Back to Journals » Cancer Management and Research » Volume 13
The Association of Clinical Outcomes with Posttreatment Changes in the Relative Eosinophil Counts and Neutrophil-to-Eosinophil Ratio in Patients with Advanced Urothelial Carcinoma Treated with Pembrolizumab
Authors Furubayashi N , Minato A, Negishi T, Sakamoto N, Song Y, Hori Y, Tomoda T , Tamura S, Kuroiwa K, Seki N, Fujimoto N , Nakamura M
Received 17 August 2021
Accepted for publication 15 October 2021
Published 24 October 2021 Volume 2021:13 Pages 8049—8056
DOI https://doi.org/10.2147/CMAR.S333823
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Nobuki Furubayashi,1 Akinori Minato,2 Takahito Negishi,1 Naotaka Sakamoto,3 Yoohyun Song,4 Yoshifumi Hori,5 Toshihisa Tomoda,6 Shingo Tamura,7 Kentaro Kuroiwa,5 Narihito Seki,4 Naohiro Fujimoto,2 Motonobu Nakamura1
1Department of Urology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan; 2Department of Urology, University of Occupational and Environmental Health, Kitakyushu, Japan; 3Department of Urology, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan; 4Department of Urology, Kyushu Central Hospital of the Mutual Aid Association of Public School Teachers, Fukuoka, Japan; 5Department of Urology, Miyazaki Prefectural Miyazaki Hospital, Miyazaki, Japan; 6Department of Urology, Oita Prefectural Hospital, Oita, Japan; 7Department of Medical Oncology, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan
Correspondence: Nobuki Furubayashi
Department of Urology, National Hospital Organization Kyushu Cancer Center, Notame 3-1-1, Minami-ku, Fukuoka, 811-1395, Japan
Tel +81-92-541-3231
Fax +81-92-551-4585
Email [email protected]
Background: To evaluate the association of clinical outcomes with posttreatment changes in the relative eosinophil count (REC) and neutrophil-to-eosinophil ratio (NER) in patients with advanced urothelial cancer (UC) treated with pembrolizumab.
Materials and Methods: We retrospectively analyzed 105 patients with advanced UC who received pembrolizumab after the failure of platinum-based chemotherapy. The REC and NER before and three weeks after pembrolizumab were recorded. A receiver operating characteristic curve was used to determine the optimal cut-off values for analyzing the risk.
Results: There were no significant differences in the overall survival (OS) between the REC ≥ 4.8% and < 4.8% groups and the NER ≥ 13.7 and < 13.7 groups before pembrolizumab (p=0.997 and 0.669, respectively). However, a significant difference in the OS was confirmed between the increased and decreased REC groups and between the decreased and increased NER groups at 3 weeks after pembrolizumab (p< 0.001 and 0.002, respectively). Multivariate analyses revealed that an Eastern Cooperative Oncology Group Performance Status ≥ 2 (P=0.003), albumin < 3.7 g/dl (p=0.002), LDH > 246 U/L (p=0.011), disease site ≥ 3 organs (p=0.019), decreased posttreatment REC (3 weeks later) (p=0.002) and increased posttreatment NER (3 weeks later) (p=0.022) were independent prognostic factors for a worse OS.
Conclusion: An increased REC and decreased NER after pembrolizumab may be significant early predictive markers of improved clinical outcomes in patients with advanced UC receiving pembrolizumab.
Keywords: urothelial carcinoma, pembrolizumab, eosinophil, neutrophil-to-eosinophil ratio
Introduction
The introduction of immune checkpoint inhibitors (ICIs) has dramatically changed the treatment for multiple malignancies, including advanced urothelial carcinoma (UC). At present in Japan, pembrolizumab (anti programmed death 1 [PD-1] antibody) is approved as second-line therapy for patients after the failure of platinum-based chemotherapy,1 and avelumab (anti–PD-L1 antibody) was recently approved as maintenance therapy for patients without progression after first-line platinum-based chemotherapy.2 However, while ICIs have markedly improved the outcomes of patients with advanced UC, the objective response rate is low, and only a minority of patients achieve a long-term benefit.3
Because of the limited response rates and concomitant immune-related adverse events, there is an urgent need for predictive biomarkers capable of predicting the clinical response to ICIs. In addition, the identification of a biomarker that is also a routinely used clinical marker would be of value, as it may represent a convenient and low-cost option that does not require special equipment.
Several predictive biomarkers for ICIs have been reported in a number of malignancies, including UC.4–7 In clinical practice, complete blood count (CBC) testing is essential for the treatment of malignant tumors, and the eosinophil count is a widely available blood parameter and part of the routine complete blood examination performed for patients on immunotherapy. Eosinophils were recently shown to be associated with the efficacy of ICIs in melanoma, lung cancer and renal carcinoma.8–10 Furthermore, an increase in eosinophil in the early stages of therapy with ICI was found be associated with an improved survival of melanoma and lung cancer patients.11–14
In the present study, we focused on the changes in the relative eosinophil count (REC) and neutrophil-to-eosinophil ratio (NER) to clarify the relationship between these changes and the clinical outcome with pembrolizumab in advanced UC.
Materials and Methods
Patient Population
We identified 125 consecutive patients with advanced (metastatic or locally advanced) UC who received pembrolizumab after the failure of platinum-based chemotherapy at 6 institutions between January 2018 and June 2021. All patients were histopathologically diagnosed with UC and showed radiologically confirmed disease progression after platinum-based chemotherapy.15 Clinical data were retrieved from the patients’ medical records. Ultimately, 20 patients were excluded from this study due to a lack of clinical data.
Pembrolizumab was administered intravenously on day 1 at a dose of 200 mg, and the cycle was basically repeated every 21 days, continuing until disease progression or the occurrence of unacceptable adverse events.
The present study protocol was approved by the ethics committee of each institution and complied with the 1964 Declaration of Helsinki and its later amendments. Tumor measurements were generally performed by computed tomography before and after every four to six cycles of pembrolizumab; however, evaluations were performed as needed when the clinical symptoms worsened. The tumor response was assessed as the best response according to the Response Evaluation Criteria in Solid Tumors, version 1.1.16
Peripheral REC and NER Measurement
Peripheral REC and NER were measured at the same time as CBC measurements before initial pembrolizumab initiation and three weeks later. Results were obtained from the medical records of each patient.
Statistical Analyses
All statistical analyses were performed using the JMP® Pro, version 15.1.0 software package (SAS Institute, Inc., Cary, NC, USA). To determine the cut-off value of the continuous variables, a receiver operating characteristics (ROC) curve analysis was performed. The Mann–Whitney U-test was used to assess the differences between the controlled disease and progressive disease groups. The Kaplan–Meier method was used to evaluate the progression‑free survival (PFS) and overall survival (OS). The PFS was calculated from the day on which pembrolizumab was started until the date when patients who were alive and without disease progression or who were lost to follow‑up had their data censored at the time of the final tumor assessment. The OS was calculated from the day on which pembrolizumab was started until the date of the last follow-up examination or death from any cause, and the difference among response groups was determined by the Log rank test. The significance of associations between the clinical parameters and OS was assessed using the Cox proportional hazards regression model. P values of <0.05 were considered to indicate statistical significance.
Results
Patient Characteristics
The clinical characteristics of the 105 patients (male, n=75; female, n=30; median age, 72 years old; interquartile range [IQR], 67–77 years old) are listed in Table 1. The median follow-up period was 8.4 months (IQR, 4.1–15.7 months). All patients received pembrolizumab for UC after the failure of platinum-based chemotherapy. According to the Eastern Cooperative Oncology Group Performance Status (ECOG PS), 64 (61.0%), 31 (29.5%) and 10 (9.5%) patients had a PS of 0, 1 and ≥2, respectively. Forty-two patients had bladder UC (40.0%), 41 had upper urinary tract UC (39.0%), and 22 had both types (21.0%). In the majority of patients, a histological examination revealed pure UC (81.0%). The number of treatments attempted before pembrolizumab was 1 (n=84, 80.0%), 2 (n=12, 11.4%) and 3 (n=9, 8.6%). Before the start of treatment with pembrolizumab, the number of diseased organs, including the primary tumor organ, was 1 in 47 patients (44.8%), 2 in 26 patients (24.8%) and ≥3 in 32 patients (30.5%). The median PFS and OS were 3.2 months (95% confidence interval [CI], 2.8–5.5) and 13.7 months (95% CI, 10.2–19.8), respectively.
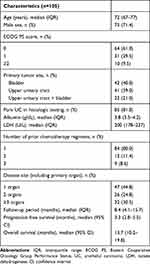 |
Table 1 Patients’ Characteristics |
Peripheral REC and NER Changes Due to Pembrolizumab
Peripheral REC and NER values before pembrolizumab initiation and three weeks later are shown according to patients who did and did not respond to pembrolizumab (Table 2). After 3 weeks of pembrolizumab administration, 65 patients (61.9%) had an increased REC, and 64 patients (61.0%) had a decreased NER compared to before pembrolizumab administration. There were no significant differences between patients with controlled disease (complete response, partial response and stable disease) and those with progressive disease in terms of the REC and NER before pembrolizumab (p=0.339 and 0.304, respectively). However, there were significant differences between patients with controlled disease and those with progressive disease in terms of the REC and NER at 3 weeks after starting pembrolizumab (p=0.008 and 0.004, respectively).
 |
Table 2 Changes in the Relative Eosinophil Count and Neutrophil-to-Eosinophil Ratio Before and Three Weeks After Pembrolizumab |
The OS According to Pre- and Posttreatment (Three Weeks Later) REC and NER Values in Patients Treated with Pembrolizumab
The OS according to the REC and NER values before and three weeks after pembrolizumab initiation are shown in Figures 1–2. The most appropriate cut-off values for patients with controlled disease and those with progressive disease were a REC of 4.8% and NER of 13.7 according to an ROC curve analysis (area under the curve [AUC]=0.554 and 0.558, respectively). There were no significant differences in the OS between the REC ≥4.8% and <4.8% groups or the NER ≥13.7 and <13.7 groups according to a Log rank test (p=0.997 and 0.669, respectively) (Figure 1). However, a significant difference in the OS was confirmed between the increased and decreased REC groups and between the decreased and increased NER groups at 3 weeks after starting pembrolizumab (p<0.001, and 0.002, respectively) (Figure 2).
 |
Figure 1 The overall survival in patients treated with pembrolizumab according to the pretreatment relative eosinophil count and neutrophil-to-eosinophil ratio. *P values are reported. |
Univariate and Multivariate Analyses of the Associations Between Various Factors and the OS with Pembrolizumab
To identify the prognostic factors associated with the OS with pembrolizumab, univariate and multivariate analyses using the Cox proportional hazards model were performed (Table 3). Univariate analyses for various factors revealed that sex, ECOG PS, albumin, lactate dehydrogenase (LDH), disease site, posttreatment REC and posttreatment NER were prognostic variables. However, the pretreatment REC and NER values were not prognostic variables. The multivariate analyses revealed that ECOG PS ≥2 (hazard ratio [HR] 4.017, 95% confidence interval [CI]: 1.618–9.975, P=0.003), albumin <3.7 g/dl (HR 2.744, 95% CI: 1.438–5.236, p=0.002), LDH >246 U/L (HR 2.436, 95% CI: 1.228–4.829, p=0.011), disease site ≥3 organs (HR 2.324, 95% CI: 1.149–4.702, p=0.019), decreased posttreatment REC (3 weeks later) (HR 31.298, 95% CI: 3.724–263.048, p=0.002) and increased posttreatment NER (3 weeks later) (HR 11.563, 95% CI: 1.430–93.481, p=0.022) were independent prognostic factors for a worse OS.
 |
Table 3 The Univariate and Multivariate Analyses of the Factors Associated with the Overalls Survival in Patients Receiving Pembrolizumab Treatment |
Discussion
We retrospectively analyzed the data of 105 patients with advanced UC who received the ICI pembrolizumab after the failure of platinum-based chemotherapy and evaluated the relationship between the change in the REC value and the OS, including the change in the NER. The present study found that an increased REC and decreased NER at three weeks after pembrolizumab were independently associated with a significantly longer OS and might be significant predictive markers of improved clinical outcomes in patients with advanced UC receiving pembrolizumab.
Several predictive biomarkers to ICIs have also been reported in advanced UC.4–7 In clinical practice, the examination of peripheral blood is a minimally invasive and commonly ordered laboratory test, suggesting its potentially reliable and predictive value. The neutrophil-to-lymphocyte (NLR) is a systemic inflammatory prognostic marker, and not only the pretreatment NLR but also the posttreatment NLR (and consequently the change in the NLR) in response to pembrolizumab have been reported to be significantly associated with the OS in advanced UC.7,17 In brief, both pre- and posttreatment factors’ changes can be significant factors in predicting an improved prognosis.
Eosinophils are known to play an important role in parasitic and allergic diseases, and the number of peripheral blood eosinophils is known to be increased in those patients.18 In addition, eosinophils can invade the tumor microenvironment and may enhance the antitumor response via degranulation with direct cytotoxic effects on cancer cells.19–21 Recently, eosinophils were shown to be associated with the efficacy of ICIs in melanoma,8 lung cancer,9 renal cell carcinoma (RCC)10 and UC.22 In UC, it was reported that a low pretreatment eosinophil count (<100 cells/μL) was associated with poorer outcomes following treatment with ICIs than a higher count. An early increase in the peripheral eosinophil count at weeks 2 or 3 was observed, but changes in eosinophil counts at weeks 2 or 3 and 6 were not clearly associated with outcomes.22 The present study did not show that the pretreatment REC (REC ≥4.8% and <4.8%) was associated with a significant difference in the OS (p=0.997), but posttreatment changes, namely an increased REC at 3 weeks after pembrolizumab, were an independent prognostic factor (P=0.002). Several factors, such as the sample size, kind of ICI, timing of the eosinophil count and the cut-off value of eosinophils, might be responsible for the discrepant clinical outcomes between the present and previous studies.
Recently, a new inflammation-based prognostic score related to eosinophils, the neutrophil-to-eosinophil ratio (NER), was reported as a predictive biomarker in RCC.23 The Phase 3 JAVELIN Renal 101 trial demonstrated improvements in the progression-free survival (PFS) and increased objective response rates for advanced RCC patients treated with avelumab and axitinib compared to those treated with sunitinib.24 A secondary analysis of the JAVELIN Renal 101 trial assessing the association between the NER and oncologic outcomes among patients receiving avelumab and axitinib and sunitinib was reported. Regarding patients randomized to avelumab and axitinib, the objective response rate was higher (63.9% vs 55.2%) and the median PFS longer (15.5 vs 11.1 months) for patients with an NER below the median than in those with a median or better NER, while there were no major differences in outcomes for patients treated with sunitinib. On assessing the PFS and OS, the stratified HR for patients with NER values below the median compared to those with NERs equal to or above the median was 0.81 (95% CI, 0.630–1.035, p=0.046) and 0.67 (95% CI, 0.481–0.940, p=0.010) for patients treated with avelumab and axitinib. To our knowledge, there have been no reports concerning the relationship between the NER and the OS in UC. In addition, the present study was the first to analyze the relationship between changes in the NER and the OS on immunotherapy in malignant tumors.
It was previously reported that a decreased NLR after pembrolizumab was significantly correlated with a better outcome in cases of UC. Therefore, theoretically, it would be expected that a decreased NER would also be correlated with a better outcome. In the present results, there was no marked difference in the OS according to the pretreatment NER value as assessed by an ROC analysis (NER ≥13.7 and <13.7), but a decreased NER after pembrolizumab treatment was significantly associated with an improved OS (p=0.022). The changes in the REC and NER at three weeks after pembrolizumab were associated with an improved OS, and changes in these factors may become a convenient and low-cost predictive marker in clinical practice, able to be evaluated early in treatment.
Several limitations associated with the present study warrant mention. This study was retrospective in nature and had a limited number of patients with a short-term follow-up period; therefore, our findings should be validated in large prospective studies with long-term follow-up. This study included patients with bladder UC alone, upper urinary tract UC alone and both together. Therefore, this was a heterogeneous group, which might have affected the results. Furthermore, the patients were heterogeneous in terms of the regimens, dosing schedule and lines of prior systemic chemotherapy due to the multicenter setting. In addition, we analyzed the early changes in REC and NER but did not analyze the long-term REC and NER values in this study.
Conclusion
Posttreatment changes related to eosinophils, namely an increased REC and decreased NER, may be significant early predictive markers of improved clinical outcomes in patients with advanced UC receiving pembrolizumab after platinum-based chemotherapy.
Abbreviations
ICIs, immune checkpoint inhibitors; UC, urothelial carcinoma; PD-1, programmed death 1; CBC, complete blood count; REC, relative eosinophil counts; NER, neutrophil-to-eosinophil ratio; ROC, receiver operating characteristics; OS, overall survival; IQR, interquartile range; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the Institutional Review Board of National Hospital Organization Kyushu Cancer Center (2020-90), and written informed consent was obtained from all patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
The authors declare that they have no conflicts of interest for this study.
References
1. Bellmunt J, Wit RD, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi:10.1056/NEJMoa1613683
2. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi:10.1056/NEJMoa2002788
3. Grisay G, Pierrard J, Confente C, et al. Future strategies involving immune checkpoint inhibitors in advanced urothelial carcinoma. Curr Treat Options Oncol. 2020;22(1):7. doi:10.1007/s11864-020-00799-9
4. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi:10.2147/OTT.S153290
5. Kawai T, Sato Y, Makino K, et al. Immune-related adverse events predict the therapeutic efficacy of pembrolizumab in urothelial cancer patients. Eur J Cancer. 2019;116:114–115. doi:10.1016/j.ejca.2019.05.017
6. Yasuoka S, Yuasa T, Nishimura N, et al. Initial experience of pembrolizumab therapy in Japanese patients with metastatic urothelial cancer. Anticancer Res. 2019;39(7):3887–3892. doi:10.21873/anticanres.13539
7. Ogihara K, Kikuchi E, Shigeta K, et al. The pretreatment neutrophil-to-lymphocyte ratio is a novel biomarker for predicting clinical responses to pembrolizumab in platinum-resistant metastatic urothelial carcinoma patients. Urol Oncol. 2020;38(6):
8. Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22(22):5487–5496. doi:10.1158/1078-0432.CCR-16-0127
9. Tanizaki J, Haratani K, Hayashi H, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13(1):97–105. doi:10.1016/j.jtho.2017.10.030
10. Zahoor H, Barata PC, Jia X, et al. Patterns, predictors and subsequent outcomes of disease progression in metastatic renal cell carcinoma patients treated with nivolumab. J Immunother Cancer. 2018;6(1):107. doi:10.1186/s40425-018-0425-8
11. Gebhardt C, Sevko A, Jiang H, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. 2015;21(24):5453–5459. doi:10.1158/1078-0432.CCR-15-0676
12. Delyon J, Mateus C, Lefeuvre D, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24(6):1697–1703. doi:10.1093/annonc/mdt027
13. Ohashi H, Takeuchi S, Miyagaki T, et al. Increase of lymphocytes and eosinophils, and decrease of neutrophils at an early stage of anti-PD-1 antibody treatment is a favorable sign for advanced malignant melanoma. Drug Discov Ther. 2020;14(3):117–121. doi:10.5582/ddt.2020.03043
14. Okauchi S, Shiozawa T, Miyazaki K, et al. Association between peripheral eosinophils and clinical outcomes in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Pol Arch Intern Med. 2021;131(2):152–160. doi:10.20452/pamw.15776
15. Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-Part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi:10.1016/j.eururo.2016.02.029
16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
17. Yamamoto Y, Yatsuda J, Shimokawa M, et al. Prognostic value of pre-treatment risk stratification and post-treatment neutrophil/lymphocyte ratio change for pembrolizumab in patients with advanced urothelial carcinoma. Int J Clin Oncol. 2021;26(1):169–177. doi:10.1007/s10147-020-01784-w
18. Klion AD, Ackerman SJ, Bochner BS. Contributions of eosinophils to human health and disease. Annu Rev Pathol. 2020;15(1):179–209. doi:10.1146/annurev-pathmechdis-012419-032756
19. Krishnan T, Tomita Y, Roberts-Thomson R. A retrospective analysis of eosinophilia as a predictive marker of response and toxicity to cancer immunotherapy. Future Sci OA. 2020;6(10):FSO608. doi:10.2144/fsoa-2020-0070
20. Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2(1):1–8. doi:10.1158/2326-6066.CIR-13-0196
21. Simon SCS, Utikal J, Umansky V. Opposing roles of eosinophils in cancer. Cancer Immunol Immunother. 2019;68(5):823–833. doi:10.1007/s00262-018-2255-4
22. Mota JM, Teo MY, Whiting K, et al. Pretreatment eosinophil counts in patients with advanced or metastatic urothelial carcinoma treated with anti-PD-1/PD-L1 checkpoint inhibitors. J Immunother. 2021;44(7):248–253. doi:10.1097/CJI.0000000000000372
23. Matthew D, Tucker MD. Association between Neutrophil-to-Eosinophil Ratio (NER) and efficacy outcomes in the JAVELIN renal 101 study. ASCO 2021. Available from: https://www.urotoday.com/conference-highlights/asco-2021/asco-2021-kidney-cancer/130142-asco-2021-association-between-neutrophil-to-eosinophil-ratio-ner-and-efficacy-outcomes-in-the-javelin-renal-101-study.html.
24. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi:10.1056/NEJMoa1816047
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

