Back to Journals » Open Access Journal of Contraception » Volume 5
The anchor of the frameless intrauterine device does not migrate over time: an analysis in over 300 women
Authors Wildemeersch D, Pett A, Jandi S, Nolte K, Albrecht W
Received 14 August 2014
Accepted for publication 3 November 2014
Published 12 December 2014 Volume 2014:5 Pages 91—96
DOI https://doi.org/10.2147/OAJC.S72664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Igal Wolman
Dirk Wildemeersch,1 Ansgar Pett,2 Sohela Jandi,2 Kilian Nolte,3 Wolfgang Albrecht4
1Gynecological Outpatient Clinic and IUD Training Centre, Ghent, Belgium; 2Gynecological Outpatient Clinic, Berlin, Germany; 3Gynecological Outpatient Clinic, Uetze, Germany; 4Gynecological Outpatient Clinic, Feldkirchen, Austria
Objective: To evaluate the correct position of the anchor at insertion and follow-up and assess if migration of the anchor occurs over time.
Materials and methods: This was an insertion-related, prospective, postmarketing study in 309 women. Following insertion, women were followed up at 4–6 weeks, 6 months, and yearly thereafter. The position of the visualized anchor in the fundus of the uterus was evaluated using ultrasound by measuring its distance from the serosal surface of the uterus (SA-distance).
Results: A total of 309 parous (n=115) and nulliparous (n=194) women were fitted with the frameless GyneFix 200 or the GyneFix 330 intrauterine device for contraception. The mean SA-distance in 306 parous and nulliparous women was 6.0 mm (range 2.0–24.0 mm) at insertion in the parous group and 5.4 mm (range 1.3–11.0 mm) in the nulliparous group. At the first follow-up in 281 women, the SA-distance was 6.0 mm (range 2.0–12.0 mm) in the parous group and 5.5 mm (range 1.1–11.0 mm) in the nulliparous group. The SA-distance was not significantly different. One patient had an exceptionally large SA-distance of 24 mm, probably due to insertion in the posterior wall. No follow-up could be done in this patient. In 77 women, the SA-distance was measured up to 42 months. The mean SA-distance at insertion in the parous group was 5.2 mm (range 3.0–8.5 mm) and 4.8 mm (range 1.3–7.0 mm) in the nulliparous group. At the last follow-up up to 36 months or longer, the SA-distance was 5.1 mm (range 3.0–8.5 mm) in the parous group and 4.9 mm (range 1.3–7.0 mm) in the nulliparous group. The SA-distance was not significantly different. The visualized anchor was highly visible on ultrasound in all cases.
Conclusion: The visualized anchor is a key element in the optimization of frameless technology, with the aim of allowing the provider to check its placement at insertion and at follow-up, which enhances provider confidence and assurance. The authors recommend measuring the thickness of the fundus prior to inserting the frameless intrauterine device and to measure the distance between the serosa and the visualized anchor following insertion and at the first follow-up examination.
Keywords: frameless IUD, anchoring technology, visualization
Introduction
The frameless, anchored, copper-releasing intrauterine device (IUD) has been the subject of considerable technical and clinical research over the past 25 years. Different sizes have been tested1,2 and histological3 and removal-force studies conducted4–6 to evaluate the anchor site and the force, as well as discomfort, to insert and remove the anchor from its position in the fundal wall of the uterus. Also, a variety of insertion instruments have been tested.7,8 The new anchoring technique was considered a valid concept to suspend active pharmaceutical agents in the interval, postabortion, and postpartum uterus, resulting in improved IUD retention or almost complete absence of expulsion if the procedure was correctly performed.9–12 Due to its one-dimensional design, the frameless IUD fits in small uterine cavities (Figure 1). The optimal IUD–uterine cavity relationship results in a high continuation rate, which contrasts with conventional IUDs, in which the transverse arm is often too wide. Standard T-shaped IUDs with a 32 mm-long transverse arm are often too long for many cavities, in which the transverse diameter is sometimes much less than the span of the IUD (Figure 2). As the frameless IUD is devoid of a plastic frame, anchoring is required. The fixation is accomplished by inserting the anchoring knot in the fundal wall with a specially designed inserter. As this technique is new for many providers, proper insertion will require some experience and skill. During the learning period, or where the provider is uncertain about the placement, it is recommended that the proper placement of the IUD in the uterine cavity be checked. Verifying the position of the IUD following insertion in the uterus can easily be performed using ultrasound by measuring the distance between the upper copper tube and the serosa of the uterus (SS-distance). However, the “anchor” itself is often difficult to visualize in many cases. The visualized anchor, as described in this paper, is developed to help the provider insert the anchor correctly and to check its correct position (serosa-anchor [SA]-distance).
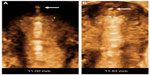  | Figure 1 The fundal transverse diameter of uterine cavities is often very narrow, even less than 20 mm (A and B). |
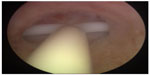  | Figure 2 A case of severe incompatibility between the intrauterine device and the narrow uterine cavity. |
Objectives
This multicenter study reports on the results of the position of the visualized anchor in relation to the serosa of the uterus at insertion and follow-up, assessed by 2-D ultrasound examination. The study also evaluated the mean penetration depth and range of the anchor to add supplementary data related to the safety of the anchoring technique. After insertion, women were followed up at 4–6 weeks, 6 months, and yearly thereafter. Patients were informed about the nature of the study and the importance of follow-up to detect changes in the position of the anchor. The use of the device was approved by the ethics committee of Ghent University, but no formal approval was requested for adding the SA-distance measurement to that of the SS-distance for evaluation of the device location in the uterus.
Materials and methods
The frameless GyneFix® IUD with visualized anchor is similar to the original GyneFix. However, a tiny stainless steel element, 2 mm long and 0.5 mm wide, is added on the anchoring thread immediately below the anchoring knot (Figure 3). The medical grade stainless steel element (AISI 316L/1.4404) is biocompatible and used in diverse medical implants. The GyneFix IUD with visualized anchor is CE-marked, and is available in the European Union.
Patients
A total of 309 women requesting the frameless copper-releasing IUD for contraception participated in this evaluation in five centers in Europe. All participants in the study were screened as to their clinical suitability for IUD insertion and compliance with World Health Organization eligibility criteria.13 All insertions were done in consecutive patients and followed up by the individual investigators. After insertion, the stainless steel element was identified on ultrasound and its SA-distance measured in 306 women. A total of 281 of the 309 women were followed up after 4–6 weeks after insertion and the SA-distance measurement was repeated (short follow-up group). Twenty-eight women were not followed up at the study centers, as they came from abroad for insertion only. They were included in the study to add to the data, although no follow-up examination could be performed. The SA-distance was again measured in 77 women 1 year or longer after insertion (long follow-up group). Women were told to return to the clinic at any time if they experienced any problems with the device, and were free to return to the clinic at any time and request removal of the device.
Data collection and analysis
All pertinent data at insertion and follow-up were recorded and included in an Excel (Microsoft, Redmond, WA, USA) file, which was sent to the data-coordinating center at the Department of Medical Informatics, University of Ghent, where they were managed according to standard procedures. Data were presented using descriptive statistics in the form of frequencies and percentages for qualitative variables, and means and standard deviations, medians and quartiles, and ranges for quantitative variables. Pair-wise comparison between insertion and follow-up was analyzed using Wilcoxon’s matched-pair signed-rank test. The comparison between nulliparous and parous women was analyzed by the Mann–Whitney U test.
Results
Table 1 (short follow-up group) shows the age and parity distribution of the 309 women who participated in this study. Participants had a mean age of 29 years (range 13–52 years); 115 of them were parous and 194 nulliparous. The SA-distances from the serosa of the uterus to the metal element at insertion and at follow-up are presented in Table 2. The mean SA-distance in the 306 of the 309 parous and nulliparous women who could be evaluated was 6.0 mm (range 2.0–24.0 mm) at insertion in the parous group and 5.4 mm (range 1.3–11.0 mm) in the nulliparous group. At the first follow-up in 281 women, the SA-distance was 6.0 mm (range 2.0–12.0 mm) in the parous group and 5.5 mm (range 1.1–11.0 mm) in the nulliparous group. The SA-distance was not significantly different. One patient had an exceptionally large SA-distance of 24 mm, probably due to insertion in the posterior wall. No follow-up could be done in this patient.
  | Table 1 Characteristics of the 309 participants (age and parity distribution, total group) |
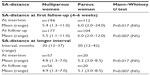  | Table 2 SA-distance at insertion and first follow-up (4–6 weeks) and SA-distance at longer interval |
Table 3 shows the age and parity distribution, as well as the duration of use in the 77 women who were followed up after 1 year up to 42 months. The mean SA-distance at insertion in the parous group was 5.2 mm (range 3.0–8.5 mm) and 4.8 mm (range 1.3–7.0 mm) in the nulliparous group. At the last follow-up, the SA-distance was 5.1 mm (range 3.0–8.5 mm) in the parous group and 4.9 mm (range 1.3–7.0 mm) in the nulliparous group. The SA-distance was not significantly different. The visualized anchor was highly visible on ultrasound in all cases, as illustrated in Figure 3.
Discussion
Intrauterine contraception has become a very important long-acting reversible contraceptive method, as an epidemiological study showed that short-acting methods, such as the pill, the dermal patch, and the vaginal ring, requiring daily attention, had a high failure rate due to incorrect and inconsistent use.14 A major advantage of long-acting hormonal methods is that they do not need specific action at the time of coitus. IUDs and intrauterine systems are particularly attractive, as they act locally, avoiding systemic effects. They also have the highest continuation of use of all contraceptives, and thus protect up to 20 times better than pills, patches, and rings to prevent pregnancy, according to Winner et al.15
Frameless IUDs have several potential advantages when compared to conventional framed IUDs. These are derived from their much-reduced space-occupying and flexible features. An IUD that fits well is likely to result in increased tolerance and higher continuation rates, and consequently less unintended pregnancies and induced abortions if use of the method is sufficiently widespread by media coverage and dedicated marketing efforts.
Significant changes have been made over the years to both the frameless IUD and the insertion instrument since its inception. Therefore, the performance of the current, frameless IUD cannot be compared with studies using prototype versions conducted during the early development phase. The evaluation of earlier studies should therefore be put in a correct context to avoid confusion and misinterpretation.7,8,16
The present study with the new visualized anchor suggests that the placement of the anchor in the uterine fundus can be checked with precision. The anchor is correctly placed in the fundus if it is inserted in its muscular wall. It is known from removal-force studies that the force required to remove the IUD from its attachment in the uterine fundus is approximately five to ten times greater than the force to remove a T-shaped IUD. Therefore, spontaneous expulsion and dislocation of the IUD is unlikely. Expulsion rates <1% are usual.17 Low rates of expulsion could help reduce unintended pregnancies, as expulsion of conventional IUDs are in the order of 5%–10% during the 1st year, with even higher expulsion rates seen in nulliparous and adolescent women.18 Dislocated IUDs also have a higher failure rate.19
The anchoring to the fundus eliminates the need for a plastic frame. This is beneficial, as the side effects (eg, bleeding, pain) caused by the frame are also eliminated. Bleeding and pain are the most common reasons for the removal of an IUD. Embedment and perforation are late consequences of disharmony between the too-big IUD and the uterine cavity.17 Many young women remember their mothers telling them about an embedded IUD that needed removal under general anesthesia, and are concerned that the same may happen to them. Embedment is unlikely with the frameless IUD, and has not yet been reported in clinical studies.
When accidental partial perforation of the anchor has occurred at insertion, attachment of the knot to the bowel, omentum, or mesentery may cause traction on the IUD due to bowel movement, resulting in progressive penetration of the IUD in the abdominal cavity. Perforation of the anchor followed by intra-abdominal displacement of the IUD occurs in approximately one in 1,000 insertions. To minimize this risk, we recommend measurement of the thickness of the fundus prior to insertion. After insertion, measuring the SA-distance will confirm if the anchor is correctly placed. If the anchor is not correctly placed, the IUD should be removed. Reinsertion can be done immediately or at a later date.
In a large multicenter clinical trial conducted by the World Health Organization with an earlier (prototype) version of the frameless copper-releasing IUD, there were no perforations.7 In two large postmarketing trials conducted in Belgium and Spain in over 15,000 women, the rate was 1.2–2.0/1,000 insertions, which is similar to the quoted perforation rate occurring with conventional IUDs.20 This rate could be further reduced with the visualized anchor.
As the frameless technology is new for many providers, becoming familiar with the insertion procedure may be acquired after only a number of insertions have been performed, depending on the skill of the provider. Experience has shown that insertion failures and expulsions in parous as well as nulliparous women, as in the present study, can be minimized to very low rates if the provider attends a training course organized by the manufacturer.10 Failed insertion means that the provider did not position the anchoring knot in the muscular wall of the uterine fundus. As a consequence, the IUD will be expelled within weeks after the attempted insertion. After training, providers can acquire proficiency by conducting training by themselves in an appropriate “home” uterine model before they start insertions in their patients. The failed-insertion rate should be minimal if the anchor is visualized after insertion.
One limitation of the present study is the relatively small number of women followed up for extended periods of time. However, experience has shown that when the frameless IUD has been properly anchored, it will remain anchored for many years, as late expulsions are rare. This study also indicates the safety of the anchoring concept.17
Conclusion
Frameless IUDs have significant potential advantages over framed IUDs, as they fit in cavities of every size and shape (“one size fits all”). The smallest frameless copper IUD version with effective copper surface area of 200 mm2 is three times smaller than T-shaped IUDs; it is therefore particularly suitable for nulliparous and adolescent women. It is expected that the possibility of visualizing the position of the anchor at insertion and follow-up will remove provider’s uneasiness to use this new-generation IUD.
Acknowledgment
The authors are grateful to Professor Dr G Van Maele, Department of Medical Informatics and Statistics, University Hospital Ghent, Belgium, for statistical analysis of the data.
Disclosure
DW is the developer of the frameless GyneFix IUD. He has also been involved in the development and optimization of new, innovative, drug-delivery systems for use in the uterus. Currently, he acts as a trainer in the insertion procedure of GyneFix during training sessions organized by the commercial companies distributing the product. He is also an advisor in devising new concepts in controlled release for contraception and gynecological treatment, and receives financial compensation for these activities. The other authors report no conflicts of interest in this work.
References
Wildemeersch D, Van der Pas H, Thiery M, Van Kets H, Parewijck W, Delbarge W. The Copper-Fix (Cu-Fix): a new concept in IUD technology. Adv Contracept. 1988;4:197–205. | |
Van Kets H, Vrijens M, Van Trappen Y, et al. The frameless GyneFix intrauterine implant: a major improvement in efficacy, expulsion and tolerance. Adv Contracept. 1995;11:131–142. | |
Coppens M, Thiery M, Delbarge W, Parewijck W, Van Der Pas H, Van Kets H. The copper-fix IUD: assessment of tissue reaction at the anchor site. Med Sci Res. 1989;17:719. | |
Batár I, Wildemeersch D. The reliability of the anchoring concept for suspension of bioactive substances in the human uterus evaluated by measuring the removal force: results after long-term use. Contraception. 2004;69:501–503. | |
Wildemeersch D. The force required to remove the frameless 0-suture anchoring system: comparison between pre- and postmenopausal women. Contraception. 2004;69:513–515. | |
Wildemeersch D. GyneFix®: study of pain caused by the removal of the GyneFix® intrauterine contraceptive device using verbal/numerical scale. Report study GF15. Ghent, Belgium: Contrel Europe NV; 2001. | |
Meirik O, Rowe PJ, Peregoudov A, Piaggio G, Petzold M. The frameless copper IUD (GyneFix) and the TCu380A IUD: results of an 8-year multicenter randomized comparative trial. Contraception. 2009;80: 133–141. | |
Wildemeersch D. Commentary – A historical note on the development of the frameless IUD. Contraception. 2010;81:172–173. | |
Wu S, Hu J, Wildemeersch D. Performance of the frameless GyneFix and the TCu380A IUDs in a 3-year multicenter randomized comparative trial in parous women. Contraception. 2003;61:91–98. | |
Cao X, Zhang W, Zhao X, et al. Three-year efficacy and acceptability of the GyneFix 200 intrauterine system (IUS). Contraception. 2004;69: 207–211. | |
Wildemeersch D, Batár I, Affandi B, et al. The ‘frameless’ intrauterine system for long-term, reversible contraception: a review of 15 years of clinical experience. J Obstet Gynaecol Res. 2003;29:164–173. | |
Batár I, Wildemeersch D, Vrijens M, Delbarge W, Temmerman M, Gbolade BA. Preventing abortion and repeat abortion with the GyneFix intrauterine implant system – preliminary results. Adv Contracept. 1989;14:91–96. | |
World Health Organization. Medical Eligibility Criteria Wheel for Contraceptive Use. 3rd ed. Geneva: WHO; 2004. Available from: http://www.who.int/reproductivehealth/publications/family_planning/9789241547710/en. Accessed May 29, 2014. | |
Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397–404. | |
Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. | |
O’Brien PA, Marfleet C. Frameless versus classical intrauterine device for contraception. Cochrane Database Syst Rev. 2001:CD003282. | |
Wildemeersch D, Pett A, Jandi S, Hasskamp T, Rowe P, Vrijens M. Precision intrauterine contraception may significantly increase continuation of use: a review of long-term clinical experience with frameless copper-releasing intrauterine contraception devices. Int J Women’s Health. 2013;5:215–225. | |
Hubacher D. Copper intrauterine device used by nulliparous women: review of side effects. Contraception. 2007;75:S8–S11. | |
Anteby E, Revel A, Ben-Chetrit A, Rosen B, Tadmor O, Yagel S. Intrauterine device failure: relation to its location within the uterine cavity. Obstet Gynecol. 1993;81:112–114. | |
Martinez F, Gimenez E, Hernández G, et al. Experience with GyneFix insertions in Spain: favorable acceptance of the intrauterine contraceptive implant with some limitations. Contraception. 2002;66:315–320. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.