Back to Journals » Cancer Management and Research » Volume 12
The Association Between Illness Acceptance and Quality of Life in Women with Breast Cancer
Authors Jankowska-Polańska B , Świątoniowska-Lonc N , Ośmiałowska E , Gałka A, Chabowski M
Received 12 May 2020
Accepted for publication 30 July 2020
Published 14 September 2020 Volume 2020:12 Pages 8451—8464
DOI https://doi.org/10.2147/CMAR.S261624
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Beata Jankowska-Polańska,1 Natalia Świątoniowska-Lonc,1 Edyta Ośmiałowska,1 Aneta Gałka,1 Mariusz Chabowski2,3
1Division of Nervous System Diseases, Department of Clinical Nursing, Faculty of Health Science, Wroclaw Medical University, Wroclaw 51-618, Poland; 2Division of Oncology and Palliative Care, Department of Clinical Nursing, Faculty of Health Science, Wroclaw Medical University, Wroclaw 51-618, Poland; 3Department of Surgery, 4th Military Teaching Hospital, Wroclaw 50-981, Poland
Correspondence: Mariusz Chabowski
Department of Surgery, 4th Military Teaching Hospital, 5 Weigla Street, Wroclaw 50-981, Poland
Tel +48 261 660 247
Fax +48 261 660 245
Email [email protected]
Introduction: Breast cancer is the most common cause of cancer death in women.
Aim: The aim of the study was to investigate the association between illness acceptance and quality of life (QoL) in patients with breast cancer.
Patients and Methods: The study included 150 patients who had undergone surgery for breast cancer. The following standardized questionnaires were used: the Acceptance of Illness Scale (AIS), the EORT QLQ–C30 (The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30), and the EORT QL–BR 23 (Quality of Life Questionnaire for Breast Cancer) for QoL evaluation. Socio-clinical data were obtained from the patients’ medical records.
Results: In the study group, the overall QoL score was 62.67± 17.11 in the BCT group, 63± 14.3 in the MTX group, and the highest: 65.5± 20.2 in the reconstruction group. Comparative analysis showed that patients in the BCT group reported significantly more fatigue (p=0.007) and appetite loss (p=0.032) than those in the MTX+R group. Patients in the MTX group were significantly less satisfied with their body image (p=0.001) and experienced more financial troubles (p=0.013) than the remaining patients. Patients in the MTX+R group reported significantly better sexual function and more sexual enjoyment than the remaining patients (p< 0.001). All patients scored high for illness acceptance, though patients in the MTX group had lower scores (28.17± 7.2) than the others: 31.84± 6.51 in the BCT group and 32.78± 7.97 in the MTX+R group. The comparative analysis of QoL according to the level of AIS showed the significantly better QoL and less intense symptoms within all the domains except for the insomnia and hair loss domains in the group of high AIS in comparison with medium and lack of AIS. Acceptance of illness significantly correlated with 4 domains of the QLQ-C30 (p< 0.05). The correlation between illness acceptance and overall QoL was positive (r=0.243; p=0.003) – the higher the acceptance, the better the QoL. Correlations with pain, diarrhoea, and financial difficulties were negative. Illness acceptance was positively correlated with QoL in 3 domains of the EORTC–BR23: body image (p< 0.001), sexual function (p=0.015), and sexual enjoyment (p=0.047), and negatively with the “treatment side effects” (p=0.011).
Conclusion: The level of illness acceptance varies depending on the treatment method, and is the lowest in the group of women having undergone a mastectomy, and the highest in patients after a mastectomy with immediate breast reconstruction. Acceptance of illness improves the QoL of women treated for breast cancer, regardless of the specific treatment method.
Keywords: breast cancer, illness acceptance, treatment, quality of life
Introduction
Breast cancer is the most common cause of cancer death in women.1 Its incidence in Poland has already exceeded 16,500 cases annually.2 Health-related quality of life (HRQoL) is the most important aspect of any person’s life especially in patients with cancer. The World Health Organization (WHO) defines the quality of life (QoL) as an individual’s perception of their position in life in the context of the culture and value systems in which they live, and in relation to their standards, tasks, and expectations, subject to environmental considerations. Meanwhile, health is broadly defined as a state of psychological, physical, and social well-being, rather than the mere absence of disease.3 Factors that affect the QoL of women with breast cancer can be classified into 4 groups: socio-demographic variables (age, sex, relationship status, education), clinical variables (TNM stage, cancer type, treatment, symptoms and side effects, pain, functional performance), psycho-social variables (acceptance of illness, coherence), and health-related beliefs (strategies for coping with the disease or with pain).4
Illness is undoubtedly a source of stress, and may be perceived by the patient as a loss (harm), threat, or challenge. Patients’ behaviours and reactions depend on a number of factors, including treatment options and conditions, disease course, and individual characteristics (temperament, coping with stress, knowledge, personality, internal resources: social support and socio-economic status). These are also associated with illness acceptance, which begins as soon as the disease is suspected, and lasts throughout the treatment process and until the end of a patient’s life. It depends not only on the type of disease, but also on the specific patient. Individual traits, health-related actions, and stress-reducing behaviours affect both the patient’s psychological state and their social functioning. All the above factors affect a person’s functioning, and as a result, their perceived QoL and illness acceptance.5 Women with breast cancer demonstrate moderate acceptance of their illness.6,7
However, literature still lacks studies on the impact that acceptance of illness has on QoL in breast cancer patients. Thus, the aim of the study was to investigate the association between illness acceptance and quality of life (QoL) in patients with breast cancer.
Patients and Methods
The study included 150 patients who had undergone surgery for breast cancer at the Surgical Oncology Department of the Regional Specialist Hospital. The study was performed between December 2016 and February 2017, and recruited patients who came in for follow-up appointments at the Oncology Clinic and the Surgical Oncology Clinic. Patients were divided into three groups depending on the treatment method used: group 1 included women after breast-conserving therapy (BCT), group 2 – mastectomy (MTX), and group 3 – mastectomy with immediate breast reconstruction (MTX+R). The study was approved by the local Bioethics Committee, approval no. KB- 223/2016. The research was conducted in accordance with the Declaration of Helsinki. Participation in the study was voluntary and anonymous. All the respondents expressed written consent, were informed about the purpose and course of the study and were aware of the possibility of withdrawing at any stage of it.
Patients were administered three standardized questionnaires:
- The Acceptance of Illness Scale (AIS), comprising eight statements describing the consequences of disease. Each statement is rated using a 5-item scale, where 1 stands for “strongly agree”, 2 – “agree”, 3 – “do not know”, 4 – “disagree”, and 5 – “strongly disagree”. The total AIS score is the sum of points from all statements and ranges between 8 (no acceptance) and 40 points (complete acceptance).8 In relation to the often used in the literature on the three levels of acceptance of illness: low acceptance of illness (8–18 points), moderate acceptance of illness (19–29 points) and good acceptance of illness (30–40 points), we also used the same in our study.9,10
- The EORT QLQ-C30 (The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30), used for evaluating the patient’s subjectively perceived health and functioning in a number of aspects: physical, emotional, and social. It comprises 30 questions regarding the current intensity of the parameters analysed, and responses are provided using a 4-item scale, where 1 stands for “never”, 2 – “sometimes”, 3 – “often”, and 4 – “very often”. These parameters are related to the patient’s functioning: physical, emotional, cognitive, and social, as well as the performance of life roles. Symptoms, such as fatigue, nausea, vomiting, pain, dyspnoea, insomnia, loss of appetite, constipation, and diarrhoea, as well as financial difficulties, are also recorded. The final items concern the respondent’s overall health.11
- The EORT QoL-BR 23 (Quality of Life Questionnaire for Breast Cancer), a breast-cancer specific questionnaire. It comprises 5 scales regarding the patient’s functional status, sexual function, treatment side effects, breast- and arm-related symptoms, sexual enjoyment, and concerns about future health and hair loss.12
Socio-clinical data were obtained from the patients’ medical records.
Statistical Methods Section
The analysis of the quantitative variables (ie, expressed as a number) was performed by the mean, standard deviation, median, quartiles, minimum and maximum. The analysis of the qualitative variables (ie, not expressed as a number) was performed by the number and the percentage of their occurrence. The comparison of the values of the qualitative variables in groups was done by chi-squared test or the Fisher exact test if there were low expected numbers in tables. The comparison of the quantitative variables in two groups was performed using t-Student test or Mann–Whitney test. The comparison of the quantitative variables in three or more groups was performed using analysis of variance ANOVA or Kruskal–Wallis test. If such a comparison revealed the significant differences, the post hoc analysis was done using HSD Tukey’s test or Dunn test. The correlation between two quantitative variables was analysed using the Pearson’s coefficient when both variables had normal distribution, or Spearman’s coefficient when at least one of the variables did not have normal distribution.
Multivariate analysis was performed using the linear regression method. The quality of the model was evaluated by the coefficient of determination (R2). Normal distribution of variables was assessed using Shapiro–Wilk W-test. The significance threshold of p-value less than 0.05 was applied.
Results
Socio-Demographic and Clinical Characteristics of the Study Group
Patient age in the study subgroups was: 37–69 years old – BTC group; 37–77 y/o – MTX; 26–70 y/o – MTX+R. Patients in the MTX+R group were significantly younger than those in the two remaining groups (p<0.05). Regarding education, residence, financial standing, or professional activity, no statistically significant differences were found between the subgroups (p>0.05). Most respondents reported having completed high school or college/university education. More than half considered their financial standing to be good or very good. Most patients were professionally active. More than 80% of patients in all groups lived in urban areas (Table 1).
 |
Table 1 Patients’ Socio-Demographic Characteristics by Treatment Method |
The subgroups differed in terms of illness duration, which was the longest in the MTX group, and the shortest in the MTX+R group. Women in the MTX+R and MTX group were more often in a relationship than women in the BCT group (respectively, 82% and 88% vs 62%; p=0.005) (Table 1).
The groups differed in terms of treatment methods used, which also constituted the basis for the analysis of QoL and illness acceptance. Adjuvant chemotherapy was most often administered in the MTX group, and the least often in the BCT group (p<0.001). Radiation therapy was most common in the BCT group, and the least common in the MTX+R group (p<0.001). As to hormonal therapy, it was most common in the MTX+R group, and the least common in the MTX group (p<0.029) (Table 2).
 |
Table 2 Patients’ Clinical Characteristics by Treatment Method |
An analysis of the patients’ clinical status and symptoms showed that upper extremity swelling was experienced most often by patients in the MTX group, and least often in the MTX+R group. Hair loss was reported most in the MTX group, and least in the BCT group (p<0.05) (Table 2). The number of secondary incidences of the disease was the highest in the MTX group and the lowest in the MTX+R group (p<0.05) (Table 2).
When broken down by TNM staging, the highest T values were found in the MTX group, and the lowest in the BCT group (p<0.05). N parameter values were the highest in the MTX group and the lowest in the BCT and MTX+R groups (p<0.05). The patients were not differentiated by the M parameter (Table 2).
Satisfaction with the treatment and its cosmetic result were the highest in the BCT group, and the lowest in the MTX group (p<0.05). Most negative impact of the treatment on personal life was reported by patients in the MTX group, and the least by patients in the MTX+R group (p<0.05) (Table 2).
Analysis of QoL Scores in the QLQ-C30 Questionnaire by Treatment Type
Post hoc analysis showed that patients in the BCT group reported significantly more fatigue (p=0.007) and appetite loss (p=0.032) than those in the MTX+R group. Patients in the MTX group experienced significantly more financial difficulties (p=0.013) than the remaining patients. No significant differences were found between the groups in terms of the remaining QLQ-C30 domains (Table 3).
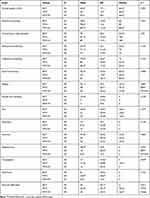 |
Table 3 Analysis of QLQ-C30 Scores by Treatment Method |
Analysis of QoL Scores in the QLQ-BR23 Questionnaire by Treatment Type
Significant differences were found in terms of body image, sexual functioning, and sexual enjoyment (p<0.05). In the post hoc analysis, MTX patients were significantly less satisfied with their body image than those in the remaining groups (p=0.001). Patients in the MTX+R group reported significantly better sexual function and more sexual enjoyment than the remaining patients (p<0.001). No significant differences were found between the groups in terms of the remaining QLQ-BR23 domains (Table 4).
 |
Table 4 Analysis of QLQ-BR23 Scores by Treatment Method |
Analysis of Illness Acceptance (AIS) Scores by Treatment Type
All patients scored high for illness acceptance. Statistically significant differences were observed between patients from the MTX group vs other patients from the MTX + R and BCT groups, among which no differences were noted. Patients in the MTX group had lower scores (28.17±7.2) than the others: 31.84±6.51 in the BCT group and 32.78±7.97 in the MTX+R (Table 5).
 |
Table 5 Analysis of AIS Scores by Treatment Method |
Correlation Between Illness Acceptance (AIS) and QoL in the QLQ-C30 and QLQ-BR23
The comparative analysis of the QoL according to AIS level, evaluated with the questionnaire QOL-BR23 showed the better functioning and the better QoL within BRBI domain (body image) in the group of patients, which better accept their disease. Similar dependence was revealed within BRSEF domain (sexual functioning): 47.4 ± 29.1 vs 43.5 ± 29.6 vs 29.2 ± 29.9 (p <0.001) and BRSEE domain (sexual enjoyment): 46.1 ± 37.5 vs 40.2 ± 31.7 vs 14.7 ± 27.4 (p = 0.001). The exception was BRFU domain (future perspective), when the maximum score had the group with moderate acceptance of the illness, and the minimum score – with high level of AIS (p = 0.010). The comparative analysis within domains focusing on the intensity of symptoms revealed the more intense level of symptoms and more negative impact on everyday functioning in the patients with low acceptance of illness and, contrary, the less impact – in the patients with high AIS. The exception was the domain “upset by hair loss (BRHL)”, where there was no significant differences between the study groups (Table 6). In terms of domains determining functioning, a higher assessment of quality of life in these domains was observed in people with a higher disease acceptance rating and lower rating in people with a lower level of acceptance (Table 6).
 |
Table 6 Results of Quality of Life Assessment (EORTC QLQ-30C and BR23 Questionnaires) in Groups of Patients with Different Levels of Illness Acceptance and Analysis of Variance |
Acceptance of illness significantly correlated only with 4 out of 15 domains of the QLQ-C30 (p <0.05). The correlation was observed between Global health status/QoL domain (r=0.243; p=0.003) and symptoms domains: pain (−0.178; p=0.029), diarrhea (−0.162; p=0.047), and financial difficulties (−0.181; p=0.027). Time from the surgery significantly correlated only with Global health status/QoL domain (r=0.126; p=0.025) (Table 7). Similar correlation between AIS and QoL was observed in the domain of QLQ-BR23 questionnaire: body image (0.301; p<0.001), sexual functioning (0.199;p=0.015), and sexual enjoyment (0.188; p=0.047) and treatment side effects (−0.207; p=0.011). Time from the surgery significantly correlated only with breast problems (r=0.204; p=0.046) (Table 8).
 |
Table 7 Impact of Illness Acceptance (AIS) and Time from the Surgery on QoL in the QLQ-C30 Questionnaire |
 |
Table 8 Impact of Illness Acceptance (AIS) and Time from the Surgery on QoL in the QLQ-BR23 Questionnaire |
Discussion
In the female population, breast cancer is the most common cause of death. Early detection and advanced diagnostics allowed for a significant reduction of mortality from this cause in recent years. Improved survival and effective diagnosis and treatment mean that patients experience not only symptoms associated with the disease, but also with the treatment. Beside surgical treatment, radiation, hormone, and chemotherapy are also used. Surgical treatment (mastectomy) is perceived by many women as a disfiguring procedure that results in a loss of their femininity. Patients’ attitude towards the impact of the procedure on their body image is strongly negative. Other complications include lymphedema, pain, restricted movement of the ipsilateral extremity, as well as psychological and emotional consequences. Chemotherapy and radiation therapy are burdened with similar emotional complications, as well as physical ones.13 Despite advances in breast cancer diagnosis and treatment, patients with the disease still experience problems in a number of areas that collectively make up their subjective QoL. Therefore, testing QoL in women with breast cancer and subsequent attempts at optimizing the treatment still seem highly relevant. There is no consensus in literature regarding the impact of socio-clinical and psycho-social variables on QoL. Such associations are often assumed, but published papers produce contradictory results. In Poland, breast-conserving therapy remains the standard in cases where no medical indications for mastectomy exist. The choice of treatment should be made jointly by the physician and the patient, and should consider both the available options and the patient’s preference.14
In the studied group, the overall QoL score in the mastectomy group was 62.67, while the highest score, 65.5, was found in the reconstruction group. The present findings are consistent with those by other authors.15–17 According to literature, the best QoL is reported by patients undergoing mastectomy with immediate breast reconstruction,18–20 which is corroborated by the present study. An analysis of QoL in relation to the treatment method used demonstrated significant differences in symptom intensity, including appetite loss, fatigue, and body image. These complaints were reported most often by patients who had undergone a mastectomy, which may be associated with the nature of the treatment. In a study by Zdończyk, women who had undergone surgical treatment reported physical symptoms (lymphedema, fatigue, dyspnoea) as well as difficulties in social functioning resulting from a lack of body self-acceptance.19 Potter et al reported that patients who had undergone immediate breast reconstruction had a significantly better QoL than those who had delayed reconstruction.21 This improvement is associated with the elimination of an external prosthesis and the major, bothersome bodily defect.22 After breast reconstruction, patients regain psychological balance faster, cope better with difficulties, and experience more self-confidence and comfort.23,24 The present study demonstrated that patients after a mastectomy experience significantly more financial troubles than those in the remaining groups. A negative perception of one’s body image seems to affect their existent ability to obtain financial means.23,24
The selected patient groups differed in terms of sexual function and sexual enjoyment, and the best results were seen in patients after immediate breast reconstruction. Available studies do not corroborate the impact of treatment type on the sexual function of women treated surgically for breast cancer.24,25 The presence of the breast may allow the studied patients to feel. Patients after a mastectomy experience poorer sexual functioning, associated with a feeling of having lost one’s physical attractiveness and desirability.25 Literature data show that even 1 year after the procedure, up to 25% of women may still experience high stress levels, and that the mastectomy affects their body image and perceived femininity, as well as sexual and social functioning.25 More than 70% of patients with breast cancer reported that their sexual relationship with their partner was slightly worse than before the disease.26 Also Poorkiani et al demonstrated that a third of women after a mastectomy experience no sexual problems in their relationship with their partner.27 In their analysis of sexual functioning in women treated for breast cancer, Słowik et al stated that 78–88% of women experienced deteriorated satisfaction with sex as a negative outcome of the cancer and its treatment. In the same analysis, though, the authors reported that in the studied group of women after a mastectomy or breast-conserving therapy, there was no significant change or deterioration of sexual function and satisfaction indicators depending on age, type of treatment, body image, or treatment side effects.17 This, however, is contrary to the present findings, which included a significantly higher level of sexual satisfaction in women after reconstructive surgery. Meanwhile, according to Słowik et al, the type of surgery had no impact on sexual functioning or sexual satisfaction.17
Patients who had undergone immediate breast reconstruction were significantly younger than those in the two remaining groups (48.9 vs 53.96 vs 56.48). Similar results were found by other authors.28,29 Perceived QoL is undoubtedly strongly associated with perceived support and being in a relationship. Such relationships were found most often among patients after immediate breast reconstruction, and least often in patients after a mastectomy. Brant and Przybyła-Basista claim that being in a relationship has no association with the treatment used.30 Literature data indicate that approx. 80% of patients treated for cancer experienced decreased satisfaction with their sexual activity as a result of the disease and its treatment.26,31,32 Sexual dysfunction is believed to be potentially associated with the perception of one’s own body. Moreover, having a partner is associated with social support, which is a strong positive factor in the treatment of cancer and other chronic diseases.
The most common reason for dissatisfaction and QoL deterioration is the negative perception and lack of acceptance of one’s body image, regardless of the actual physical condition and symptoms associated with treatment.33,34 Mastectomy has a negative impact on the aesthetic satisfaction with one’s body, resulting in embarrassment, lower acceptance of illness, and poorer QoL.35,36 In their study on the QoL of women treated for breast cancer, Graja and Grodecka-Gazdowska demonstrated that a deteriorated body image produces concerns about the disruption of the patient’s family, marital and sexual relations.37
The studied patients had a high level of illness acceptance overall, though the highest scores were found among women treated surgically with a mastectomy and immediate breast reconstruction. The women who had undergone mastectomy alone, the illness acceptance score was the lowest (32.78±7.97 vs 28.17±7.2). This may also be associated with body image and the loss of a feminine attribute. Patients in the immediate reconstruction group were better educated, younger, and received more support in coping with the disease from their partners. Thus, the higher acceptance may be due to better psychological preparation for the procedure.4
Illness acceptance improves adaptation to any dysfunction or limitation caused by the disease or its treatment, and increases control over one’s health or disease.38 In the present study, patients after a mastectomy were less accepting of their disease than those in the two remaining groups. Similar findings were reported by Nowicki et al, who compared groups of women undergoing standard (conservative) surgical treatment and mastectomy. Patients after a conservative procedure were found to have a higher level of illness acceptance.7
Illness acceptance had a positive impact on the studied women’s QoL, which is consistent with literature data.39 Greater illness acceptance reduced the intensity of symptoms such as pain or diarrhoea, but was also associated with better sexual function, better body image, increased sexual satisfaction, and improved financial standing. In the correlation analysis, pain had a negative impact on QoL and illness acceptance. According to Ferenc et al, significant determinants of pain intensity in breast cancer patients include time from diagnosis, illness acceptance, and source of pain.40 In their study on the QoL of women treated for breast cancer, Graja and Grodecka-Gazdowska demonstrated that a deteriorated body image produces concerns about the disruption of the patient’s family, marital and sexual relations.37 Illness acceptance enables viewing one’s situation rationally and making efforts to preserve one’s health. It may also improve QoL and determine treatment outcomes.41,42 The level of illness acceptance is often correlated with the intensity of symptoms and personal control of pain.43 The present findings are consistent with those by Pawlik and Karczmarek-Borowska, where 46% of women having undergone a mastectomy declared a very high level of illness acceptance.44
Illness acceptance has a positive impact on patients’ self-reported QoL, irrespective of the Global health status/QoL score. In comparative analysis, patients with higher levels of illness acceptance had better perceived QoL, while the intensity of their symptoms associated with the disease and its treatment were lower. Illness acceptance alleviates the negative emotions associated with the disease and increases patients’ sense of security. The greater the acceptance, the better patients adapt to the disease, and the less discomfort they experience.
Both the type of treatment and the scope of surgery have an impact on QoL. The diagnostic process and the identification of a life-threatening condition already have a negative impact on the patient’s mood. They also shape the patient’s attitude towards the proposed therapy. A major surgery significantly deteriorates QoL, similarly to treatment side effects. In the studies analysed, patients undergoing BCT are the most satisfied with the treatment outcome and the cosmetic effect, while patients after a mastectomy are the least satisfied. The latter also report the most negative impact of the treatment on their personal life, and tend to have the least impact on the choice of treatment method. Patients who had undergone immediate breast reconstruction are the ones who had had the greatest impact on the choice of treatment. This decision, however, cannot be made by the patient alone – it involves a complex process requiring the participation of a specialist surgical oncologist, and must be made on a case by case basis. Musial et al and Potter et al report that patients who decided to have a mastectomy with immediate breast reconstruction experienced a significant improvement of QoL.21,28
The own research showed that the short time after surgery had an impact on the overall quality of life measured by the QLQ-C30 questionnaire and on the domain breast problems of the BR23 questionnaire. Meanwhile, the quality of life depending on the duration of the disease did not bring the expected results, and one can assume that the initial period of treatment is most intensive in chemotherapy after surgery. The results indicate the significant differences only in everyday life functioning, pain and constipation in C30 and body image in BR 23. In a multicenter study of patients who underwent breast cancer therapy twenty years earlier, it was noted that the problems most frequently observed are breast symptoms – 27% lymphatic edema and 20% numbness.45 In the study of Tsai et al the patients after the mastectomy who underwent the treatment less than a year ago reported less frequent side effects such as nausea and vomiting compared to patients with BCT, but they were more satisfied with their body image after 1–2 and 2–5 years after the treatment, compared to the group after the mastectomy at the same time.46 According to the available research more than 5 years after treatment, no significant differences in quality of life are observed.47 Less than 1 year after mastectomy, significantly fewer patients reported hair loss, in 1–2 years after mastectomy – higher cognitive functioning, and after 2–5 years after mastectomy – higher sexual satisfaction.47 According to our knowledge, there are no studies which prove the association between disease acceptance (AIS) and the method of surgical treatment.
Physical and psychological comfort contributes to better functioning and more satisfaction with treatment. Multiple authors demonstrate a positive impact of breast reconstruction on the patient’s QoL. In their comparison of women after a traditional mastectomy and after a mastectomy with reconstruction, Goldberg et al found major differences in terms of self-acceptance that allow the latter group to function freely in their daily life.48
Conclusions
The specific surgical treatment method does not directly influence QoL. The level of illness acceptance in the whole-studied group of women treated for breast cancer was high. Between study groups, the lowest was recorded in the women having undergone a mastectomy, and the highest in the patients after a mastectomy with immediate breast reconstruction.
In comparative analyses, people with a high level of acceptance had the highest level of quality of life in the domain of functioning and the lowest severity of disease symptoms and side effects of the applied treatment.
Acceptance of the disease correlated significantly with the domains: body image, sexual functioning and enjoyment and treatment side effects in the BR 23 questionnaire and the Global health status/QoL domain and symptoms domains: pain, diarrhea, and financial difficulties.
Study Limitation
The main limitation of this study is the lack of analysis that would allow to show what level of disease acceptance was obtained depending on the time that has elapsed since the surgical procedure was used. The study design might be confounding by indication of the three different interventions. There is no analysis of illness acceptance depending on the treatment method. In our study, we evaluated only the global illness acceptance for the whole group, but the method of surgical treatment was not the cause of concern. However, knowing that the time after the surgery itself was not a significant determinant related to the assessment of the quality of life, we did not decide to show these results in this work, due to the extensive manuscript. Similarly, the limitation of the study should be considered the lack of analysis determining the quality of life depending on the use of other methods of treatment besides surgical treatment of the examined patients. As we know, each of them had at least one or even more types of therapy used during cancer treatment (next to surgical treatment). However, we assumed that there is a lot of work on the relationship between chemo and radiotherapy in breast cancer in quality of life, and in our research, the subject of chemo and radiation therapy was not as important as the issue of accepting illness discussed. Another limitation is that the patient came from one clinical center.
Ethics Approval and Consent to Participate
The study was approved by the Commission of Bioethics at Wroclaw Medical University (No KB - 223/2016). The research was conducted in accordance with the Declaration of Helsinki.
Informed Consent for Publication
Written informed consent in Polish was obtained from all the patients for the publication. All the patients provided written informed consent to participate in this study.
Funding
The authors declare that they have received no external funding for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Momenimovahed Z, Salehiniya H. Epidemiological characteristics and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019;11:151–164. doi:10.2147/BCTT.S176070
2. Malicka I, Hanuszkiewicz J, Wozniewski M. Barriers of physical activity of women post breast cancer treatment in rural Poland. Health Care Women Int. 2019;40(6):682–695. doi:10.1080/07399332.2019.1608208
3. Bickenbac J. WHO’s definition of health: philosophical analysis. Available from: https://link.springer.com/referenceworkentry/10.1007%2F978-94-017-8706-2_48-1.
4. Ośmiałowska E, Świątoniowska N, Homętowska H. Quality of life in patients diagnosed with breast cancer. Palliat Med Pract. 2018;12(3):143–150. doi:10.5603/PMPI.2018.0003
5. Megari K. Quality of life in chronic disease patients. Health Psychol Res. 2013;1(3):e27. doi:10.4081/hpr.2013.932
6. Cipora E, Konieczny M, Sobieszczański J. Acceptance of illness by women with breast cancer. Ann Agric Environ Med. 2018;25(1):167–171. doi:10.26444/aaem/75876
7. Nowicki A, Krzemkowska E, Rhone P. Acceptance of illness after surgery in patients with breast cancer in the early postoperative period. Pol Przegl Chir. 2015;87(11):539–550. doi:10.1515/pjs-2016-0001
8. Felton BJ, Revenson TA, Hinrichsen GA. Stress and coping in the explanation of psychological adjustment among chronically ill adults. Soc Sci Med. 1984;18(10):889–898. doi:10.1016/0277-9536(84)90158-8
9. Cybulski M, Cybulski L, Krajewska-Kulak E, Cwalina U. Illness acceptance, pain perception and expectations for physicians of the elderly in Poland. BMC Geriatr. 2017;17(1):46. doi:10.1186/s12877-017-0441-4
10. Jankowska-Polańska B, Kasprzyk M, Chudiak A, Uchmanowicz I. Relation between illness acceptance and quality of life in patients with chronic obstructive pulmonary disease (COPD). Pneumonol Alergol Pol. 2016;84:3–10. doi:10.5603/PiAP.a2015.0079
11. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi:10.1093/jnci/85.5.365
12. Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for research and treatment of cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768. doi:10.1200/JCO.1996.14.10.2756
13. D’Egidio V, Sestili C, Mancino M, et al. Counseling interventions delivered in women with breast cancer to improve health-related quality of life: a systematic review. Qual Life Res. 2017;26(10):2573–2592. doi:10.1007/s11136-017-1613-6
14. Kenny P, King LM, Shiell A, et al. Early stage breast cancer: costs and quality of life one year after treatment by mastectomy or conservative surgery and radiation therapy. Breast. 2000;9:37–44. doi:10.1054/brst.1999.0111
15. Kamińska M, Ciszewski T, Kukiełka-Budny B, et al. Life quality of women with breast cancer after mastectomy or breast conserving therapy treated with adjuvant chemotherapy. Ann Agric Environ Med. 2015;22(4):724–730. doi:10.5604/12321966.1185784
16. Gokgoz S, Sadikoglu G, Paksoy E, et al. Health related quality of life among breast cancer patients: a study from Turkey. Glob J Health Sci. 2011;3(2):140–152. doi:10.5539/gjhs.v3n2p140
17. Słowik AJ, Jabłoński MJ, Michałowska-Kaczmarczyk AM, Jach R. Evaluation of quality of life in women with breast cancer, with particular emphasis on sexual satisfaction, future perspectives and body image, depending on the method of surgery. Psychiatr Pol. 2017;51(5):871–888. doi:10.12740/PP/OnlineFirst/63787
18. Lee C, Sunu C, Pignone M. Patient-reported outcomes of breast reconstruction after mastectomy: a systematic review. J Am Coll Surg. 2009;209(1):123–133. doi:10.1016/j.jamcollsurg.2009.02.061
19. Zdończyk SA. The effect of selected socio-medial factors on quality of life and psychosexual functioning in women after surgical treatment of breast cancer. Pom J Life Sci. 2015;61(2):199–206. doi:10.21164/pomjlifesci.79
20. Gałka A, Świątoniowska N, Kolasińska J, Hanczyc P, Jankowska-Polanska B. Assessment of the quality of life of women with breast cancer depending on the surgical treatment method used. Palliat Med Pract. 2018;12(2):76–85.
21. Potter S, Thomson HJ, Greenwood RJ, Hopwood P, Winters ZE. Health-related quality of life assessment after breast reconstruction. Br J Surg. 2009;96(6):613–620. doi:10.1002/bjs.6605
22. Sehati Shafaee F, Mirghafourvand M, Harischi S, Esfahani A, Amirzehni J. Self-Confidence and Quality of Life in Women Undergoing Treatment for Breast Cancer. Asian Pac J Cancer Prev. 2018;19(3):733–740. doi:10.22034/APJCP.2018.19.3.733
23. Paterson CL, Lengacher CA, Donovan KA, Kip KE, Tofthagen CS. Body image in younger breast cancer survivors: a systematic review. Cancer Nurs. 2016;39(1):E39–E58. doi:10.1097/NCC.0000000000000251
24. Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: relationships with global, cancer-related, and life event stress. Psychooncology. 2004;13(3):211–220. doi:10.1002/pon.736
25. Boswell EN, Dizon DS. Breast cancer and sexual function. Transl Androl Urol. 2015;4(2):160–168. doi:10.3978/j.issn.2223-4683.2014.12.04
26. Milik A. Mental adjustment to cancer in group of women with breast cancer before and after mastectomy and before and after breast conserving surgery. Psychoonkologia. 2013;17(2):50–55.
27. Poorkiani M, Abbaszadeh A, Hazrati M, Jafarati P, Sadeghi M, Mohammadianpanah M. The effect of rehabilitation on quality of life in female breast cancer survivors in Iran. Indian J Med Paediatr Oncol. 2010;31(4):105–109. doi:10.4103/0971-5851.76190
28. Kim Z, Min SY, Yoon CS, et al. The basic facts of Korean breast cancer in 2012: results from a nationwide survey and breast cancer registry database. J Breast Cancer. 2015;18:103–111. doi:10.4048/jbc.2015.18.2.103
29. Park SH, Han W, Yoo TK, et al. Oncologic safety of immediate breast reconstruction for invasive breast cancer patients: a matched case control study. J Breast Cancer. 2016;19(1):68–75. doi:10.4048/jbc.2016.19.1.68
30. Brant A, Przybyła-Basista H. Decision on breast reconstruction in women after mastectomy – motivation, concerns, effects’ perception. Psychoonkologia. 2016;20(1):17–26. doi:10.5114/pson.2016.60926
31. Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: an eighteen months follow-up study. BMC Cancer. 2008;8:330–333. doi:10.1186/1471-2407-8-330
32. Abbashr SM. Sexual health issues in Sudanese women before and during hormonal treatment for breast cancer. Psychooncology. 2009;18(8):858–865. doi:10.1002/pon.1489
33. Mond J, Mitchison D, Latner J, Hay P, Owen C, Rodgers B. Quality of life impairment associated with body dissatisfaction in a general population sample of women. BMC Public Health. 2013;13:920. doi:10.1186/1471-2458-13-920
34. Hosseini SA, Padhy RK. Body Image Distortion. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; December 12, 2019.
35. Brunault P, Champagne AL, Huguet G, et al. Major depressive disorder, personality disorders, and coping strategies are independent risk factors for lower quality of life in non-metastatic breast cancer patients. Psychooncology. 2016;25(5):513–520. doi:10.1002/pon.3947
36. Arora NK, Gustafson DH, Hawkins RP, et al. Impact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma; a prospective study. Cancer. 2001;92(5):1288–1298. doi:10.1002/1097-0142(20010901)92:5<1288::AID-CNCR1450>3.0.CO;2-E
37. Graja T, Grodecka-Gazdowska S. Factors influencing the quality of life in women treated due to breast cancer. Przegl Ginekol Pol. 2005;5:115–120.
38. Obiegło M, Uchmanowicz I, Wleklik M, Jankowska-Polańska B, Kuśmierz M. The effect of acceptance of illness on the quality of life in patients with chronic heart failure. Eur J Cardiovasc Nurs. 2016;15:241–247. doi:10.1177/1474515114564929
39. Chabowski M, Polański J, Jankowska-Polanska B, Lomper K, Janczak D, Rosinczuk J. The acceptance of illness, the intensity of pain and the quality of life in patients with lung cancer. J Thorac Dis. 2017;9(9):2952–2958. doi:10.21037/jtd.2017.08.70
40. Ferenc W, Kulik T, Pacian A, Krawczyk M. Assessment of pain experienced by women with breast cancer. Disability. 2016;2(19):109–127.
41. Begovic A, Chmielewski A, Iwuagwu S, Chapman LA. Impact of body image on depression and quality of life among women with breast cancer. J Psychosoc Oncol. 2012;30:446–460. doi:10.1080/07347332.2012.684856
42. Ashing KT, Padilla GV, Bohorquez DE, Tejero JS, Garcia M. Understanding the breast cancer experience of Latina women. J Psychosoc Oncol. 2006;24:19–52. doi:10.1300/J077v24n03_02
43. Grobstein R. Breast Cancer. Warszawa: Wab Publishing House; 2007.
44. Pawlik M, Karczmarek-Borowska B. Acceptance of cancer in women after mastectomy. Prz Med Uniw Rzesz Ins Leków. 2013;2:203–211.
45. Kornblith AB, Herndon JE, Weiss RB, et al. Long-term adjustment of survivors of early stage breast cancer 20 years after adjuvant chemotherapy. Cancer. 2003;98:679689. doi:10.1002/cncr.11531
46. Tsai HY, Kuo RNC, Chung KP. Quality of life of breast cancer survivors following breast-conserving therapy versus mastectomy: a multicenter study in Taiwan. Jpn J Clin Oncol. 2017;47(10):909–918. doi:10.1093/jjco/hyx099
47. Klein D, Mercier M, Abeilard E, et al. Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Res Treat. 2011;129:125–134. doi:10.1007/s10549-011-1408-3
48. Goldberg P, Stolzman M, Goldberg HM. Psychological considerations in breast reconstruction. Ann Plast Surg. 1984;13(1):38–45. doi:10.1097/00000637-198407000-00008
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
