Back to Journals » Infection and Drug Resistance » Volume 15
The Analysis of Drug-Resistant Bacteria from Different Regions of Anhui in 2021
Authors Liu Y , Wang W, Guo M, Xu Z, Yang Y, Yu L, Li Y, Hu L , Ye Y, Li J
Received 17 October 2022
Accepted for publication 14 December 2022
Published 21 December 2022 Volume 2022:15 Pages 7537—7553
DOI https://doi.org/10.2147/IDR.S393760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yanyan Liu,1– 3,* Wei Wang,1,* Mingjuan Guo,4,* Zhicheng Xu,1 Yi Yang,1– 3 Liang Yu,1– 3 Yasheng Li,1– 3 Lifen Hu,1– 3 Ying Ye,1– 3 Jiabin Li1– 4
1Department of Infectious Diseases, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 2Anhui Center for Surveillance of Bacterial Resistance, Health Commission of Anhui Province, Hefei, People’s Republic of China; 3Institute of Bacterial Resistance, Anhui Medical University, Hefei, Anhui, People’s Republic of China; 4Department of Infectious Diseases, The Chaohu Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiabin Li; Ying Ye, Department of Infectious Diseases, The First Affiliated Hospital of Anhui Medical University, Jixi Road no. 218, Hefei, 230022, People’s Republic of China, Tel +86-551-2922713, Fax +86-551-2922281, Email [email protected]; [email protected]
Purpose: To analyze the differences in clinical distribution and antimicrobial resistance of pathogens among northern Anhui, central Anhui, and southern Anhui in 2021, and to provide a basis for the rational use of drugs for clinicians in different regions.
Methods: Nonrepetitive pathogens isolated from clinical samples of inpatients and outpatients from 59 member units with qualified data in 2021 were obtained from the Anhui Province Antimicrobial Resistance Surveillance System, which was divided into northern Anhui, central Anhui, and southern Anhui by region. Identification and antimicrobial susceptibility analyses were carried out using the Vitek 2 Compact and standard disc diffusion method. The results were determined according to the American Clinical Laboratory Standards Institute in 2021 with data analyzed using WHONET 5.6 and SPSS 17.0.
Results: A total of 133,268 pathogenic bacteria were isolated from clinical samples. Staphylococcus aureus (S. aureus) was the most common gram-positive bacterium and Escherichia coli (E. coli) was the most common gram-negative bacterium. Sputum was the main source of clinical specimens. The detection rates of methicillin-resistant S.aureus, methicillin-resistant coagulase-negative Staphylococcus, carbapenem-resistant E. coli, carbapenem-resistant Klebsiella pneumoniae (K. pneumoniae), carbapenem-resistant Acinetobacter baumannii, third-generation cephalosporin-resistant E. coli, and third-generation cephalosporin-resistant K. pneumoniae were higher in northern Anhui than in southern Anhui (P< 0.0001). E. coli, K. pneumoniae, and Pseudomonas aeruginosa were sensitive to amikacin. Strains resistant to vancomycin, linezolid, and teicoplanin were not isolated until 2021.
Conclusion: There were significant differences in bacterial resistance in different regions of Anhui Province. Antibiotic resistance in northern Anhui was the most serious in 2021. Antimicrobial agents must be used according to the resistance of the bacteria in the local region.
Keywords: pathogens, antibacterial agents, drug resistance, different regions
Introduction
Bacterial drug resistance has become a major public health concern worldwide. Many infectious agents have acquired resistance to most, and in some cases, all of these Drugs.1 Under the influence of different time and regional factors, there are obvious differences in the distribution of pathogens and the drug resistance spectrum, especially in the distribution of special drug-resistant bacteria,2 including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant coagulase-negative Staphylococcus (MRCNS), vancomycin-resistant Enterococcus faecalis (VREA), vancomycin-resistant Enterococcus faecium (VREM), third-generation cephalosporin-resistant Escherichia coli (CTX/CRO-R ECO), third-generation cephalosporin-resistant Klebsiella pneumoniae (CTX/CRO-R KPN), carbapenem-resistant Escherichia coli (CR-ECO), carbapenem-resistant Klebsiella pneumoniae (CR-KPN), carbapenem-resistant Pseudomonas aeruginosa (CR-PAE), and carbapenem-resistant Acinetobacter baumannii (CR-ABA). However, there were few reports on distribution of special drug-resistant bacteria in different areas of Anhui Province. The aim of this study was to analyze the data on bacterial drug resistance in northern Anhui, central Anhui, and southern Anhui in 2021, to understand the distribution and drug resistance differences of clinically isolated pathogens and special drug-resistant bacteria in the three areas, to provide an effective reference for administrative departments in different areas, and to make relevant policies and for clinicians to make reasonable diagnoses and treatments.
Materials and Methods
Data Sources
All data were obtained from 59 member units of the Anhui Province Bacterial Resistance Surveillance Network (HuiNet) in 2021. According to the geographical location of the hospitals, they were divided into northern Anhui (Fuyang, Bozhou, Bengbu, Suzhou, Huaibei), central Anhui (Hefei, Chuzhou, Lu’an, Huainan), and southern Anhui (Anqing, Wuhu, Tongling, Huangshan, Chizhou, Xuancheng, Ma ‘anshan). After the review, the number of hospitals in northern Anhui, central Anhui, and southern Anhui were 18, 24, and 17, respectively.
Bacterial Identification and Drug Susceptibility Test
Bacteria were identified using manual or automated testing instruments. The duplicate strains were excluded according to the principle of retaining the first strain of the same bacteria in the same patient. Antimicrobial susceptibility tests were performed using the disc diffusion, automated instrument, and broth dilution methods. Antimicrobial varieties were determined according to the monitoring technology scheme of the China Antimicrobial Resistance Surveillance System (CARSS, http://www.carss.cn/). The susceptibility test results were interpreted according to the American Clinical Laboratory Standards Institute (CLSI) in 2021.3 The detection rates of 10 special drug-resistant bacteria in different areas were analyzed, including MRSA, MRCNS, VREA, VREM, CTX/CRO-R ECO, CTX/CRO-R KPN, CR-ECO, CR-KPN, CR-PAE, and CR-ABA.
Statistical Analysis
Data were analyzed using WHONET 5.6 software and SPSS version 17.0. Qualitative data were described by frequency. Univariate analysis was performed using the chi-square test or Fisher’s exact test when appropriate. P-values were based on two-tailed test results, and P-values <0.05 were considered statistically significant.
Ethics
This study is conducted on already available data from HuiNet. Ethical approval was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Number: Quick-PJ 2022-12-26). The ethics committee approved the waiver of informed consent. The research data accessed were de-identified and anonymously analyzed. The study was conducted according to the Declaration of Helsinki.
Results
Number of Bacteria
The total number of bacteria analyzed in 2021 was 133,268. In total, 39,315 strains were analyzed from northern Anhui, among which the proportion of gram-positive bacteria was 26.2%, and that of gram-negative bacteria was 73.8%. A total of 54,783 strains were analyzed in central Anhui, among which the proportion of gram-positive bacteria was 27.2% and that of gram-negative bacteria was 72.8%. Of the 39,170 strains analyzed from southern Anhui, the proportion of gram-positive bacteria was 24.4% and that of gram-negative bacteria was 75.6%.
Composition Ratio of Strains
Staphylococcus aureus (S. aureus) was the most common gram-positive bacterium in 2021, with 33.2% in northern Anhui, 33.4% in central Anhui, and 32.5% in southern Anhui. There was no significant difference about the detection rate of S. aureus among the three regions (χ2=2.281, P=0.32) (Table 1). Staphylococcus epidermidis (S. epidermidis) was the second most common gram-positive bacterium, with 15.6% in northern Anhui, 12.8% in central Anhui, and 13.4% in southern Anhui. There was a significant difference about the detection rate of S. epidermidis among the three regions (χ2=40.998, P<0.0001) (Table 1). Staphylococcus hominis (S. hominis) was the third most common gram-positive bacterium, with 8.8% in northern Anhui, 7.4% in central Anhui, and 6.3% in southern Anhui. There was a significant difference about the detection rate of S. hominis among the three regions (χ2=45.144, P<0.0001) (Table 1). Enterococcus faecium (E. faecium) was the fourth most common gram-positive bacterium, with 8.8% in northern Anhui, 9.7% in central Anhui, and 10.6% in southern Anhui. There was a significant difference about the detection rate of E. faecium among the three regions (χ2=17.427, P<0.0001) (Table 1). Enterococcus faecalis (E. faecalis) was the fifth most common gram-positive bacterium, with 7.7% in northern Anhui, 11.3% in central Anhui, and 10.9% in southern Anhui, with a significant difference about the detection rate of E. faecalis among the three regions (χ2=93.118, P<0.0001) (Table 1).
 |
Table 1 The Composition of Pathogenic Bacteria in Different Areas of Anhui Province in 2021 |
Escherichia coli (E. coli) was the most common gram-negative bacterium in 2021, with 30.5% in northern Anhui, 30.4% in central Anhui, and 30.7% in southern Anhui. There was no significant difference about the detection rate of E. coli among the three regions (χ2=0.869, P=0.648) (Table 1). Klebsiella pneumoniae (K. pneumoniae) was the second most common gram-negative bacterium, with 21.2% in northern Anhui, 20% in central Anhui, and 22.4% in southern Anhui. There was a significant difference about the detection rate of K. pneumoniae among the three regions (χ2=55.103, P<0.0001) (Table 1). Pseudomonas aeruginosa (P. aeruginosa) was the third most common gram-negative bacterium, with 14.2% in northern Anhui, 14.2% in central Anhui, and 13.5% in southern Anhui. There was a significant difference about the detection rate of P. aeruginosa among the three regions (χ2=9.498, P=0.009) (Table 1). Acinetobacter baumannii (A. baumannii) was the fourth most common gram-negative bacterium, with 8.5% in northern Anhui, 9.6% in central Anhui, and 8.8% in southern Anhui. There was a significant difference about the detection rate of A. baumannii among the three regions (χ2=28.06, P<0.0001) (Table 1). Enterobacter cloacae (E. cloacae) was the fifth most common gram-negative bacterium, with 3.2% in northern Anhui, 4.1% in central Anhui, and 4.4% in southern Anhui. There was a significant difference about the detection rate of E. cloacae among the three regions (χ2=54.607, P<0.0001) (Table 1).
Source of Bacterial Specimens
Sputum was the most common specimen source of bacteria in 2021, with 40.9% in northern Anhui, 37.1% in central Anhui, and 38.1% in southern Anhui. Urine was the second most common specimen source of bacteria, with 15.9% in northern Anhui, 21.5% in central Anhui, and 26.5% in southern Anhui. Blood was the third most common specimen source of bacteria, with 12.5% in northern Anhui, 11.8% in central Anhui, and 9.6% in southern Anhui. Pus was the fourth most common specimen source of bacteria, with 10.6% in northern Anhui, 7.2% in central Anhui, and 6.4% in southern Anhui. Ascites was the fifth most common gram-negative bacterium, with 2% in northern Anhui, 1.5% in central Anhui, and 2.4% in southern Anhui. The proportion of sputum, blood and pus specimen in northern Anhui were higher than those in central and southern Anhui (χ2=142.671, P<0.0001; χ2=181.478, P<0.0001; χ2=534.805, P<0.0001, respectively) (Table 2). The proportion of urine and ascites specimen in southern Anhui were higher than those in northern Anhui and central Anhui (χ2=1300.016, P<0.0001; χ2=98.44, P<0.0001, respectively) (Table 2).
 |
Table 2 Constituent of Major Specimen Sourses of Bacteria in Different Areas of Anhui Province in 2021 |
Drug Susceptibility of Gram-Positive Bacteria
The resistance rates of penicillin (PEN), sulfamethoxazole (SMX), clindamycin (CLI), and erythromycin (ERY) among S. aureus in northern Anhui were 96%, 14.4%, 34.1%, and 64.2%, respectively, which were higher than those in central Anhui and southern Anhui (χ2=43.84, P<0.0001; χ2=162.44, P<0.0001; χ2=166.10, P<0.0001; χ2=235.47, P<0.0001, respectively) (Table 3). The resistance rate of gentamicin (GEN) among S. aureus in southern Anhui was 4.9%, which was lower than that in northern Anhui and central Anhui (χ2=2588.077, P<0.0001) (Table 3). The resistance rates of PEN, SMX, CLI, and ERY among coagulase-negative Staphylococcus in northern Anhui were 92.8%, 45%, 34.6%, and 80.7%, respectively, which were higher than those in central Anhui and southern Anhui (χ2=17.318, P<0.0001; χ2=170.30, P<0.0001; χ2=39.882, P<0.0001; χ2=45.395, P<0.0001, respectively) (Table 3). The resistance rate of GEN among coagulase-negative Staphylococcus in southern Anhui was 18.5%, which was higher than that in northern Anhui and central Anhui (χ2=19.554, P<0.0001) (Table 3).
 |
Table 3 Resistance Rate of Staphylococcus aureus and Coagulase-Negative Staphylococcus in Different Regions of Anhui Province |
The resistance rates of gentamicin-high (GEH) and levofloxacin (LVX) among E. faecalis in northern Anhui were 41.8% and 38.2%, respectively, which were higher than those in central Anhui and southern Anhui (χ2=25.131, P<0.0001; χ2=21.019, P<0.0001, respectively) (Table 4). The resistance rate of streptomycin-high (STH) among E. faecalis in central Anhui was 19.7%, which was lower than that in northern Anhui and southern Anhui (χ2=11.884, P=0.003) (Table 4). The resistance rate of linezolid (LNZ) among E. faecalis in central Anhui was 2.5%, which was higher than that in northern Anhui and southern Anhui (χ2=21.019, P<0.0001) (Table 4). The resistance rates of ampicillin (AMP) and STH among E. faecium in northern Anhui were 90.8% and 32.9%, respectively, which were higher than those in central Anhui and southern Anhui (χ2=11.348, P=0.003; χ2=19.158, P<0.0001, respectively) (Table 4). The resistance rate of rifampicin (RIF) among E. faecium in southern Anhui was 85.4%, which was higher than that in northern Anhui and central Anhui (χ2=72.93, P<0.0001) (Table 4). The resistance rate of E. faecium to LVX in southern Anhui was 80.4%, which was lower than that in northern Anhui and central Anhui (χ2=9.199, P=0.01) (Table 4).
 |
Table 4 Resistance Rate of Enterococcus faecalis and Enterococcus faecium in Different Regions of Anhui Province |
Drug Susceptibility of Gram-Negative Bacteria
The resistance rates of amikacin (AMK), GEN, amoxicillin/clavulanic acid (AMC), ampicillin/sulbactam (SAM), piperacillin/tazobactam (TZP), cefuroxime (CXM), ceftriaxone (CRO), ceftazidime (CAZ), cefepime (FEP), cefoxitin (FOX), aztreonam (ATM), ciprofloxacin (CIP), LVX, and SMX among E. coli in northern Anhui were 5%, 44%, 17.5%, 57.7%, 5.9%, 62.7%, 60.8%, 29.5%, 30%, 14.1%, 43%, 62.3%, 56.6%, and 61.3%, respectively, which were higher than those in central Anhui and southern Anhui (χ2=160.182, P<0.0001; χ2=198.679, P<0.0001; χ2=72.482, P<0.0001; χ2=211.422, P<0.0001; χ2=74.321, P<0.0001; χ2=258.757, P<0.0001; χ2=376.829, P<0.0001; χ2=100.618, P<0.0001; χ2=90.259, P<0.0001; χ2=82.623, P<0.0001; χ2=82.465, P<0.0001; χ2=161.376, P<0.0001; χ2=206.918, P<0.0001; χ2=437.602, P<0.0001, respectively) (Table 5). The resistance rates of cefoperazone/sulbactam (CSL), cefotaxime (CTX), and imipenem (IPM) among E. coli in southern Anhui were 4.4%, 45.6% and 1.2%, respectively, which were lower than those in northern Anhui and central Anhui (χ2=22.198, P<0.0001; χ2=51.674, P<0.0001; χ2=32.652, P<0.0001, respectively) (Table 5).
 |
Table 5 Resistance Rate of Escherichia coli and Klebsiella pneumoniae in Different Regions of Anhui Province |
The resistance rates of AMK, GEN, CRO, FOX, LVX, and SMX among K. pneumoniae in northern Anhui were 13.7%, 29%, 36.9%, 23.2%, 28% and 29.2%, which were higher than those in central Anhui and southern Anhui (χ2=235.193, P<0.0001; χ2=47.919, P<0.0001; χ2=67.987, P<0.0001; χ2=9.338, P=0.009; χ2=164.768, P<0.0001; χ2=36.657, P<0.0001, respectively) (Table 5). The resistance rates of SAM, TZP, CAZ, FEP, ATM, IPM, and ciprofloxacin (CIP) among K. pneumoniae in southern Anhui were 31.6%, 14.2%, 22.1%, 21.3%, 24.7%, 9.1%, and 17.4%, which were lower than those in northern and central Anhui (χ2=85.492, P<0.0001; χ2=126.189, P<0.0001; χ2=103.946, P<0.0001; χ2=70.227, P<0.0001; χ2=74.419, P<0.0001; χ2=197.123, P<0.0001; χ2=192.112, P<0.0001, respectively) (Table 5). The tigecycline (TGC) resistance rate among K. pneumoniae in southern Anhui was 4.4%, which was higher than that in northern Anhui and central Anhui (χ2=56.811, P<0.0001) (Table 5).
The resistance rates to AMK, GEN, tobramycin (TOB), and CIP in P. aeruginosa in northern Anhui were 5.8%, 15.1%, 8.4%, and 17.7%, respectively, which were higher than those in central Anhui and southern Anhui (χ2=28.1, P<0.0001; χ2=45.449, P<0.0001; χ2=23.24, P<0.0001; χ2=29.719, P<0.0001, respectively) (Table 6). The resistance rate of IPM among P. aeruginosa in northern Anhui was 16.6%, which was lower than that in central Anhui and southern Anhui (χ2=31.036, P<0.0001) (Table 6). The resistance rates of TZP, CAZ, and FEP among P. aeruginosa in southern Anhui were 17.6%, 21.4%, and 15.8%, respectively, which were higher than those in northern Anhui and central Anhui (χ2=76.953, P<0.0001; χ2=39.476, P<0.0001; χ2=57.223, P<0.0001, respectively) (Table 6). The resistance rates to cefoperazone/sulbactam (CSL) and meropenem (MEM) among P. aeruginosa in central Anhui were 17.5% and 19.6%, respectively, which were higher than those in northern Anhui and central Anhui (χ2=21.71, P<0.0001; χ2=65.615, P<0.0001, respectively) (Table 6).
 |
Table 6 Resistance Rate of Pseudomonas aeruginosa and Acinetobacter baumannii in Different Regions of Anhui Province |
The resistance rates of AMK, GEN, TOB, TZP, CSL, CAZ, FEP, IPM, MEM, CIP and LVX among A. baumannii in northern Anhui were 46.1%, 69.9%, 67.3%, 73.2%, 56.2%, 70.3%, 63.2%, 69.8%, 73.4%, 76.7%, and 58.8%, respectively, which were higher than those in central Anhui and southern Anhui (χ2=11.442, P=0.003; χ2=86.556, P<0.0001; χ2=217.177, P<0.0001; χ2=104.601, P<0.0001; χ2=56.309, P<0.0001; χ2=181.713, P<0.0001; χ2=122.779, P<0.0001; χ2=263.425, P<0.0001; χ2=31.008, P<0.0001; χ2=207.692, P<0.0001; χ2=106.187, P<0.0001, respectively) (Table 6). The TGC resistance rate among A. baumannii in northern Anhui was 2%, which was higher than that in central Anhui and southern Anhui (χ2=25.558, P<0.0001) (Table 6).
Differential Analysis of Special Drug-Resistant Bacteria
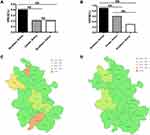
The detection rates of MRSA in northern Anhui, central Anhui, and southern Anhui were 51.4%, 39.2%, and 24.8%, respectively. There was a significant difference in the detection rate of MRSA among the three regions (χ2=460.281, P<0.0001) (Figure 1A and C). The detection rates of MRCNS in northern Anhui, central Anhui, and southern Anhui were 75.8%, 75.8%, and 72.3%, respectively. The detection rate of MRCNS in southern Anhui was lower than that in northern Anhui and central Anhui (χ2=15.206, P<0.0001) (Figure 1B and D). The detection rates of VREA in northern Anhui, central Anhui, and southern Anhui were 0.4%, 0.2%, and 0.2%, respectively. The detection rates of VREM in northern Anhui, central Anhui, and southern Anhui were 0.9%, 0.6%, and 0.3%, respectively. There were no significant differences in the detection rates of VREA and VREM among the three regions (Figure 2).
The detection rates of CTX/CRO-R ECO in northern Anhui, central Anhui, and southern Anhui were 60.5%, 55.0%, and 46.1%, respectively. There was a significant difference in the detection rate of CTX/CRO-R ECO among the three regions (χ2=376.829, P<0.0001) (Figure 3A and C). The detection rates of CTX/CRO-R KPN in northern Anhui, central Anhui, and southern Anhui were 37.0%, 34.2%, and 29.9%, respectively. There was a significant difference in the detection rate of CTX/CRO-R KPN among the three regions (χ2=69.495, P<0.0001) (Figure 3B and D). The detection rates of CR-ECO in northern Anhui, central Anhui, and southern Anhui were 2.5%, 2.4%, and 1.5%, respectively. The detection rate of CR-ECO in southern Anhui was lower than that in northern Anhui and central Anhui (χ2=25.495, P<0.0001) (Figure 4A and C). The detection rates of CR-KPN in northern Anhui, central Anhui, and southern Anhui were 16.5%, 17.4%, and 9.7%, respectively. The detection rate of CR-KPN in southern Anhui was also lower than that in northern Anhui and central Anhui (χ2=197.123, P<0.0001) (Figure 4B and D). The detection rates of CR-PAE in northern Anhui, central Anhui, and southern Anhui were 16.6%, 21.0%, and 20.4%, respectively. The detection rate of CR-PAE in northern Anhui was lower than that in central Anhui and southern Anhui (χ2=31.036, P<0.0001) (Figure 5A and C). The detection rates of CR-ABA in northern Anhui, central Anhui, and southern Anhui were 69.9%, 64.2%, and 60.8%, respectively. The detection rate of CR-ABA in northern Anhui was higher than that in central Anhui and southern Anhui (χ2=263.425, P<0.0001) (Figure 5B and D).
Discussion
Our data showed that 133,268 strains of bacteria were isolated from clinical specimens in 2021. The detection rates of gram-negative bacteria in northern Anhui, central Anhui, and southern Anhui were higher than those of gram-positive bacteria, which is consistent with other reports from China.4 S. aureus was the most common gram-positive bacterium in northern Anhui, central Anhui, and southern Anhui, and E. coli was the most common gram-negative bacterium. There were no significant differences in the detection rates of S. aureus and E. coli among the three regions. The detection rates of S. epidermidis and S. hominis in northern Anhui were higher than those in central Anhui and southern Anhui, while the detection rates of E. faecium, E. faecalis, and E. cloacae in northern Anhui were lower than those in central Anhui and southern Anhui. Our data also showed that the sputum, blood, pus, and cerebrospinal fluid specimens in northern Anhui were higher than those in central Anhui and southern Anhui, while the urine and ascites samples in southern Anhui were higher than those in northern Anhui and central Anhui. The above results indicate that the distribution of bacteria and specimen sources differed among the different regions.
The present study showed that the resistance rates of Staphylococci to PEN, SMX, CLI, and ERY in northern Anhui were higher than those in central Anhui and southern Anhui, which may be related to the degree of antibiotic use in northern Anhui. Interestingly, the resistance rate of S. aureus to PEN in southern Anhui was lower than that in northern Anhui and central Anhui, whereas the resistance rate of coagulase-negative Staphylococcus to PEN in southern Anhui was higher than that in northern Anhui and central Anhui, indicating that the resistance rate of Staphylococcus to PEN in different regions was significantly different. The detection rate of MRSA in northern Anhui was higher than that in central Anhui, and the detection rate of MRSA in central Anhui was higher than that in southern Anhui. The obvious regional differences should be carefully considered. In addition, the detection rate of MRCNS in southern Anhui was lower than that in northern Anhui and central Anhui. The resistance rates of MRCNS to most antibacterial agents were high. However, the sensitivity rates of MRCNS to vancomycin (VAN), LNZ, and teicoplanin were 100%, indicating that the three antibacterial agents could still be used as the treatment of choice. The resistance rate of Staphylococcus to PEN in the three regions was greater than 90%, indicating that PEN was no longer suitable for Staphylococcus in Anhui Province. Shariati et al found moderate VAN-resistant S. aureus in Asia, and the detection rate was on the rise in recent years.5 At present, VAN-resistant S. aureus has not been found in Anhui province. However, the presence of VAN-mediated and VAN-resistant staphylococci should be monitored with the widespread use of VAN.6,7 Control and treatment guidelines should be formulated according to geographical areas to prevent the further spread of VAN-resistant S. aureus.
Our results showed that the resistance rates of E. faecalis to GEH and LVX in northern Anhui were higher than those in central Anhui and southern Anhui. Meanwhile, the resistance rates of E. faecium to AMP and STH in northern Anhui were higher than those in central Anhui and southern Anhui. The above results indicate that the resistance rates of enterococci to relevant antimicrobial agents were significantly different in the three regions, and antimicrobial agents should be carefully selected. The resistance rates of E. faecium to VAN in northern Anhui, central Anhui, and southern Anhui were 0.9%, 0.6%, and 0.3%, respectively. There were no significant differences in the detection rates among the three regions. Although the detection rate of VAN-resistant enterococci (VRE) was low, the problems such as high mortality, prolonged hospital stay and increased medical expenditure caused by VRE should not be ignored.8 At present, the activities of LNZ, daptomycin (DAP) and TGC in vitro against enterococcus were high.9,10 However, LNZ was easy to produce erythrocyte, hemoglobin, platelet reduction and other hematological adverse reactions.11,12 DAP has more advantages in safety, efficacy and prognosis, therefore, the clinician may choose DAP to treat Enterococcus.13
This study showed that E. coli was the main gram-negative bacterium isolated in Anhui province, following K. pneumoniae and P. aeruginosa, which is consistent with previous studies.14,15 The resistance rates of most antibiotics among E. coli and A. baumannii in northern Anhui were higher than those in central Anhui and southern Anhui, indicating that antibiotics should be carefully selected for the treatment of infections caused by E. coli and A. baumannii in northern Anhui. At present, carbapenem-resistant Enterobacteriaceae (CRE) is a serious situation in China.16 CRE is widely, or even fully, resistant to most clinical antibiotics, and its resistance is related to a variety of molecular mechanisms. Therefore, CRE has become a major challenge in clinical anti-infective therapy and an important reason for its high mortality.16–18 This study showed that the detection rates of CR-ECO, CR-KPN, and CR-ABA in northern Anhui were higher than those in southern Anhui in 2021, suggesting that the use of carbapenems in clinical work in northern Anhui was higher and should be more standardized and rational for clinical anti-infection treatment in the future. Carbapenemase is the main resistance mechanism of carbapenems antibacterial agents in Enterobacteriaceae, including KPC, NDM, OXA-48, VIP, and IMP.19 It was reported that E. coli mainly produced NDM metalloenzyme and K. pneumoniae produced KPC enzyme.20 However, in recent years, class D OXA-48 enzymes have been prevalent in CR-KPN.21 Ceftazidime/avibactam showed high antibacterial activity against KPC or OXA-48 carbapenemase strains, but poor activity against NDM metalloenzyme strains.22 Therefore, it is recommended to carry out combined antibacterial agents for carbapenem-resistant strains in order to provide a more accurate regimen for clinicians. The high sensitivity of E. coli, K. pneumoniae, and P. aeruginosa to amikacin in Anhui suggests that amikacin could be used as an antimicrobial agent of choice for these infections. However, amikacin has ototoxicity and nephrotoxicity,23 which still need to be carefully considered in clinical treatment.
Conclusion
Gram-negative bacteria were the main pathogens in the different regions of Anhui Province in 2021. The resistance rates of bacteria to antibiotics were different in northern Anhui, central Anhui, and southern Anhui, with the resistance rate in northern Anhui being the highest. Therefore, it is very important to conduct long-term dynamic monitoring and understand the trends in drug susceptibility in real time. The differences of bacterial resistance in different regions suggest that attention should be given to the rational selection of antimicrobial agents in clinical anti-infection treatment according to the situation of local bacterial resistance to reduce the emergence of drug-resistant strains and delay the rate of bacterial resistance.
Acknowledgments
We gratefully acknowledge the contributions of the members of HuiNet for collection of the isolates tested in this study. Their names and affiliations are as follows: Ying Huang from the First Affiliated Hospital of Anhui Medical University; Zhou Liu from the Second Affiliated Hospital of Anhui Medical University; Qiang Jin from the Third People’s Hospital of Bengbu; Xiaoyan Zhu from the Second People’s Hospital of Hefei; Yao Chen from the First People’s Hospital of Bengbu; Yun Wang from the Ningguo People’s Hospital; Yindi Zhou from the First People’s Hospital of Hefei; Pu Guo from the First Affiliated Hospital of Bengbu Medical College; Fei Ying from the First People’s Hospital of Wuhui; Juan Wang from the Lujiang People’s Hospital; Wenjiao Chang from the First Affiliated Hospital of USTC; Feng Zhong from the Second Affiliated Hospital of Wannan Medical College; Xiu Tu from the First People’s Hospital of Chuzhou; Li Huang from the Tongling Municipal Hospital; Hongjuan Liu from the Anhui Provincial Children’s Hospital; Yonghong Chen from the Xuancheng People’s Hospital; Zongguang Li from the Anqing Municipal Hospital; Baohua Zhang from the Huangshan People’s Hospital; Qishan Sun from the Huainan Chaoyang Hospital; Lu Wang from the Lu’an People’s Hospital; Shuo Wang from the Guoyang People’s Hospital; Huilin Yao from the Huaibei Miner General Hospital; Xiaowu Wang from the Second People’s Hospital of Fuyang; Chengcheng Ling from the Second People’s Hospital of Wuhu; Changfeng Zhang from the First Affiliated Hospital of Anhui University of Chinese Medicine; Xia Cai from the First People’s Hospital of Huainan; Qingsong from the Xuancheng City Central Hospital; Dongping Wang from the Anhui Chest Hospital; Fei Du from the Binhu Hospital of Hefei; Zhenghai Yang from the the First Affiliated Hospital of Wannan Medical College; Qi Zhang from the Wanbei People’s Hospital; Meiling Yin from the Anhui Cancer Hospital; Zhijun Hu from the Tongling People’s Hospital; Wei Li from the Fuyang People’s Hospital; Yuanyan Xu from the Wanbei Coal and Electricity Group General Hospital; Fei Luo from the Chizhou People’s Hospital; Yaping Wang from the Anqing People’s Hospital; Yulong Wang from the Suzhou Municipal Hospital; Guoping Lu from the Fuyang Hospital of Anhui Medical University; Li Liang from the First People’s Hospital of Suzhou; Fengming Jin from the Affiliated Hospital of West Anhui Health Vocational College; Shuang Liu from the Lu ‘an Hospital of Chinese Medicine; Xiufang Zhang from the Bozhou People’s Hospital; Jing Zhang from the Taihe Hospital of Chinese Medicine; Yan Gao from the Yingshang People’s Hospital; Fang Wang from the Ma’anshan People’s Hospital; Wanqi Men from the First Affiliated Hospital of Anhui Medical University North District.
Funding
This study was supported by the Natural Science Research Project of Universities in Anhui Province (No. KJ2020A0176), the Natural Science Foundation in Anhui Province (No. 2208085MH264), the Project Supported by Anhui Medical University (2021xkj138), the National Natural Science Foundation of China (No. 81973983), Collaborative Tackling and Public Health Collaborative Innovation Project in Anhui Province (No. GXXT-2020-018), the joint construction project of clinical medicine university and hospital (No. 2021lcxk006), and China Primary Health Care Foundation (No. MTP2022A015).
Disclosure
All authors have no conflicting interests in this work.
References
1. McEwen SA, Collignon PJ. Antimicrobial resistance: a one health perspective. Microbiol Spectr. 2018;6(2). doi:10.1128/microbiolspec.ARBA-0009-2017
2. Ferri M, Ranucci E, Romagnoli P, Giaccone V. Antimicrobial resistance: a global emerging threat to public health systems. Crit Rev Food Sci Nutr. 2017;57(13):2857–2876. doi:10.1080/10408398.2015.1077192
3. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100.
4. Hu F, Guo Y, Yang Y, et al.; China Antimicrobial Surveillance Network (CHINET) Study Group. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281. doi:10.1007/s10096-019-03673-1
5. Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. 2020;10(1):12689. doi:10.1038/s41598-020-69058-z
6. Zhang DX, Li Y, Yang XQ, et al. In vitro antibiotic susceptibility, virulence genes distribution and biofilm production of Staphylococcus aureus isolates from bovine mastitis in the Liaoning Province of China. Infect Drug Resist. 2020;11(13):1365–1375. doi:10.2147/IDR.S247765
7. Liang B, Mai J, Liu Y, et al. Prevalence and characterization of Staphylococcus aureus isolated from women and children in Guangzhou, China. Front Microbiol. 2018;16(9):2790. doi:10.3389/fmicb.2018.02790
8. Cheah AL, Spelman T, Liew D, et al. Enterococcal bacteraemia: factors influencing mortality, length of stay and costs of hospitalization. Clin Microbiol Infect. 2013;19(4):E181–E189. doi:10.1111/1469-0691.12132
9. Bender JK, Cattoir V, Hegstad K, et al. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: towards a common nomenclature. Drug Resist Updat. 2018;40:25–39. doi:10.1016/j.drup.2018.10.002
10. Asgin N, Otlu B. Antibiotic resistance and molecular epidemiology of vancomycin-resistant enterococci in a tertiary care hospital in Turkey. Infect Drug Resist. 2020;21(13):191–198. doi:10.2147/IDR.S191881
11. Wei Y, Zhang H, Fu M, Ma R, Li R, Kong L. Plasma and intrapulmonary pharmacokinetics, and dosage regimen optimization of linezolid for treatment of gram-positive cocci infections in patients with pulmonary infection after cerebral hemorrhage. Infect Drug Resist. 2022;8(15):1733–1742. doi:10.2147/IDR.S357300
12. Cattaneo D, Orlando G, Cozzi V, et al. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with gram-positive infections. Int J Antimicrob Agents. 2013;41(6):586–589. doi:10.1016/j.ijantimicag.2013.02.020
13. Lee BJ, Vu BN, Seddon AN, Hodgson HA, Wang SK. Treatment considerations for CNS infections caused by vancomycin-resistant Enterococcus faecium: a focused review of linezolid and daptomycin. Ann Pharmacother. 2020;54(12):1243–1251. doi:10.1177/1060028020932513
14. Hu F, Zhu D, Wang F, Wang M. Current status and trends of antibacterial resistance in China. Clin Infect Dis. 2018;67(suppl_2):S128–S134. doi:10.1093/cid/ciy657
15. Wang J, Zhou M, Huang G, et al. Antimicrobial resistance in southern China: results of prospective surveillance in Dongguan city, 2017. J Hosp Infect. 2020;105(2):188–196. doi:10.1016/j.jhin.2020.03.029
16. Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019;25(8):943–950. doi:10.1016/j.cmi.2019.04.013
17. Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis. 2018;66(8):1290–1297. doi:10.1093/cid/cix893
18. Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2016;29(6):583–594. doi:10.1097/QCO.0000000000000314
19. Han R, Shi Q, Wu S, et al.; China Antimicrobial Surveillance Network (CHINET) Study Group. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. doi:10.3389/fcimb.2020.00314
20. Lu MC, Chen YT, Tang HL, et al. Transmission and evolution of OXA-48-producing Klebsiella pneumoniae ST11 in a single hospital in Taiwan. J Antimicrob Chemother. 2020;75(2):318–326. doi:10.1093/jac/dkz431
21. Chen Y, Fang L, Yang Y, et al. Emergence of carbapenem-resistant Klebsiella pneumoniae harbouring bla OXA-48-like genes in China. J Med Microbiol. 2021;70(3):001306. doi:10.1099/jmm.0.001306
22. Yin D, Dong D, Li K, et al. Clonal dissemination of OXA-232 carbapenemase-producing Klebsiella pneumoniae in neonates. Antimicrob Agents Chemother. 2017;61(8):e00385–17. doi:10.1128/AAC.00385-17
23. Jenkins A, Thomson AH, Brown NM, et al.; (BSAC Working Party on Therapeutic Drug Monitoring). Amikacin use and therapeutic drug monitoring in adults: do dose regimens and drug exposures affect either outcome or adverse events? A systematic review. J Antimicrob Chemother. 2016;71(10):2754–2759. doi:10.1093/jac/dkw250
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.