overall, 64 patients (22.9%) developed post-operative CI-AKI.
 |
Figure 1 Study flow chart. |
Baseline clinical characteristics are summarized in Table 1. The patients with CI-AKI were older, and they were more likely to be smokers and to have received pre-PCI on admission (p<0.05). Body mass index (BMI) and preoperative lactate levels were higher, while eGFR, left ventricular ejection fraction (LVEF), hemoglobin, and albumin levels were lower. There was no significant difference in gender, systolic/diastolic blood pressure, heart rate, hypertension, diabetes, stroke, old myocardial infarction (OMI), the prevalence of STEMI, contrast volume and in-hospital medications between the groups (p>0.05). Participants with CI-AKI on average were more likely to have higher hospitalization time and costs, and had more left anterior descending vessels for culprit lesion, and a higher percentage use of IABP during emergency PCI (p<0.05).
 |
Table 1 Baseline Clinical Characteristics Between Patients with CI-AKI and Those without CI-AKI |
Correlation Between Preoperative Lactate and the Occurrence of CI-AKI
Spearman correlation analysis revealed that there was a significantly positive correlation between preoperative lactate and the occurrence of CI-AKI (r=0.416, p<0.001). In univariate regression analysis, age, BMI and smoking, pre-PCI, levels of LVEF and lactate, hemoglobin, albumin, the culprit lesion for LAD, and the use of IABP were significantly associated with the development of CI-AKI. A multivariable logistic regression model was used after univariate analysis. Multivariate logistic regression analysis revealed that baseline lactate level and IABP were the independent risk factors for the development of CI-AKI. However, the levels of eGFR, LVEF, and albumin were the protective factors for the CI-AKI. See Table 2 for details.
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis of CI-AKI Risk Factors |
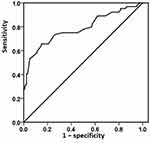
On ROC curve analysis (Figure 2), the lactate level was an accurate predictor for the development of CI-AKI; the AUC was 0.786 for the baseline lactic acid level (95% CI: 0.712–0.860, p<0.001). The optimum cut-off point of lactate was 3.02mmol/L, with sensitivity of 65.6% and specificity of 85.2%.
According to the lactate level based on the optimal cut-off value, we divided the patients into 2 groups: the high lactate group, 160 patients (lactate ≥3.02mmol/L), and the control group, 120 patients (lactate <3.02mmol/L). Noteworthily, the level of eGFR in the high lactate group was significantly lower than that in the control group [(71 ± 23) vs (83± 22), p<0.001]. Lactate was negatively correlated with eGFR (r=−0.268, p<0.001), The correlations of lactate with eGFR are shown in Figure 3A. We found the incidence of CI-AKI in high lactate group was significantly increased compared with the control group [31.3% (50/160) vs 11.7% (14/120), p<0.001, Figure 3B].
 |
Figure 3 The correlation between lactate and eGFR (A), and the occurrence of CI-AKI among 2 groups (B). |
Analysis of Lactate and Long-Term Clinical Outcomes
The 1-year follow-up results for each group are shown in Table 3. Patients with high preoperative lactate levels (≥3.02mmol/L) had worse clinical outcomes, with a greater incidence of primary endpoints [26.3% (42/160) vs 15.8% (19/120), p=0.035], demonstrating that elevated lactate level was associated with poor cardiorenal outcomes. There were no significant differences in all-cause death, non-fatal myocardial infarction, target vascular revascularization and MACE between the two groups. However, the incidence of re-hospitalization due to heart failure and worsening renal function was significantly increased in the high lactate group. The respective Kaplan–Meier curves are shown in Figure 4.
 |
Table 3 The 1-Year Follow-Up Clinical Outcomes |
 |
Figure 4 The Kaplan–Meier analysis curve of clinical outcomes after 1-year follow-up among 2 groups. |
The results of Cox regression analysis are presented in Table 4. After adjusting for potential confounding factors, such as male, elderly (≥60y), smoking, hypertension, diabetes mellitus, OMI, pre-PCI, stroke, STEMI, LAD-culprit, lower LVEF (<40%), hypoalbuminemia (albumin <40 g/L), eGFR<60mL/min, IABP, CI-AKI and high lactate, multivariate Cox regression analysis showed that high lactate was an independent predictor for primary endpoint outcomes at 1-year follow-up (hazard ratio [HR], 1.916; 95% CI, 1.118–3.285; p=0.018). Other variables showing an independent prognostic impact were CI-AKI (HR, 2.075; 95% CI, 1.180–3.649; p=0.011) and elderly (HR, 2.537; 95% CI, 1.332–4.833; p=0.005) in AMI patients undergoing emergency PCI after 1 year.
 |
Table 4 Univariate and Multivariate Cox Regression Analysis for Predictors of Primary Endpoints |
Discussion
In this study, we investigated the predictive value of preoperative lactate level on CI-AKI and long-term adverse cardiorenal outcomes in patients with AMI after emergency PCI. In agreement with many other studies,12–14 the occurrence of CI-AKI caused enormous influences in clinical practice, prolonging the length of stay and increasing the cost of hospitalization. Our study also demonstrated that lactate level was independently associated with an increased risk of CI-AKI, and then found that a lactate cut-off of ≥3.02 mmol/L was an important predictor of long-term poor cardiorenal outcomes in AMI patients undergoing emergency PCI.
Kidneys are prone to ischemic injury because of their unique blood circulation, in which renin is usually injected with hypoxic tension and has limited reserve. Ischemia-reperfusion injury induced by revascularization, low cardiac output and renal vasoconstriction after myocardial infarction are inevitable Other possible mechanisms include formation of reactive oxygen species, the inflammatory response, medullary hypoxia, and direct cytotoxicity on glomerular cells during the use of iodine contrast agents.15 All these factors lead to a primary tubular damage in the development of CI-AKI.16
Serum lactate is a well-known biomarker of tissue ischemia, and is commonly used as a diagnostic and prognostic tool in the intensive care environment. Not only were measurements at a single point in time associated with prognosis, but the duration and area under the curve of increased lactate levels in different patient groups were also associated with morbidity (organ failure) and mortality.17 For example, Schollin-Borg et al found blood lactate was a useful indicator for the medical emergency team,18 and Shetty et al demonstrated that a serum lactate cut-off of ≥2 mmol/L screened for suspected sepsis was a risk stratification tool for in-hospital adverse outcomes in emergency department patients.8 Zhang and Ni used a normalized lactate load model to account for the magnitude and time of lactate in the measurement duration and demonstrated that normalized lactate load was independently associated with acquiring cardiac surgery-associated AKI in patients undergoing cardiopulmonary bypass.19
To our knowledge, AMI is usually associated with the effect of tissue oxygenation, which may be due to insufficient oxygen supply or inhibition of mitochondrial pyruvate dehydrogenase complex.20 When oxygen supply exceeds the capacity of diseased vessels, hypoxia forces myocardium to change from an aerobic metabolism to an anaerobic metabolism, leading to lactate accumulation.10 As previously reported, lactate was the strongest predictor as a determinant of global tissue hypoxaemia. The marked increase of serum lactate may be due to the hypoperfusion of spleen and kidney, resulting in the decrease of lactate clearance during cardiac revascularization procedures.19 Gatien et al demonstrated that intravenous lactate levels were very sensitive to the diagnosis of AMI, especially in patients with chest pain for more than two hours.21 Moreover, Vermeulen et al found higher lactate levels were independently related to 30-day mortality and an overall worse response to PCI. Rapid point-of-care measurement of arterial lactate at presentation in patients with STEMI has the potential to improve acute risk stratification.22 Janine et al found arterial blood lactate at admission of >5 mmol/L was an independent predictor for 30-day mortality in patients with cardiogenic shock after AMI.23 Liu et al found that a lactic acid level of ≥4.5 mmol/L was an independent predictor of death in AMI-cardiogenic shock patients at 1 year.24 Despite the specificity of such a cut-off being addressed, this finding suggested a sensitive power of lactate in predicting cardiac death with AMI patients during different follow-up periods. However, our results were inconsistent with those previous studies. We found that higher lactate was associated with postoperative major cardiorenal complications after 1 year, mainly re-hospitalizations for congestive heart failure or worsening renal function. But there were no statistically significant differences in the incidence of MACE including all-cause death, non-fatal myocardial infarction and target vessel revascularization between patients with and without high lactate. It’s worth emphasizing that, contrary to the studies mentioned above, most of our patients do not have hemodynamic instability, and we recruited nearly 55% of NSTEMI patients all of whom had emergency PCI. Therefore, the increase in lactate levels caused by insufficient perfusion of heart and kidney due to hemodynamic instability is not obvious. Our results also show that patients with poor baseline renal function (lower eGFR) have higher levels of lactate, which supports our findings related to lactate as an indicator of kidney function. Therefore, it is easy to conclude that patients with poor baseline renal function have a significantly worse cardiorenal prognosis, especially a higher rate of re-hospitalization due to heart failure or worsening renal function.
There are also many shortcomings in this study. (1) As a single-center, observational study, with a small sample size, the conclusion had certain limitations; use of multivariable regression analysis meant it was difficult to fully control the differences in baseline characteristics between different groups. (2) This study only tested for a single lactate, and did not observe dynamically the changes in lactate and creatinine levels after PCI. (3) The level of lactate is affected by many factors. Hypotension, stress and glycometabolic dysregulation may also lead to increased lactate levels. One single lactate measurement can provide insight into the hemodynamic condition of the patient, but the fact that patients with high patency rates have lower lactate levels could mean that, after successful primary PCI, lactate levels normalize. (4) The evaluation of renal function after PCI was limited to the change of creatinine level within 72 hours, and the long-term monitoring of renal function such as one month or even one year was lacking. (5) This study did not compare with other markers such as Cystin C, myeloperoxidase, etc., and the mechanism of CI-AKI occurrence was not deep enough. (6) We collected patients’ serum creatinine and lactate levels at baseline during coronary angiography. However, baseline serum creatinine and lactate measured on admission cannot be considered true baseline values as an increase may have already occurred before hospital admission.
Conclusions
The results of this study suggest that preoperative high lactate levels may be an independent risk factor in patients who developed CI-AKI and are the important predictor of long-term poor cardiorenal outcomes with AMI undergoing emergency PCI. This finding needs to be confirmed by further large samples and prospective randomized controlled trials in other institutional settings.
Data Sharing Statement
Data related to this paper can be made available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
I confirm that I have read the Editorial Policy pages. This study was conducted with approval from the Ethics Committee of Zhongda Hospital, Southeast University Medical School. This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81600227), Jiangsu Provincial Key Medical Discipline (Laboratory ZDXKA2016023) and Jiangsu Provincial Key Research and Development Program (No. BE2016785). These funding agencies had no influence on the study design, data collection or analysis, or decision to publish or preparation of the article.
Disclosure
The authors declare that they have no competing interests concerning this paper.
References
1. Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi:10.1053/ajkd.2002.32766
2. James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78(8):803–809. doi:10.1038/ki.2010.258
3. Narula A, Mehran R, Weisz G, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014;35(23):1533–1540. doi:10.1093/eurheartj/ehu063
4. Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7(1):1–9. doi:10.1016/j.jcin.2013.06.016
5. Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. doi:10.1186/2110-5820-3-12
6. Moran JL, Santamaria J, Garcia de Frutos P. Reconsidering lactate as a sepsis risk biomarker. PLoS One. 2017;12(10):e0185320. doi:10.1371/journal.pone.0185320
7. Radovic M, Bojic S, Kotur-Stevuljevic J, et al. Serum lactate as reliable biomarker of acute kidney injury in low-risk cardiac surgery patients. J Med Biochem. 2019;38(2):118–125. doi:10.2478/jomb-2018-0018
8. Shetty AL, Thompson K, Byth K, et al. Serum lactate cut-offs as a risk stratification tool for in-hospital adverse outcomes in emergency department patients screened for suspected sepsis. BMJ Open. 2018;8(1):e015492. doi:10.1136/bmjopen-2016-015492
9. Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009;37(10):2827–2839. doi:10.1097/CCM.0b013e3181a98899
10. Attana P, Lazzeri C, Picariello C, Dini CS, Gensini GF, Valente S. Lactate and lactate clearance in acute cardiac care patients. Eur Heart J Acute Cardiovasc Care. 2012;1(2):115–121. doi:10.1177/2048872612451168
11. Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. 2011;21(12):2527–2541. doi:10.1007/s00330-011-2225-0
12. Aronson S, Fontes ML, Miao Y, et al. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation. 2007;115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
13. Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23(6):1970–1974. doi:10.1093/ndt/gfm908
14. Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. doi:10.1161/CIRCULATIONAHA.108.800011
15. Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy: from pathophysiology to preventive strategies. Can J Cardiol. 2016;32(2):247–255. doi:10.1016/j.cjca.2015.05.013
16. Mariscalco G, Lorusso R, Dominici C, Renzulli A, Sala A. Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg. 2011;92(4):1539–1547. doi:10.1016/j.athoracsur.2011.04.123
17. Jansen TC, van Bommel J, Woodward R, Mulder PG, Bakker J. Association between blood lactate levels, sequential organ failure assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med. 2009;37(8):2369–2374. doi:10.1097/CCM.0b013e3181a0f919
18. Schollin-Borg M, Nordin P, Zetterstrom H, Johansson J. Blood lactate is a useful indicator for the medical emergency team. Crit Care Res Pract. 2016;2016:5765202. doi:10.1155/2016/5765202
19. Zhang Z, Ni H. Normalized lactate load is associated with development of acute kidney injury in patients who underwent cardiopulmonary bypass surgery. PLoS One. 2015;10(3):e0120466. doi:10.1371/journal.pone.0120466
20. Aleksandar J, Vladan P, Markovic-Jovanovic S, Stolic R, Mitic J, Smilic T. Hyperlactatemia and the outcome of type 2 diabetic patients suffering acute myocardial infarction. J Diabetes Res. 2016;2016:6901345. doi:10.1155/2016/6901345
21. Gatien M, Stiell I, Wielgosz A, Ooi D, Lee JS. Diagnostic performance of venous lactate on arrival at the emergency department for myocardial infarction. Acad Emerg Med. 2005;12(2):106–113. doi:10.1197/j.aem.2004.10.012
22. Vermeulen RP, Hoekstra M, Nijsten MW, et al. Clinical correlates of arterial lactate levels in patients with ST-segment elevation myocardial infarction at admission: a descriptive study. Critical Care. 2010;14(5):R164. doi:10.1186/cc9253
23. Janine P, Koster J, Fuernau G, et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(15):1913–1920. doi:10.1016/j.jacc.2017.02.027
24. Liu Y, Li CP, Lu PJ, et al. Percutaneous coronary intervention assisted by invasive mechanical ventilation and intra-aortic balloon pump for acute myocardial infarction with cardiogenic shock: retrospective cohort study and meta-analyses. Bosn J Basic Med Sci. 2020;20(4):514–523. doi:10.17305/bjbms.2019.4500
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.