Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis
Authors Zhu M, Wang T, Wang C, Ji Y
Received 26 November 2015
Accepted for publication 5 May 2016
Published 19 October 2016 Volume 2016:11(1) Pages 2597—2607
DOI https://doi.org/10.2147/COPD.S101382
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Min Zhu, Ting Wang, Chengdi Wang, Yulin Ji
Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
Background: In recent years, the pleiotropic roles of vitamin D have been highlighted in various diseases. However, the association between serum vitamin D and COPD is not well studied. This updated systematic review and meta-analysis aimed to assess the relationship between vitamin D and the risk, severity, and exacerbation of COPD.
Methods: A systematic literature search was conducted in PubMed, Medline, EMBASE, Chinese National Knowledge Infrastructure, Wanfang, and Weipu databases. The pooled risk estimates were standardized mean difference (SMD) with 95% confidence interval (CI) for vitamin D levels and odds ratio (OR) with 95% CI for vitamin D deficiency. Meta-regression and subgroup analyses were performed on latitude, body mass index, and assay method.
Results: A total of 21 studies, including 4,818 COPD patients and 7,175 controls, were included. Meta-analysis showed that lower serum vitamin D levels were found in COPD patients than in controls (SMD: -0.69, 95% CI: -1.00, -0.38, P<0.001), especially in severe COPD (SMD: -0.87, 95% CI: -1.51, -0.22, P=0.001) and COPD exacerbation (SMD: -0.43, 95% CI: -0.70, -0.15, P=0.002). Vitamin D deficiency was associated with increased risk of COPD (OR: 1.77, 95% CI: 1.18, 2.64, P=0.006) and with COPD severity (OR: 2.83, 95% CI: 2.00, 4.00, P<0.001) but not with COPD exacerbation (OR: 1.17, 95% CI: 0.86, 1.59, P=0.326). Assay methods had significant influence on the heterogeneity of vitamin D deficiency and COPD risk.
Conclusion: Serum vitamin D levels were inversely associated with COPD risk, severity, and exacerbation. Vitamin D deficiency is associated with increased risk of COPD and severe COPD but not with COPD exacerbation. It is worth considering assay methods in the heterogeneity sources analysis of association between vitamin D deficiency and COPD.
Keywords: vitamin D, COPD, risk, severity, exacerbation, meta-analysis
Introduction
COPD is the fourth leading cause of mortality worldwide,1 posing a big threat to public health. This is a progressive disease characterized by persistent airflow limitation, as a consequence of chronic inflammation and structural changes.2 COPD patients suffer from progressive reduction of lung function, loss of exercise capacity, frequent disease exacerbations, and development of extrapulmonary comorbidities (such as osteoporosis, infection, and cardiovascular disease).3
Vitamin D is traditionally known for its roles in bone health and homeostasis of calcium and phosphorus.4 However, vitamin D is not just a vitamin. It is recognized as a pleiotropic prohormone with its receptor (vitamin D receptor [VDR]) ubiquitously distributed.5 As an immunomodulatory effector, vitamin D can not only boost innate immune responses upon infection but also regulate adaptive immune responses.6 Moreover, vitamin D is related to cell proliferation, cell differentiation, apoptosis, and intercellular adhesion.7 The majority of vitamin D originates from skin with sunlight exposure, and the remaining can be obtained from diet or supplements.8 Epidemiologic studies reported that vitamin D deficiency is a global and important health issue.9 Vitamin D deficiency can underpin the etiology of broad range of diseases, including autoimmune diseases, allergy diseases, endocrine and metabolic disorders, cancer, infections, and cardiovascular disorders.10,11
With a focus on the association between COPD and vitamin D, evidence from some studies indicated a possible link between vitamin D and COPD.12–14 However, the conclusion was not definite. Two meta-analyses on the roles of vitamin D in COPD have been conducted.15,16 However, the studies did not include enough articles, did not extract correct data, pooled the levels of vitamin D from serum and plasma, included participants with vitamin D supplement, or did not analyze the sources of high heterogeneity. Therefore, we performed a further systematic review and meta-analysis to clarify the roles of vitamin D in COPD. In this study, the associations between circulating 25(OH)D levels and risk, severity, and exacerbations of COPD were addressed.
Methods
Bibliographic search
This meta-analysis was conducted in accordance with the statement of meta-analysis of observational studies in epidemiology.17 Two investigators (MZ and TW) independently conducted a literature search in PubMed, Medline, EMBASE, Chinese National Knowledge Infrastructure, Wanfang, and Weipu databases for publications targeting vitamin D and COPD (up to September 15, 2015). The search terms were “vitamin D”, “cholecalciferol”, “D3”, “ergocalciferol”, “D2”, “hydroxycholecalciferols”, “25-hydroxyvitamin D2”, “dihydrotachysterol” or “25(OH)D” in combination with “COPD”, “pulmonary emphysema”, “chronic obstructive pulmonary disease”, or “chronic bronchitis”. Additionally, the reference lists of retrieved articles and reviews on target topic were manually checked for potential eligible studies.
Inclusion criteria and exclusion criteria
Two authors (MZ and TW) independently screened the titles and abstracts and further reviewed the full text for potentially eligible studies according to the following inclusion and exclusion criteria. The inclusion criteria were as follows: 1) cohort, case–control, or cross-section design; 2) analyzed the association between serum 25(OH)D and COPD; 3) the effect size and its 95% confidence interval (CI) were provided or could be estimated; 4) high-quality study; and 5) reported in English or Chinese. The exclusion criteria were as follows: 1) reviews, case reports, conference abstracts, letters, or editorials; 2) cells or animal models; 3) involved individuals who had vitamin D supplement; and 4) the levels of vitamin D were quantified by 1,25(OH)2D only, other than 25(OH)D, as 25(OH)D was the best indicator of vitamin D status. For duplicate publications, the most complete one was included.
Data extraction
The following information was extracted from the included studies: first author, year of publication, study design, country, latitude of the region, sample size, body mass index (BMI), assay method, serum vitamin D levels, and distribution of vitamin D deficiency. The quality of included studies was assessed by 9-star Newcastle–Ottawa Scale. The following three major study components were judged: selection (0–4 stars), comparability (0–2 stars), and exposure/outcome (0–3 stars). This meta-analysis included studies with ≥6 stars representing the better methodological quality. Any discrepancies were resolved by discussion or consulting a senior author. If necessary data were not offered, we mailed the corresponding author for details.
Statistical analysis
For the continuous data, the standardized mean difference (SMD) and 95% CI were computed. For the dichotomous data, the odds ratio (OR) and 95% CI were calculated. Heterogeneity was assessed by the Cochran Q test and the I2 statistic. For the Q statistic, P<0.10 level indicated statistically significant heterogeneity. For the I2 statistic, I2>50% suggested substantial heterogeneity. When heterogeneity was confirmed, the results were pooled using random effects model; otherwise, the fixed effects model was used. To explore potential sources of heterogeneity, meta-regression analyses and subgroup analyses were used according to latitude (low latitude: 0°–30°, middle latitude: 30°–60°, and high latitude: 60°–90°), BMI (normal: 18.5–24.99, overweight: 25.0–29.99, and obese: ≥30.0), and assay method (enzyme-linked immunosorbent assay (ELISA), immunoassay, liquid chromatography electrospray ionisation tandem mass spectrometry (LC-MS/MS), and electrocheminluminescence). Sensitivity analysis was applied by omitting single study in turn and recalculating the pooled estimates to test the robustness of the pooled estimate. Publication bias was assessed by funnel’s plot and Egger’s test. All statistical analyses were conducted using Stata 12.0 (StataCorp LP, College Station, TX, USA). A two-tailed P-value was considered statistically significant, unless explicitly stated.
Results
Study selection and study characteristics
The study selection process is presented in a flow chart (Figure 1). Briefly, a total of 528 related publications were identified based on the searching strategy, among which 166 were duplicates. After screening the titles and abstracts, 110 articles remained for full text review. Finally, 21 eligible articles18–38 were included in this study. The characteristics of the included studies are shown in Table 1. The 21 articles were published from 2010 to 2015 covering 4,818 COPD patients and 7,175 controls. For vitamin D levels and COPD, 13 articles18,20,22,23,27–29,32–35,37,38 were recruited. For vitamin D deficiency and COPD, 12 articles18–20,22,25,27,28,32,34,35,37,38 were included. For vitamin D and COPD severity, nine articles18,20,23–26,30,33,35 were used. For vitamin D and COPD exacerbation, seven articles20,21,26,31,32,36,38 were identified.
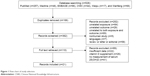  | Figure 1 Flow chart of study selection. |
Serum vitamin D levels in COPD patients
Thirteen studies18,20,22,23,27–29,32–35,37,38 reported the levels of serum vitamin D in 1,981 COPD patients and 1,283 control subjects. The pooled effect sizes showed that the serum vitamin D levels in COPD patients were lower than the levels in control subjects (SMD: −0.69, 95% CI: −1.00, −0.38, P<0.001; Figure 2), with significant heterogeneity among these studies (I2=94.0%, P<0.001). The regression analyses showed that latitude degree was a source of heterogeneity (R2=25.21%, P=0.061). The latitude degree-special subgroup analyses showed that high heterogeneity remained and subjects in low latitude region had lower serum vitamin D levels (SMD: −1.22, 95% CI: −2.08, −0.36, P=0.005) than subjects in middle latitude region (SMD: −0.43, 95% CI: −0.67, −0.20, P<0.001) and high latitude region (SMD: −0.02, 95% CI: −0.12, 0.16, P=0.781). Sensitivity analyses showed that no individual study significantly influenced the pooled results. Publication bias was detected by Egger’s test (P=0.011), but no missing studies were reported with the trim and fill method.
Vitamin D deficiency and COPD risk
Twelve studies18–20,22,25,27,28,32,34,35,37,38 reported the association between vitamin D deficiency and COPD in 3,224 COPD patients and 6,699 control subjects. Vitamin D deficiency was defined as serum 25(OH)D <20 ng/mL (50 nmol/L).39 Significant heterogeneity was among the 12 studies (I2=83%, P<0.001); thus, a random effect model was selected. Results showed that vitamin D deficiency patients had 77% higher odds of COPD compared with control subjects (OR: 1.77, 95% CI: 1.18, 2.64, P=0.006; Figure 3). To explore the sources of heterogeneity, meta-regression analyses were conducted. The results indicated that assay methods had significant influence on heterogeneity (R2=69.36%, P=0.001). The subgroup analyses based on assay methods were carried out. Four studies applying immunoassay showed 116% higher odds of COPD in vitamin D deficiency patients compared with control subjects (OR: 2.16, 95% CI: 1.29, 3.63, P=0.004, I2=13.7%, P=0.324). Meanwhile, four studies using ELISA showed 240% higher odds of COPD in vitamin D deficiency patients compared with control subjects (OR: 3.40, 95% CI: 2.30, 5.01, P<0.001, I2=0%, P=0.90). Sensitivity analyses showed no excessive change in summarized results. Neither Begg’s test (P=0.837) nor Egger’s test (P=0.324) indicated publication bias.
Vitamin D and COPD severity
Seven studies18,20,23,24,26,33,35 (2,091 COPD patients) investigated the association between serum vitamin D levels and COPD severity. All studies defined COPD severity based on the Global Initiative for Obstructive Lung Disease (GOLD) classification. Meta-analysis was conducted to analyze the vitamin D levels between mild–moderate (GOLD 1 and 2) COPD and severe–very severe (GOLD 3 and 4) COPD. Severe–very severe COPD patients had lower serum vitamin D levels compared to mild–moderate COPD patients (SMD: −0.87, 95% CI: −1.51, −0.22, P=0.001; Figure 4). However, high degree of heterogeneity was assessed (I2=97.6%, P<0.001). The subgroup analyses based on latitude, BMI, and assay method showed that there was only a slight drop in the heterogeneity, which was still high. Sensitivity analyses showed that overall results were not obviously altered by individual studies. Begg’s test (P=0.293), as well as Egger’s test (P=0.251), did not indicate evidence of publication bias.
Only three studies18,25,30 reported the vitamin D deficiency by COPD severity. Two of the studies reported the vitamin D deficiency status in mild (GOLD 1) COPD patients and moderate–very severe (GOLD 2–4) COPD patients. And another two provided the vitamin D deficiency status in moderate (GOLD 2) COPD patients and severe–very severe (GOLD 3–4) COPD patients. No association was found between vitamin D deficiency and moderate–very severe COPD compared to mild COPD (OR: 1.08, 95% CI: 0.31, 3.74, P=0.908, I2=94.4%, P<0.001). However, meta-analysis reported that vitamin D deficiency patients had higher risk of severe–very severe COPD than moderate COPD (OR: 2.83, 95% CI: 2.00, 4.00, P<0.001, I2=42.3%, P=0.188). The sensitivity analyses and publication bias analyses were not conducted for the limited amount of studies.
Vitamin D and COPD exacerbation
Five studies,20,21,31,32,36 including 278 acute exacerbation COPD (AECOPD) patients and 563 stable COPD patients, were under meta-analysis. AECOPD was defined as worsening or new symptoms from stable state and requiring additional treatment.40 Combined by the random effect model, the pooled results reported that AECOPD patients had lower levels of serum vitamin D compared to stable COPD patients (SMD: −0.43, 95% CI: −0.70, −0.15, P=0.002, I2=65.5%, P=0.021; Figure 5). The sensitivity analyses indicated consistency of overall results. No publication bias was detected (Begg’s test: P=0.806, Egger’s test: P=0.426).
For the association between vitamin D deficiency and COPD exacerbation, only three studies26,32,38 were included. No significant association was detected between vitamin D deficiency and COPD exacerbation (OR: 1.17, 95% CI: 0.86, 1.59, P=0.326, I2=23.2%, P=0.272). The sensitivity analyses and publication bias analyses were not conducted for the limited amount of studies.
Discussion
The roles of vitamin D in COPD have been highlighted in recent years. Vitamin D plays a role in the pathogenesis of COPD via several biological processes.41 Previously, two meta-analyses were conducted to assess the association between vitamin D and COPD. Zhang et al15 just included two studies, and no statistical significance was found in the analysis. Zhu et al16 conducted a meta-analysis of 18 studies. The analysis showed that the serum levels of 25(OH)D were lower in COPD patients and vitamin D deficiency was associated with COPD severity rather than COPD risk. However, the vitamin D levels in the included studies were measured either in serum or in plasma. And participants with vitamin D supplement were included. Furthermore, the high source of heterogeneity was not analyzed. To explore more accurately the association between vitamin D and COPD, current studies including 21 studies were conducted on the vitamin D roles in COPD risk, severity, and exacerbation. Serum vitamin D levels were inversely associated with COPD risk, severity, and exacerbation. Vitamin D deficiency was associated with increased risk of COPD and severe COPD. Furthermore, assay methods is the sources of high heterogeneity in the analysis of association between vitamin D deficiency and COPD risk. There was no enough evidence to support the association between vitamin D deficiency and COPD exacerbation.
This meta-analysis found certain evidence for the association between low serum vitamin D levels and COPD. It may be due to the reduction in outdoor activity inducing the absence of sun exposure, reduced dermal vitamin D synthesis due to aging skin and smoking, increased vitamin D catabolism by glucocorticoids, and lower vitamin D storage capacity in COPD patients.42 The latitude degree-special subgroup analysis showed a tendency toward lower serum vitamin D levels in lower latitude degree region with high heterogeneity. This result ran counter to the traditional theory in which lower latitude degree region gains more sun exposure, thus more vitamin D synthesis. The point of Kimlin et al could account for this discrepancy. They suggested that latitude did not influence vitamin D levels for the majority of the year and only has a affect in the coldest months of year.43 The season variation, as a confusion factor, may impact the overall results.
This meta-analysis demonstrated that subjects with vitamin D deficiency had an increased risk of COPD. Vitamin D deficiency is prevalent in the elderly with atrophic skin reducing vitamin D production and inadequate outdoor activity, insufficient diet intake, decreased intestinal absorption, as well as hydroxylation in the liver and kidneys.44 The majority of COPD patients are elderly people.45 Thus, the proportion of vitamin D deficiency comorbid COPD was large, especially in the elderly. Besides, many chronic disease such as cardiovascular diseases which are usually co-morbid COPD relate to vitamin D deficiency. Maybe, vitamin D deficiency and COPD could mutually promote bridging by those co-morbid chronic diseases.11,14
Several biological mechanisms may explain the contribution of vitamin D deficiency to COPD. First, vitamin D acts as a potent inhibitor in either innate or adaptive immune response via activation of VDR.46 VDR is expressed in various types of inflammatory and structural cells. Vitamin D deficiency fails to restrain the maturation of dendritic cell and macrophage by regulating the major histocompatibility complex class II molecules,47 decrease the production of proinflammatory cytokines and chemokines,48 promote monocyte and neutrophil recruitment depending on NFκB-mediated pathway,49 and shift Th1 T-cell toward Th2 and regulatory T-cell.50,51 The dysregulated immune-inflammatory response leads to the development of chronic inflammation and lung structural destruction, which in turn, promotes the onset and progress of COPD. Second, vitamin D can upregulate the expression of antimicrobial peptides in response to infections.52,53 Vitamin D deficiency increases the susceptibility to respiratory infections, which in turn, contributes to airway colonization and chronic inflammation. Third, vitamin D deficiency has an effect on airway smooth muscle by regulating the expression of genes related to cell proliferation, glucocorticoid response, and smooth muscle contraction.54 In addition, vitamin D deficiency contributes to the remodeling of airway smooth muscle and lung tissue by inducing fibroblast proliferation, promoting collagen synthesis, and increasing levels of matrix metalloproteinase.55 Fourth, vitamin D is associated with the metabolism of bone and muscle. Vitamin D deficiency plays a role in the development of osteoporosis and skeletal muscle weakness, indicating the loss of lung function.56,57
Vitamin D deficiency was defined as serum 25(OH)D <20 ng/mL (50 nmol/L) in this meta-analysis and in the included studies. Due to the various sensitivities and accuracies of different assay methods, interassay and interlaboratory variations in vitamin D measurements can contribute to the heterogeneity of vitamin D deficiency and COPD.58,59 Thus, it is optimal that cut-point data are assayed by special assay method. In this study, we performed meta-regression and subgroup analyses on assay method to remove the influence of assay methods on heterogeneity. Substantial drop in heterogeneity was found. This result supported the point that assay method factor can affect vitamin D measurement and should be taken into account in vitamin D deficiency-related analysis. The present study indicates that the serum vitamin D levels were inversely associated with the severity of COPD and severe COPD was more likely to develop in individuals who suffered vitamin D deficiency. Severe COPD patients suffer more disabling pulmonary function, poor nutritional status, comorbid cardiovascular diseases, rib fractures and psychological distress, are an economic and society burden, and have impaired quality of life.60,61 They are more likely to stay indoors, have longer smoking history,62 be anorectic,63 and take oral glucocorticoids, which reduces the levels of vitamin D. Vitamin D deficiency contributes to severe COPD by magnifying inflammation, enhancing structural changes, decreasing lung function, and allowing microbe infection.
The present study suggests that COPD patients with exacerbation had lower levels of vitamin D than stable COPD patients. A higher risk of COPD exacerbation in vitamin D deficiency patients was observed but not statistically significant. Exacerbations are mainly triggered by microorganism infections leading to an amplified inflammation. Vitamin D benefits the anti-inflammation effects by activating monocytes and macrophages, inducing antimicrobial peptides as well as enhancing the chemotactic and phagocytic capacity of inflammatory cells. Low levels of vitamin D are unable to upregulate the innate immune defense system and reduce pathogen load and colonization. The consequence is frequent exacerbations with worse airway flow and even dyspnea. The association between vitamin D deficiency and COPD exacerbation is controversial. Puhan et al26 reported a trend that severe vitamin D deficiency patients were susceptible to exacerbations without statistically significant association. Martineau et al64 suggested that vitamin D supplementation protected against moderate or severe exacerbation in COPD patients with vitamin D deficiency. However, the study by Lehouck et al65 held inconsistent results that vitamin D supplementation had no effect on the rate of COPD exacerbations. A study exploring the effect of vitamin D supplementation on the incidence of exacerbations in vitamin D deficiency comorbid COPD patients is in progress.66 Maybe, it can broaden our knowledge of vitamin D and COPD exacerbation.
The limitations of this study should be acknowledged. First, many of the included studies were case–control or cross-sectional. The nature of study design resulted in the identification of association but not causality link. The evidence just addressed the association between abnormal levels of vitamin D and COPD. However, it was still not clear whether abnormal level of vitamin D was a consequence of COPD or a contributor to COPD. Hence, further prospective, longitudinal, and well-designed cohort studies are needed. Second, potential confounders, such as sunlight exposure, seasonal variation, and diet intake, can affect vitamin D status. However, insufficient information regarding these factors in included studies limited the adjustment of the results. Statistical heterogeneity was still assessed, even with stratified analyses based on latitude degree, assay method, and BMI. Thus, the potential confounders may be the source of heterogeneity. Third, the limited number of eligible studies on the association between vitamin D and COPD severity as well as COPD exacerbations confined the analyses.
Conclusion
This meta-analysis suggests that, as compared to controls, serum vitamin D levels were lower in patients with COPD, severe COPD, and COPD exacerbation. Vitamin D deficiency is associated with increased risk of COPD and severe COPD but not COPD exacerbation. The results provided an improved understanding of the roles of vitamin D in COPD development and progression. Further prospective, large, and well-designed studies are needed to confirm the results. Awareness of association between vitamin D and COPD risk, severity, and exacerbation in clinical practice may benefit disease outcomes.
Acknowledgment
This study was supported by grant nos 81171320 and 31450007 from the National Natural Science Foundation of China.
Disclosure
The authors report no conflicts of interest in this work.
References
Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. | ||
Jeffery PK. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax. 1998;53:129–136. | ||
Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. | ||
Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383(9912):146–155. | ||
Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619–4628. | ||
Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–2521. | ||
Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev. 2008;66(10 suppl 2):S116–S124. | ||
Finklea JD, Grossmann RE, Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr. 2011;2(3):244–253. | ||
Edwards MH, Cole ZA, Harvey NC, Cooper C. The global epidemiology of vitamin D status. J Aging Res Clin Prac. 2014;3:148–158. | ||
Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95(2):471–478. | ||
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. | ||
Baldrick FR, Elborn JS, Woodside JV, et al. Vitamin D status in chronic obstructive pulmonary disease. Proc Nutr Soc. 2012;71(OCE2):E98. | ||
Shaheen SO, Martineau AR. Vitamin D and chronic obstructive pulmonary disease: justified optimism or false hope? Am J Respir Crit Care Med. 2012;185:239–241. | ||
Foong RE, Zosky GR. Vitamin D deficiency and the lung: disease initiator or disease modifier? Nutrients. 2013;5(8):2880–2900. | ||
Zhang LL, Gong J, Liu CT. Vitamin D with asthma and COPD: not a false hope? A systematic review and meta-analysis. Genet Mol Res. 2014;13(3):7607–7616. | ||
Zhu B, Xiao C, Zheng Z. Vitamin D deficiency is associated with the severity of COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1907–1916. | ||
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. | ||
Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–220. | ||
Duckers JM, Evans BA, Fraser WD, Stone MD, Bolton CE, Shale DJ. Low bone mineral density in men with chronic obstructive pulmonary disease. Respir Res. 2011;12:101. | ||
Persson LJ, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TM. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One. 2012;7(6):e38934. | ||
Quint JK, Donaldson GC, Wassef N, Hurst JR, Thomas M, Wedzicha JA. 25-hydroxyvitamin D deficiency, exacerbation frequency and human rhinovirus exacerbations in chronic obstructive pulmonary disease. BMC Pulm Med. 2012;12:28. | ||
Zhou X, Han J, Song Y, Zhang J, Wang Z. Serum levels of 25-hydroxyvitamin D, oral health and chronic obstructive pulmonary disease. J Clin Periodontol. 2012;39:350–356. | ||
Berg I, Hanson C, Sayles H, et al. Vitamin D, vitamin D binding protein, lung function and structure in COPD. Respir Med. 2013;107(10):1578–1588. | ||
Holmgaard DB, Mygind LH, Titlestad IL, et al. Serum vitamin D in patients with chronic obstructive lung disease does not correlate with mortality – results from a 10-year prospective cohort study. PLoS One. 2013;8(1):e53670. | ||
Lee HM, Liu M, Lee K, Luo Y, Wong ND. Does low vitamin D amplify the association of COPD with total and cardiovascular disease mortality? Clin Cardiol. 2014;37(8):473–478. | ||
Puhan MA, Siebeling L, Frei A, Zoller M, Bischoff-Ferrari H, Ter Riet G. No association of 25-hydroxyvitamin D with exacerbations in primary care patients with COPD. Chest. 2014;145(1):37–43. | ||
Wang XM, Xiao H, Liu LL, Li XJ. Bone metabolism status and associated risk factors in elderly patients with chronic obstructive pulmonary disease (COPD). Cell Biochem Biophys. 2014;70(1):129–134. | ||
Heidari B, Javadian Y, Monadi M, Dankob Y, Firouzjahi A. Vitamin D status and distribution in patients with chronic obstructive pulmonary disease versus healthy controls. Caspian J Intern Med. 2015;6(2):93–97. | ||
Yumrutepe T, Aytemur ZA, Baysal O, Taskapan H, Taskapan CM, Hacievliyagil SS. Relationship between vitamin D and lung function, physical performance and balance on patients with stage I–III chronic obstructive pulmonary disease. Rev Assoc Med Bras. 2015;61(2):132–138. | ||
Persson LJ, Aanerud M, Hiemstra PS, et al. Vitamin D, vitamin D binding protein, and longitudinal outcomes in COPD. PLoS One. 2015;10(3):e0121622. | ||
Zhang P, Zhu YQ, Li Z, He LP, Fan D, Cai Q. Expression and correlation of vitamin D and MMP-9 in chronic obstructive pulmonary disease. Int J Respir. 2014;34:649–651. | ||
Huang JH. Effects of vitamin D to the pulmonary function and living quality in COPD. Jiangxi Med J. 2013;48(8):668–670. | ||
Lu JW, Wu SQ, Zhang KB, Bao QY. Correlation between 25(OH) vitamin D concentration and lower respiratory tract infection in chronic obstructive pulmonary disease. Chin J Nosocomiol. 2014; 24(24):6131–6133. | ||
Lu YX, Liu JF, Qin XF. The association between serum vitamin D and CAT scores in stable chronic obstructive pulmonary disease. J Cummun Med. 2014;12(23):50–52. | ||
Tan J, Tong Q, Dong XX, Fang XZ, Lu ZH. Relationship between lung function and bone mineral density, serum vitamin D in elderly patients with chronic obstructive disease. Acta Univ Med Nan Jing (Nat Sci). 2013;13(11):1584–1586. | ||
Ye J, Chang J, Sun QM. The relationship among 25-hydroxy vitamin D level in serum with the lung function and CAT score of elderly patients with COPD. Chin Med Rec. 2014;15(7):78–80. | ||
Said AF, Abd-Elnaeem EA. Vitamin D and chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2015;64(1):67–73. | ||
Zhang P, Luo H, Zhu YQ. Prevalence of vitamin D deficiency and impact on quality of life in patients with chronic obstructive pulmonary disease. J Cent South Univ (Med Sci). 2012;37(8):802–806. | ||
Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. | ||
Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s. | ||
Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179(8):630–636. | ||
Janssens W, Mathieu C, Boonen S, Decramer M. Vitamin D deficiency and chronic obstructive pulmonary disease: a vicious circle. Vitam Horm. 2011;86:379–399. | ||
Kimlin MG, Olds WJ, Moore MR. Location and vitamin D synthesis: is the hypothesis validated by geophysical data? J Photochem Photobiol B. 2007;86(3):234–239. | ||
Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75(4):611–615. | ||
Afonso AS, Verhamme KM, Sturkenboom MC, Brusselle GG. COPD in the general population: prevalence, incidence and survival. Respir Med. 2011;105(12):1872–1884. | ||
Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients. 2015;7(10):8251–8260. | ||
Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7(9):8127–8151. | ||
Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. | ||
Trujillo G, Habiel DM, Ge L, Ramadass M, Cooke NE, Kew RR. Neutrophil recruitment to the lung in both C5a- and CXCL1-induced alveolitis is impaired in vitamin D-binding protein-deficient mice. J Immunol. 2013;191(2):848–856. | ||
Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29(6):369–375. | ||
Barrera D, Díaz L, Noyola-Martínez N, Halhali A. Vitamin D and inflammatory cytokines in healthy and preeclamptic pregnancies. Nutrients. 2015;7(8):6465–6490. | ||
Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–1165. | ||
Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240–4270. | ||
Banerjee A, Panettieri R. Vitamin D modulates airway smooth muscle function in COPD. Curr Opin Pharmacol. 2012;12(3):266–274. | ||
Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011;406(1):127–133. | ||
Sarkar M, Bhardwaj R, Madabhavi I, Khatana J. Osteoporosis in chronic obstructive pulmonary disease. Clin Med Insights Circ Respir Pulm Med. 2015;9:5–21. | ||
Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33–83. | ||
Lai JK, Lucas RM, Clements MS, Harrison SL, Banks E. Assessing vitamin D status: pitfalls for the unwary. Mol Nutr Food Res. 2010;54(8):1062–1071. | ||
Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21(11):2708–2717. | ||
Pinnock H, Kendall M, Murray SA, et al. Living and dying with severe chronic obstructive pulmonary disease: multi-perspective longitudinal qualitative study. BMJ. 2011;342:d142. | ||
Maleki-Yazdi MR, Kelly SM, Lam SY, Marin M, Barbeau M, Walker V. The burden of illness in patients with moderate to severe chronic obstructive pulmonary disease in Canada. Can Respir J. 2012;19(5):319–324. | ||
Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor’s diagnosis, symptoms, age, gender, and smoking habits. Respiration. 2005;72(5):471–479. | ||
Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151–1156. | ||
Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3(2):120–130. | ||
Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114. | ||
Rafiq R, Aleva FE, Schrumpf JA, et al. Prevention of exacerbations in patients with COPD and vitamin D deficiency through vitamin D supplementation (PRECOVID): a study protocol. BMC Pulm Med. 2015;15:106. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





