Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
The association of neutrophil-to-lymphocyte ratio and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage: possible involvement of cerebral blood perfusion
Authors Wu Y, He Q, Wei Y, Zhu J, He Z , Zhang XD, Guo ZD, Xu R, Cheng C, Huang Z, Sun XC
Received 11 October 2018
Accepted for publication 14 February 2019
Published 26 April 2019 Volume 2019:15 Pages 1001—1007
DOI https://doi.org/10.2147/NDT.S190477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Yue Wu,1 Qiuguang He,2 Yuling Wei,3 Ji Zhu,1 Zhaohui He,1 Xiaodong Zhang,1 Zongduo Guo,1 Rui Xu,1 Chongjie Cheng,1 Zhijian Huang,1 Xiaochuan Sun1
1Department of Neurosurgery, First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Neurosurgery, Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 3Department of Pharmacy, Chongqing Traditional Chinese Medicine Hospital, Chongqing, People’s Republic of China
Background and purpose: Emerging evidence suggests that systemic inflammation is associated with the pathophysiological process of delayed cerebral ischemia (DCI) after aneurysmal subarachnoid hemorrhage (aSAH). This study aimed to investigate the association of white blood cell (WBC) count and neutrophil-to-lymphocyte ratio (NLR) with the occurrence of DCI in SAH patients.
Methods: A total of 122 patients diagnosed with aSAH within 72 h of onset were retrospectively enrolled. The count of WBC, neutrophil count (NC), and lymphocyte (LC) was collected on admission. Computed tomography perfusion was performed within 7 days after SAH. The occurrence of DCI was recorded during the hospitalization.
Results: Among enrolled patients, 43 (35.2%) developed DCI during hospitalization. Patients who developed DCI had a higher count of WBC, NC, and NLR as well as a lower count of LC. NC and NLR were independently associated with the occurrence of DCI, while NLR was the best predictive parameter according to the receiver operating characteristic curve. Moreover, there was a strong correlation between NLR and mean cerebral blood flow, mean transit time and mean time to peak.
Conclusion: Leukocytosis is an early pathology of SAH, and NLR may be a practical predictor for the occurrence of DCI in SAH patients.
Keywords: aneurismal subarachnoid hemorrhage, neutrophil-to-lymphocyte ratio, cerebral blood flow, delayed cerebral ischemia
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a fatal subtype of hemorrhagic stroke with an overall mortality of about 50%, and over 30% of the survivors remain severely disabled.1 Delayed cerebral ischemia (DCI) was considered to be the major cause of poor outcome in aSAH patients.2 However, aneurismal rebleeding is less common now because of acute surgical repair of the aneurysms.3 DCI is reported to occur in about 30% of aSAH cases, which is the most important adverse prognostic factor for the poor outcome of aSAH.4 The pathophysiology of DCI includes angiographic vasospasm, microcirculatory disturbance, microthrombosis, and abnormal vascular autoregulation.5 These pathophysiological processes contribute eventually to the attenuated cerebral perfusion and ischemia.
Prediction of the occurrence of DCI is of critical value for the evaluation of the prognosis of aSAH patients. Many factors have been reported to be predictive for the outcome of aSAH, including clinical scales, radiological signs, and laboratory findings.4,6,7 Laboratory biomarkers attracted much attention for their practicality, sensitivity, and convenience. However, most biomarkers need special laboratory test or collection of CSF sample which may increase additional complications. Therefore, a sensitive and convenient biomarker is valuable for the clinical practice in aSAH treatment.
There is increasing evidence that inflammation plays a critical role in the pathogenesis of DCI after aSAH. More specifically, the leukocyte endothelial interaction has drawn much attention in the progression of vasospasm.8 Up to 60% of aSAH patients develop a systemic inflammatory response syndrome, including leukocytosis and leukopenia.9 However, the relationship between peripheral white blood cell (WBC) count and the occurrence of DCI in aSAH remains unclear. In the present study, the WBC count, neurotrophil count (NC), and lymphocyte count (LC) were collected in the acute phase of aSAH, and the neutrophil-to-lymphocyte ratio (NLR) was calculated. The association between these systemic inflammatory parameters and the occurrence of DCI was examined, and the possible involvement of cerebral perfusion was evaluated.
Methods
Patient population
This study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, in accordance with the Declaration of Helsinki. Written informed consent was provided by all patients or their relatives on admission. We retrospectively identified consecutive patients hospitalized at the Department of Neurosurgery of the First Affiliated Hospital of Chongqing Medical University from January to December 2015 for SAH caused by intracranial aneurysm rupture. Existence of SAH was determined on admission by computed tomography (CT) scan. The intracranial aneurysms were identified by CT angiography (CTA) or digital subtraction angiography (DSA). Patients with traumatic SAH, recent infectious diseases, prior neurological conditions, including ischemic stroke, hemorrhagic stroke, or brain trauma were excluded from this study. Early treatment of ruptured aneurysms was performed by surgical clipping or endovascular coiling according to an interdisciplinary decision-making process. All enrolled patients received standard medical treatment including intravenous nimodipine on admission and triple-H therapy after occlusion of the intracranial aneurysms.
Clinical assessment
The baseline characteristics of all patients were collected on admission. The severity of SAH was determined by Hunt and Hess grade (HH grade). The clinical severity was divided into good grade (with the HH grades I – III) and poor grade (with the HH grades IV–V) according to the HH grade on admission. The bleeding extent was determined by modified Fisher grade which was divided into low grade (with Fisher grades I–II) and high grade (with Fisher grades III–IV). Total WBC, NC, and LC were collected, and the NLR was calculated based on the admission blood work within 72 hr after SAH onset. Other clinical features of all patients were collected including time-to-admission, hypertension, diabetes, smoking, aneurysm location (anterior circulation or posterior circulation), and development of hydrocephalus. The outcome measure was the occurrence of DCI diagnosed according to the previous report.10 Briefly, DCI was defined as the occurrence of focal impairment or a decrease in at least 2 points on the Glasgow Coma Scale, which cannot be attributed to other causes by means of clinical assessment, imaging, and appropriate laboratory studies.
Cerebral perfusion assessment
All included patients received CT perfusion (CTP) scan before surgical or interventional treatment. Imaging was performed on a 64 slice CT scanner (GE Light speed VCT). An ROI was drawn in the cortical gray matter corresponding to the vascular territories for the anterior cerebral artery, middle cerebral artery, and posterior cerebral artery for each of the right and left hemisphere.11 The mean cerebral blood flow (mCBF), mean cerebral blood volume (mCBV), mean transit time (MTT), and mean time to peak (mTTP) were obtained according to measurements of all ROIs.
Statistics
Values were presented as mean ± SD or number (%) of subjects. Comparisons were made through Student t-test or χ2 test. Variables were subjected to univariate and multivariate analysis by logistic regression. Adjustment was made on the baseline characteristics (eg, age, sex, Hunt–Hess grade, hydrocephalus, hypertension, etc.). The receiver operating characteristic (ROC) analysis was undertaken to evaluate the predictive ability of WBC, NC, LC, and NLR. Spearman correlation was used to analyze the correlation between NLR and mCBF, mCBV, MTT, and mTTP. Difference was considered as significant when P<0.05. All statistical analysis was performed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
Results
Baseline information on patients
According to the exclusion criteria, 102 patients were excluded from the final analysis. A total of 122 patients were included in the study. The mean age of the participants was 55.3±10.6 years (ranging from 34 to 80), and 39.3% were male. Forty-three (35.2%) of these patients developed DCI during hospitalization (Table 1). Patients who were more likely to develop DCI tend to be male in gender, in higher clinical grade (HH grade and Fisher grade), and accompanying hydrocephalus (P<0.05). No difference was found in age, time-to-admission, hypertension, diabetes, and smoking between DCI and non-DCI patients. We further investigated the initial laboratory findings. The results showed that patients who developed DCI had a higher WBC count, NC, and NLR. However, no difference was found in LC between DCI and non-DCI patients (P=0.074).
 | Table 1 Baseline characteristics according to the prevalence of DCI |
The association of WBC, NC, LC, NLR, and DCI
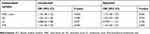
Multivariate logistic regression analysis indicated that WBC, NC, and NLR were associated with the occurrence of DCI (P<0.001). However, LC was not associated with DCI (P=0.077). With the adjustment of baseline characteristics, including sex, age, HH grade, hydrocephalus, and hypertension, the NC and NLR remained significantly associated with the occurrence of DCI (P=0.022 and P=0.002, respectively), whereas WBC count was not associated with the occurrence of DCI after adjustment (P=0.056) (Table 2).
 | Table 2 Logistic regression analysis according to the occurrence of DCI |
To further investigate the values of the abnormality of WBC, NC, LC, and NLR in predicting the occurrence of DCI, a higher WBC count, higher NC, and lower LC were classified according to the normal references.12 The results showed that higher WBC count and lower LC were associated with the occurrence of DCI (P<0.001 and P=0.043, respectively), whereas only higher WBC was exhibited to be associated with DCI after adjustment with baseline characteristics (P=0.013) (Table 3).
 | Table 3 WBC count in predicting the occurrence of DCI |
Efficacy of NLR and its relation to CTP parameters
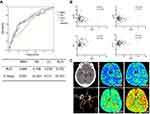
The ROC analysis showed that NLR was the best predicting variable which possessed the largest area under the curve (AUC) (AUC =0.722). The best predictive cutoff value of NLR was 11.47. The sensitivity and specificity of NLR were 58.1% and 82.3%, respectively, for predicting DCI (Figure 1A). All patients received early CTP assessment (72.6 hr after onset on average) before surgical treatment. Spearman correlation analysis of NLR and CTP parameters (mCBF, mCBV, MTT, and mTTP) implicated its potential influence on cerebral perfusion. There was a strong negative correlation between NLR and mCBF (P=0.001), and a positive correlation between NLR and MTT (P<0.001), mTTP (P=0.031) was observed. However, no correlation was found between NLR and mCBV (P=0.198) (Figure 1B). The associations between CTP parameters and DCI were also analyzed (Figure 1C showing the CT, CTA, and CTP images of an aSAH patient). All CTP parameters exhibited an association with DCI in univariate regression, while mCBF, MTT, and mTTP showed association with DCI after adjustment with baseline characteristics (Table 4).
 | Table 4 CTP parameters in predicting the occurrence of DCI |
Discussion
The main findings of this study include (1) aSAH induced a systemic inflammatory response characterized by leukocytosis and lymphopenia in the early phase. (2) NC and NLR on admission were independently associated with the occurrence of DCI in aSAH patients. (3) NLR was correlated with cerebral perfusion in the early phase of aSAH, indicating a potential correlation between inflammatory response and cerebral perfusion after aSAH. As a systemic inflammatory marker, WBC count increase is a characteristic acute phase response after SAH.5 Growing evidence supports that inflammation plays important roles in DCI after SAH.13 Elevation of WBC count has been proved to be associated with poor outcomes and increased risk of symptomatic vasospasm.14 Different subtypes of WBC may exert different effects in acute neurovascular diseases. Neutrophils, macrophages, and monocytes are the major effector cells of the acute inflammatory reaction. In the present study, we observed that NC elevated evidently within 72 hr after aSAH. The neutrophil increase reflects the degree of tissue inflammation and the severity of hemorrhagic irritation, which may be the basis of the prediction of DCI by systemic inflammatory indicators.
Besides angiographic vasospasm, DCI involves complicated pathophysiology, including microcirculatory constriction, microthrombosis, cortical ischemia, and spreading depolarization.5 Leukocytes are involved in the development of these processes which eventually induce dysfunction of microcirculation of the brain. It is reported that neutrophils infiltrate in the brain tissue as early as 10 mins after hemorrhage in an experimental model of SAH, and inhibition of neutrophil infiltration attenuates vascular injury and increases survival.15 Moreover, NLR is reported to be a prognostic marker in acute ischemic stroke.16 The accumulation of neutrophils attenuates the microvascular perfusion, and recruited neutrophils cause the release of inflammatory mediators which induces vascular injury.17,18 As DCI shares the common pathological process of perfusion deficit with acute cerebral ischemia, the underlying mechanisms may explain the value of NLR for the prediction of DCI in aSAH. In the present study, we observed an early increase of neutrophils that were independently associated with the occurrence of DCI, indicating the inflammatory influence of neutrophils on the vascular homeostasis. On the other hand, in spite of no predictive value for DCI, a decrease in lymphocytes was observed. However, it is reported that SAH induces lymphocytopenia and immunological impairment of lymphocyte functions, which may be associated with infectious complications in SAH.19 As lymphocytes are the primary mediator of chronic inflammation, they were usually found to decrease in the acute phase and then elevated after the increase in neutrophils. Therefore, long-term monitoring of the quantity and function of these cells may be beneficial in predicting outcomes of aSAH. NLR involving both characteristics of neutrophils and lymphocytes may be a more comprehensive and stable parameter for the prediction of DCI.
CTP is a noninvasive tool for diagnosing cerebral vasospasm. Instead of detecting the areas of arterial narrowing as CTA and DSA work, CTP provides direct visualization of brain tissue perfusion. Early CTP evaluation (on day 3) is reported to be reliable for the identification of risk for DCI.20 Although earlier CTP is valueless in predicting DCI, a wider time window of 4–14 days after SAH for CTP exam is valuable for the diagnosis of DCI.21 Moreover, CBF is more sensitive than CBV in the prediction of DCI in the early phase of SAH.6 Our findings indicated a strong correlation between NLR and CTP parameters, including CBF, MTT, and TTP rather than CBV within 7 days, which may be due to a more sensitive reflection of these parameters for cerebral perfusion in the early stage of aSAH in which cerebral vasospasm has not yet influenced CBV. This may imply the fact that the predictive value of NLR may rely on its correlation to the condition of cerebral perfusion. Our results showed that CTP parameters were the predictors for DCI, and mCBF, MTT, and mTTP were the independent factors. However, compared to CTP, NLR is routinely accessible and cost-effective, making it a practical parameter for the prediction of DCI after aSAH.
Several limitations should be taken into consideration when interpreting our findings. Firstly, a relatively small sample size and retrospective design may attenuate the strength of the conclusions. Secondly, WBC count was collected on admission. Therefore, dynamic monitoring of leukocyte change would be more valuable as aforementioned. Thirdly, other inflammatory indicators such as C-reactive protein were not provided as a control of nonspecific inflammatory parameter.
In conclusion, leukocytosis is an early pathology of aSAH. NLR on admission may be a potential marker for the prediction of DCI after aSAH. Correlation of NLR and cerebral perfusion may account for the value of NLR for predicting DCI. Further investigation is needed for better understanding of the dynamic changes of WBC count and cerebral perfusion after aSAH.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China: numbers 81571159 (XCS), 81870927 (ZHH), and 81671160 (ZDG), and the Cultivating Fund of the First Affiliated Hospital of CQMU: PYJJ2017-21 (YW).
Disclosure
The authors report no conflicts of interest in this work.
References
1. van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124:249–278.
2. Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347.
3. Starke RM, Connolly ES
4. Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. doi:10.1161/STROKEAHA.107.484360
5. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58. doi:10.1038/nrneurol.2013.246
6. Sun H, Li W, Ma J, Liu Y, You C. Ct perfusion diagnoses delayed cerebral ischemia in the early stage of the time-window after aneurysmal subarachnoid hemorrhage. J Neuroradiol [Journal De Neuroradiologie]. 2017;44:313–318. doi:10.1016/j.neurad.2016.12.013
7. Cai H, Zheng S, Cai B, et al. Neuroglobin as a novel biomarker for predicting poor outcomes in aneurysmal subarachnoid hemorrhage. World Neurosurg. 2018;116:e258–e265. doi:10.1016/j.wneu.2018.04.184
8. Chaichana KL, Pradilla G, Huang J, Tamargo RJ. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg. 2010;73:22–41. doi:10.1016/j.surneu.2009.05.027
9. Tam AK, Ilodigwe D, Mocco J, et al. Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: exploratory analysis of conscious-1 database. Neurocrit Care. 2010;13:182–189. doi:10.1007/s12028-010-9402-x
10. Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi:10.1161/STROKEAHA.110.589275
11. Tatu L, Moulin T, Vuillier F, Bogousslavsky J. Arterial territories of the human brain. Front Neurol Neurosci. 2012;30:99–110. doi:10.1159/000333602
12. Behrouz R, Hafeez S, Miller CM. Admission leukocytosis in intracerebral hemorrhage: associated factors and prognostic implications. Neurocrit Care. 2015;23:370–373. doi:10.1007/s12028-015-0128-7
13. Provencio JJ, Vora N. Subarachnoid hemorrhage and inflammation: bench to bedside and back. Semin Neurol. 2005;25:435–444. doi:10.1055/s-2005-923537
14. McGirt MJ, Mavropoulos JC, McGirt LY, et al. Leukocytosis as an independent risk factor for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;98:1222–1226. doi:10.3171/jns.2003.98.6.1222
15. Friedrich V, Flores R, Muller A, Bi W, Peerschke EI, Sehba FA. Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage. J Neuroinflammation. 2011;8:103. doi:10.1186/1742-2094-8-72
16. Xue J, Huang W, Chen X, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:650–657. doi:10.1016/j.jstrokecerebrovasdis.2016.11.010
17. Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. 2009;22:294–301.
18. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47.
19. Sarrafzadeh A, Schlenk F, Meisel A, Dreier J, Vajkoczy P, Meisel C. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42:53–58. doi:10.1161/STROKEAHA.110.594705
20. Malinova V, Dolatowski K, Schramm P, Moerer O, Rohde V, Mielke D. Early whole-brain ct perfusion for detection of patients at risk for delayed cerebral ischemia after subarachnoid hemorrhage. J Neurosurg. 2016;125:128–136. doi:10.3171/2015.6.JNS15720
21. Cremers CH, van der Schaaf IC, Wensink E, et al. Ct perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 2014;34:200–207. doi:10.1038/jcbfm.2013.208
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.