Back to Journals » OncoTargets and Therapy » Volume 11
The application of a three-dimensional visualized seed planning and navigation system in 125I seed implantation for pancreatic cancer
Authors Hu YY , Qi EP, Liu FY, Lu YH, Tan SL, Sun Y, Han ZY, Liang P , Yu XL
Received 6 May 2017
Accepted for publication 3 August 2017
Published 31 January 2018 Volume 2018:11 Pages 619—627
DOI https://doi.org/10.2147/OTT.S141245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samir Farghaly
Yanyan Hu,1,2 Erpeng Qi,1 Fangyi Liu,1 Yuhan Lu,1 Shuilian Tan,1 Ya Sun,1 Zhiyu Han,1 Ping Liang,1 Xiaoling Yu1
1Department of Interventional Ultrasound, General Hospital of People’s Liberation Army, Beijing, China; 2Medical Department, First Affiliated Hospital of PLA General Hospital, Beijing, China
Objectives: To evaluate the effectiveness of iodine-125 (125I) seed implantation for pancreatic cancer (PC), and preliminarily evaluate the clinical value of a self-developed three-dimensional (3D) visualized seed planning and navigation system in 125I seed implantation for treatment of PC.
Patients and methods: Our team retrospectively reviewed 25 PC patients who underwent 125I seed implantation between December 2010 and November 2016. The patients were divided into two groups: 3D visualization preoperative planning group (12 patients, 13 lesions) and two-dimensional (2D) regular group (13 patients, 14 lesions). We compared and analyzed the parameters of the two groups, such as number of needle insertions, one-time treatment success rate, proportion of added seeds, local control rate, rate of complications, rate of pain relief, and the survival rate and risk factors of the two groups. There was no significant difference in clinical data of the two groups.
Results: 125I seed implantation was performed successfully in all PC patients, with no occurrence of serious complications during and after the procedure. The one-time treatment success rate of 3D group (80%) was higher than that of 2D group (45.5%) (P<0.05), and the proportion of added seed number of 3D group was lower than that of 2D group (P<0.05). The local control rate of 3D group (76.9%) was higher than that of 2D group (35.7%) (P<0.05). The survival rate of 3D group was significantly higher than that of 2D group (P=0.026), and the median survival of 2D group vs 3D group was 5.00 vs 10.80 months. The median survival of all 25 patients was 7.10 months (95% confidence interval: 4.43–9.77). The rate of pain relief was 77.8% (7/9) in 2D group and 88.9% (8/9) in 3D group.
Conclusion: Ultrasound-guided, 3D visualized seed planning and navigation system assisted 125I seed implantation is a safe and effective method for the treatment of PC, with a prolonged survival of patients and better local control of tumor.
Keywords: 125I seed, pancreatic cancer, intraoperative implantation, ultrasound-guided, three-dimensional visualization
Introduction
Pancreatic cancer (PC) is one of the most lethal forms of cancer, with a 5-year survival rate of less than 5%.1 Although surgical resection of the primary tumor is currently the first selected treatment for PC and offers the best survival rate, ~80% of the patients are not candidates for surgical resection because at the time of appearance of the first symptoms, most PC patients will have advanced-stage disease.2–4 Even in PC patients who qualify for radical resection, which is now the only potential cure, the 5-year survival rate is only 25%, owing to the high incidence of undiscovered metastatic lesions.5,6 For patients presenting with advanced PC, chemotherapy and radiation therapy are the most commonly used frontline clinical strategies. However, the overall survival of these patients remains poor, as the highly heterogeneous and aggressive pancreatic tumors easily develop resistance, highlighting the dire need for other treatment options.7
In recent years, Iodine-125 (125I) seed implantation, a form of radiotherapy, has been accepted as a useful and minimally invasive interventional modality and provides a new treatment option for unresectable PC. The local tumor control is improved with fewer complications.8,9 In this procedure, 125I radioactive seed is permanently implanted into the tumor or region of interest. This miniature radioactive source continuously delivers low doses of X-rays and γ-rays, irradiates the G2- and M-phase tumor cells, and destroys double-stranded DNA to inhibit their proliferative ability, leading to the ultimate death of the cells. Because the radiation dose of 125I seed decreases rapidly with increasing distance from the source, a large proportion of radioactive dose is localized to the tumor region and damage to the surrounding normal tissues is avoided, thereby achieving conformal radiation therapy.10 Recently, the clinical efficacies of 125I seed implantation have been encouraging, especially in pain relieving and local tumor control.11–14 Wang et al15 have reported that in a clinical investigation of 125I seed implantation as a salvage modality for unresectable PC, the local control of tumor was achieved in 85.7% (24/28) of patients, and a good to medium pain relief was achieved in 94.1% (16/17) of patients. The median survival was 10.1 months (95% CI: 9.0–10.9).
To date, computed tomography (CT) and ultrasound are the most commonly used imaging modalities to guide the procedure of 125I seed implantation. Because of the advantages of direct vision, real-time dynamic imaging, and no radiation, ultrasound is now widely used in the percutaneous delivery of 125I seeds in the treatment of PC, with an improvement in accuracy and a relative reduction of complications. Precise implantation of 125I seeds and even radioactive dose distribution in three-dimensional (3D) space are the preconditions for clinical effectiveness. However, at present, even dose distribution of seed implantation is often affected by the operator’s experience and the lack of 3D information of the tumor and the adjacent tissues or vessels, yielding incomplete coverage of the tumors and failed local control. In order to solve this problem, more and more researchers are trying to bring in the application of 3D image processing and analysis technology, 3D visualization and computer-aided technology, in the treatment of tumor.
Therefore, our team and Hokai company (Zhuhai Hokai Biomedical Electronics Co., Ltd., Zhuhai, China) have developed a 3D visualized seed planning and navigation system. This 3D system with rapid image processing and image fusion technologies is able to visualize the 3D space relationship between tumors and surrounding structures, guide the path and position of needle insertion, and display 3D distribution of the radioactive dose. This study aimed to evaluate the effectiveness, safety, and patient survival of ultrasound-guided 125I seed implantation in the management of PC with or without the assistance of this 3D visualized seed planning and navigation system.
Patients and methods
Patients
From December 2010 to November 2016, 25 patients with unresectable pancreatic cancer who met our inclusion criteria and underwent ultrasound-guided 125I seed implantation in the Department of Interventional Ultrasound, General Hospital of People’s Liberation Army were reviewed. Twelve patients (with 13 lesions) who underwent the procedure received the assistance of the 3D visualized seed planning and navigation system (hereinafter 3D group), and 13 patients (with 14 lesions) did not (hereinafter 2D group). This retrospective study was approved by the Chinese PLA General Hospital Institutional Review Board and all patients provided informed written consent.
Of the 13 patients in 2D group, 23% (3/13) had jaundice, 69% (9/13) suffered from pain, and 38% experienced (5/13) weight loss. Two patients had chemotherapy, one had molecular-targeted therapy, two had high-intensity focused ultrasound (HIFU), one had transcatheter arterial chemoembolization, and one had percutaneous transhepatic cholangial drainage (PTCD) before the procedure. After the procedure, one patient in 2D group received two cycles of concurrent chemotherapy and molecular-targeted therapy, and another patient received immunotherapy two times (dendritic cell- cytokine-induced killers). According to the clinical TNM staging, five patients were diagnosed with stage II disease, six had stage III disease, and two had stage IV disease. Of the 14 lesions in 2D group, eight were located in the head, two in the neck, and four in the body or tail of the pancreas. Of the 12 patients in 3D group, 42% (5/12) had jaundice, 69% (9/12) suffered from pain, 50% experienced (6/12) weight loss, and 25% (3/12) had nausea. One patient had surgery, one had radiotherapy, two had chemotherapy, one had molecular-targeted therapy, one had HIFU, and two had PTCD before the procedure. Five patients were diagnosed with stage II disease, three had stage III disease, and four had stage IV disease. Of the 13 lesions in 3D group, nine were located in the head and two in the body or tail of the pancreas, and two metastatic lesions were located in the liver. Nine patients of the 2D group and eight patients of the 3D group had pathological diagnosis and four patients of each group were clinically diagnosed with pancreatic cancer based on clinical characteristics and imaging features. Nine patients of the 2D group and eight patients of the 3D group accepted 125I seed implantation procedure once, while four patients of each group received additional procedures for one or several times because of the progression of the lesions after at least 1-month of follow-up. One patient in 2D group and one patient in 3D group failed to follow-up 6 months post-treatment. Summaries of the patients’ characteristics and tumor characteristics of both groups are presented in Table 1.
Inclusion and exclusion criteria
Inclusion criteria for the patients of 3D or 2D group in our study were as follows: 1) lesion diameter ≤7 cm; 2) patients with unresectable lesions or cannot tolerate surgery; 3) local anesthesia can be tolerated; and 4) platelet count >40×109/L, prothrombin activity >40%, prothrombin time <25 s.
Exclusion criteria were as follows: 1) evidence of widespread tumor metastasis; 2) no safe needle insertion path under ultrasound guidance; 3) lesion diameter >7 cm; 4) patients with cachexia or refractory ascites; 5) severe hemorrhagic tendency not improved after blood transfusion or use of hemostatics; 6) patients with acute pancreatitis; and 7) patients with severe cardiovascular disease or mental illness.
Treatment planning protocol
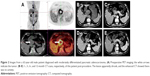
For the 3D group, Digital Imaging and Communications in Medicine data obtained from CT scans of the PC patients were used to do the preoperative treatment planning for 125I seed implantation procedure with assistance of the developed 3D visualized seed planning and navigation system. The system assisted the doctors in 3D reconstruction of the tumor, surrounding tissues, and vessels; calculation of the required number of 125I seeds; and planning of the needle insertion path. 125I radioactive seed (29.6 MBq, 0.8 mCi) (Beijing Atom and HighTechnique Industries Inc, Beijing, China) has a half-life of 59.4 days with a low energy level of 27.4 KeV and the seed distance of 1 cm.16 The matched peripheral dose (MPD) was 120 Gy and D90> MPD; D90 is defined as the dose such that at least 90% of the tumor volume received the reference dose. The preoperative treatment planning for seed implantation is shown in Figure 1.
For the 2D group, CT scans and ultrasound were done before the procedure to evaluate the detailed tumor location and safe path for needle insertion. The implanted seed number was calculated by the formula: seed number = (length + width + height) ÷ 3×5.
Ultrasound-guided 125I seed implantation for pancreatic cancer
Before the procedure, a detailed understanding of the treatment history was required. For patients with obstructive jaundice, PTCD was necessary. Anticoagulant or antiplatelet drugs should be discontinued for 5–7 days. Preoperative examination of blood routine, liver and kidney function, blood coagulation, pancreatin, and CA19-9 level should be done. One day before the procedure, gastrointestinal preparation and intravenous infusion of somatostatin (0.25 mg/h) were performed.
The seed implantation procedure was performed in our department, and local anesthesia was used. Patient’s position was dependent on the location of the tumors. Electrocardiography monitoring was used when necessary during the procedure. Conventional gray scale ultrasound or contrast-enhanced ultrasound (CEUS) was used to evaluate the lesion size, find a safe puncture path, and guide the procedure. An 18-gauge needle was placed into the tumor lesions and the radioactive seeds were implanted using a Mick applicator and spaced at intervals of 1.0 cm using the same needle.17 After the seed implantation, the needle was withdrawn and then the puncture point was sterilized with alcohol. The presence of abnormal effusion or hematoma around the treated lesion was checked with ultrasound.
Patient follow-up
Postoperative evaluation was performed. According to the preoperative and postoperative CT scans (at 1, 2, and 6 months post-treatment), whether there was a “cold zone” of the radioactive dose, whether the distribution of seeds was as planned, or whether there was a shift of seeds was assessed. If the radioactive dose coverage of the tumor was incomplete, the complementary 125I seed implantation was done within 5 days.
The follow-up included laboratory tests (blood count, pancreatin, and CA19-9 level), routine physical examination, CEUS, or abdominal CT imaging at 1, 2, 3, and 6 months post-treatment and then at 6-month intervals for both groups. Follow-up was closed at the time of death or the last follow-up date which was March 1, 2017. Survival was calculated from the date of treatment to the date of death or last follow-up.
Definition of tumor response
Tumor response was assessed according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) guideline.32 Briefly, there are four levels of the response: 1) complete remission (CR) was defined as disappearance of all target lesions and any pathological lymph node (whether target or non-target) must show a reduction in short axis to <10 mm; 2) partial response (PR) was defined as at least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum of diameters, time ≥4 weeks; 3) stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR or sufficient increase to qualify for PD, taking as reference the smallest sum of diameters while on study; 4) progressive disease (PD) was defined as at least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum of diameters while on study, or the occurrence of one or more new lesions. In addition to the relative 20% increase, the sum must also demonstrate an absolute increase of at least 5 mm. The local control rate was defined as the proportion of patients with a CR or PR.
Statistics analysis
SPSS 20.0 (SPSS Inc, Chicago, IL, USA) was used to perform the statistical analysis. Comparison between 2D and 3D groups was conducted by using Pearson’s χ2 test or Fisher’s exact test. Overall survival rate and the univariate analysis to identify predictors of survival were calculated by using the Kaplan–Meier method and compared by the log-rank test. Data were presented by average ± standard deviation. A P-value <0.05 was considered to be statistically significant.
Results
Patient characteristics
As shown in Table 1, there were no significant differences between 2D group and 3D group with regard to sex, age, lesion diameter, primary tumor location, treatment times (number of seed implantations), clinical staging, and pathology of the tumor.
Treatment parameter and local control
The seed implantation procedures proceeded well in both groups, and no major complications occurred during the procedure. The number of implanted 125I seeds in 2D group ranged from 3 to 57, and the average number was 18.59±2.167. The average number of needle insertions was 5.059±0.4794 (ranged from 2 to 12 times). The number of implanted seeds in 3D group ranged from 5 to 42, and the average number was 15.50±1.653. The average number of needle insertions was 4.423±0.4936 (ranged from 2 to 11 times). In 2D and 3D groups, the implantation procedure was performed successfully in one session in 45.5% of patients (10/22) and 80.0% of patients (16/20), respectively. The rest of the patients in the two groups had to accept additional 125I seed implantation within 5 days after the first implantation, owing to the unclear images of ultrasound caused by too much gas coming into the lesions while inserting the needle or incomplete dose coverage of lesions by 125I seeds showed by postoperative CT scans. Therefore, the one-time treatment success rate of 2D and 3D groups was 45.5% and 80.0%, respectively. As shown in Table 2, there was no significant difference in the number of implanted seeds and needle insertions between 2D and 3D groups (P>0.05). However, one-time treatment success rate of the 3D group was significantly higher than the 2D group (P<0.05), and the proportion of added seeds in the second session within 5 days in 3D group was significantly lower than that in 2D group (P=0.034).
Patients of both groups were followed up for 1 month after the procedure to evaluate the tumor response. According to RECIST criteria, among the 14 lesions in 2D group, the number of CR, PR, SD, and PD cases were 1, 4, 8, and 1, respectively. Among the 13 lesions in 3D group, CR, PR, SD, and PD cases were 1, 9, 3, and 0, respectively. Therefore, the short-term local control rate in the 3D group was significantly higher than in the 2D group (P<0.05) (Table 2). The follow-up and tumor responses of a patient in 3D group after the 125I implantation procedure are shown in Figure 2.
Survival
The Kaplan–Meier survival curve shows that the survival rate of 3D group was significantly higher than 2D group (P=0.026) (Figure 3). The median survival time of 2D and 3D groups was 5.00 months (95% CI: 0.76–9.24) and 10.8 months (95% CI: 0.00–28.40), respectively. The median survival of all the 25 patients was 7.10 months (95% CI: 4.43–9.77).
Univariate analysis
The influence of patient and tumor-related factors on survival is shown in Table 3. The results of univariate analysis revealed that the survival of PC patients was significantly correlated with treatment times (P=0.043). Therefore, those PC patients whose tumors do not grow fast and have the chance to receive more 125I implantation procedures might survive longer.
  | Table 3 Single factor analysis of prognostic factors for overall survival |
Pain relief
Pain is one of the most common symptoms of PC.17 Among the 13 patients in 2D group, nine patients suffered from pain before 125I seed implantation. Seven patients (7/9, 77.8%) achieved good to medium pain relief post-treatment and three patients achieved complete pain relief, and it occurred on days 2, 4, and 5 after the procedure, respectively. Among the 12 patients in 3D group, nine of them suffered from pain. Eight patients (8/9, 88.9%) achieved pain relief and five achieved complete pain relief, and it occurred on days 1, 3, 4, 5, and 7, respectively. There was no significant difference between the two groups in pain relief rate.
Complications
No vital structures around the lesions were injured during the procedure and no severe intraoperative or postoperative complications occurred in all patients. During the procedure, five patients (5/13, 38.5%) had mild pain and a small amount of hemorrhage was observed in one patient (1/13, 7.7%) in 2D group. The hemorrhage stopped after the intravenous infusion of thrombin. In 3D group, two patients (2/12, 16.7%) experienced mild pain and no one had hemorrhage.
After the procedure, three patients (3/13, 23.1%) in 2D group and two patients (2/12, 16.7%) in 3D group had postoperative fever. The body temperature decreased to normal by using oral antipyretics. One patient (1/12, 8.3%) in 3D group suffered from postoperative nausea and turned to be better after treatment. No seed displacement and obvious leukocyte decreasing were observed. There was no significant difference between intraoperative (P=0.202) and postoperative complications (P=1.000) in the two groups.
Discussion
PC is one of the five most lethal malignancies in the world, and the overall survival has not been significantly improved in the past 30 years.1,18 Although there are a lot of advances in radiotherapy, chemotherapy, combined therapy, and targeted therapy, the therapeutic effects of these nonsurgical therapies are not satisfactory. Conventional radiotherapy for locally advanced PC may prolong survival, and the 1- and 2-year survival rates are 30% and 10%, respectively.19,20 The therapeutic effects of new options in radiotherapy such as stereotactic body radiation therapy (SBRT), 3D conformal radiotherapy, intensity-modulated radiotherapy, intraoperative radiation therapy, high-dose rate radiation, or low-dose rate have all been explored in treating unresectable PC. Some reports indicated that SBRT combined with chemotherapy might be useful in treating PC patients, achieving a median survival time of 10.6–14.3 months with acceptable complications;21–23 however, some researchers still believe the benefits of SBRT are questionable. Nagai et al reviewed 198 patients with unresectable PC and concluded that intraoperative radiotherapy followed by gemcitabine (GEM)-based chemotherapy was the recommended treatment strategy for unresectable PC.24 Ogawa et al25 reported that the median survival time for 144 patients treated with intraoperative radiotherapy with or without external beam radiotherapy, was 10.5 months. Zhang et al26 performed a meta-analysis comparing the therapeutic effects of GEM alone and radiotherapy combined with GEM for PC patients and found out that the clinical efficacy of radiotherapy combined with GEM was no better than GEM alone. But Chen et al27 reported that the 6-, 12-, and 18-month survival rate of combined radiochemotherapy was higher than radiotherapy or chemotherapy alone, despite the higher possibility of treatment-related toxicity. Molecular-targeted therapies have shown promising effects for killing PC cells in vitro and in animal experiments, but the results of several clinical trials of targeted therapies have not shown any clinical benefit.28–31
Recently, a new treatment modality, 125I seed implantation, has been accepted as an effective, minimally invasive, local-regional, and interventional therapy for locally advanced PC. Treatment Planning System (TPS) is a software system that can assist the operators to do the preoperative planning of 125I seed implantation. However, there are some disadvantages of the existing TPS systems. Most of the domestic TPS systems are just a set of computer software, which can only be used to do the preoperative treatment planning while ignoring the connection and coordination between the intraoperative implantation and preoperative treatment plan, and perform no function in positioning and orientation. TPS developed abroad have considered the above-mentioned factors, but the positioning and orientation hardware has been designed based on the treatment site, ie, prostatic cancer; therefore, it is difficult to be expanded to treat tumors in other body parts, such as liver or pancreas. Although some commercial navigation systems have emerged recently, none of them has reached a wide clinical application, especially in the area of interstitial brachytherapy.
In this study, we use our self-developed 3D visualized seed planning and navigation system to assist the operators to perform the 125I seed implantation procedure for PC patients. This system can acquire a 3D visualization of the patient’s anatomy by reconstructing the images acquired from magnetic resonance or CT scans, identify safe and operable needle trajectories, and calculate the number of seeds needed so as to achieve the calculated dosimetric goals. The results of this study comparing the outcomes between the 3D and 2D group indicate the potential value of this 3D system in clinical application.
However, since the patient number in this preliminary study is quite small, larger patient cohort and randomized control trial are still needed to validate the results of the study and the clinical value of this 3D system. In addition, the 3D system remains to be optimized in many aspects. In the near future, we shall focus more on the improvement of the real-time navigation guidance accuracy and the smooth connection among preoperative treatment planning, intraoperative navigation, and postoperative evaluation for seed implantation.
Acknowledgments
We would like to thank Professor Zhigang Cheng and Professor Jie Yu for their kind assistance in the acquisition of original data. This work was funded by the National Natural Science Foundation of China (numbers 81471683 and 81671710).
Disclosure
All of the authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19(1):169–175. | ||
Spadi R, Brusa F, Ponzetti A, et al. Current therapeutic strategies for advanced pancreatic cancer: a review for clinicians. World J Clin Oncol. 2016;7(1):27–43. | ||
Puleo F, Marechal R, Demetter P, et al. New challenges in perioperative management of pancreatic cancer. World J Gastroenterol. 2015;21(8):2281–2293. | ||
Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. | ||
Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10–15. | ||
Long J, Zhang Y, Yu X, et al. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets. 2011;15(7):817–828. | ||
Zhongmin W, Yu L, Fenju L, et al. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol. 2010;20(7):1786–1791. | ||
Xiang Z, Li G, Liu Z, et al. 125I brachytherapy in locally advanced nonsmall cell lung cancer after progression of concurrent radiochemotherapy. Medicine. 2015;94(49):e2249. | ||
Qin QH, Huang BS, Tan QX, et al. Radiobiological effect induced by different activities of (125)I seed brachytherapy in a hepatocellular carcinoma model. Int J Clin Exp Med. 2014;7(12):5260–5267. | ||
Badakhshi H, Graf R, Budach V, et al. Permanent interstitial low-dose-rate brachytherapy for patients with low risk prostate cancer: an interim analysis of 312 cases. Strahlenther Onkol. 2015;191(4):303–309. | ||
Wang G, Zhang F, Yang B, et al. Feasibility and clinical value of CT-guided (125)I brachytherapy for bilateral lung recurrences from colorectal carcinoma. Radiology. 2016;278(3):897–905. | ||
Jin Z, Du Y, Li Z, et al. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40(4):314–320. | ||
Grimm PD, Blasko JC, Sylvester JE, et al. 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int J Radiat Oncol Biol Phys. 2001;51(1):31–40. | ||
Wang H, Wang J, Jiang Y, et al. The investigation of 125I seed implantation as a salvage modality for unresectable pancreatic carcinoma. J Exp Clin Cancer Res. 2013;32:106. | ||
Shipley WU, Nardi GL, Cohen AM, et al. Iodine-125 implant and external beam irradiation in patients with localized pancreatic carcinoma: a comparative study to surgical resection. Cancer. 1980;45(4):709–714. | ||
Wang J, Jiang Y, Li J, et al. Intraoperative ultrasound-guided iodine-125 seed implantation for unresectable pancreatic carcinoma. J Exp Clin Cancer Res. 2009;28:88. | ||
Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6(12):699–708. | ||
Gutt R, Liauw SL, Weichselbaum RR. The role of radiotherapy in locally advanced pancreatic carcinoma. Nat Rev Gastroenterol Hepatol. 2010;7(8):437–447. | ||
Andre T, Balosso J, Louvet C, et al. Combined radiotherapy and chemotherapy (cisplatin and 5-fluorouracil) as palliative treatment for localized unresectable or adjuvant treatment for resected pancreatic adenocarcinoma: results of a feasibility study. Int J Radiat Oncol Biol Phys. 2000;46(4):903–911. | ||
Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78(3):735–742. | ||
Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):181–188. | ||
Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010;17(8):2092–2101. | ||
Nagai S, Fujii T, Kodera Y, et al. Prognostic implications of intraoperative radiotherapy for unresectable pancreatic cancer. Pancreatology. 2011;11(1):68–75. | ||
Ogawa K, Karasawa K, Ito Y, et al. Intraoperative radiotherapy for unresectable pancreatic cancer: a multi-institutional retrospective analysis of 144 patients. Int J Radiat Oncol Biol Phys. 2011;80(1):111–118. | ||
Zhang X, Huang HJ, Feng D, et al. Is concomitant radiotherapy necessary with gemcitabine-based chemotherapy in pancreatic cancer? World J Gastroenterol. 2014;20(46):17648–17655. | ||
Chen Y, Sun XJ, Jiang TH, et al. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: a meta-analysis. World J Gastroenterol. 2013;19(42):7461–7471. | ||
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. | ||
Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–3622. | ||
Overall CM, Kleifeld O. Tumour microenvironment – opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6(3):227–239. | ||
Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.