Back to Journals » Journal of Pain Research » Volume 15
The Analgesic Efficacy of Intradiscal Injection of Bone Marrow Aspirate Concentrate and Culture-Expanded Bone Marrow Mesenchymal Stromal Cells in Discogenic Pain: A Systematic Review
Authors Her YF , Kubrova E , Martinez Alvarez GA, D'Souza RS
Received 4 May 2022
Accepted for publication 5 October 2022
Published 20 October 2022 Volume 2022:15 Pages 3299—3318
DOI https://doi.org/10.2147/JPR.S373345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sudhir Diwan
Yeng F Her,1 Eva Kubrova,2 Gabriel A Martinez Alvarez,2 Ryan S D’Souza1
1Department of Anesthesiology and Perioperative Medicine, Mayo Clinic Hospital, Rochester, MN, 55905, USA; 2Department of Physical Medicine and Rehabilitation, Mayo Clinic Hospital, Rochester, MN, 55905, USA
Correspondence: Ryan S D’Souza, Mayo Clinic, 200 1st St SW, Rochester, MN, 55905, USA, Tel +507-284-9696, Email [email protected]
Abstract: Pain originating from the intervertebral disc (discogenic pain) is a prevalent manifestation of low back pain and is often challenging to treat. Of recent interest, regenerative medicine options with injectable biologics have been trialed in discogenic pain and a wide variety of other painful musculoskeletal conditions. In particular, the role of bone marrow aspirate concentrate (BMAC) and culture-expanded bone marrow derived mesenchymal stromal cells (BM-MSCs) in treating discogenic pain remains unclear. The primary objective of this systematic review was to appraise the evidence of intradiscal injection with BMAC and culture-expanded BM-MSCs in alleviating pain intensity from discogenic pain. Secondary outcomes included changes in physical function after intradiscal injection, correlation between stromal cell count and pain intensity, and anatomical changes of the disc assessed by radiographic imaging after intradiscal injection. Overall, 16 studies consisting of 607 participants were included in qualitative synthesis without pooling. Our synthesis revealed that generally intradiscal autologous or allogeneic BMAC and culture-expanded BM-MSCs improved discogenic pain compared to baseline. Intradiscal injection was also associated with improvements in physical functioning and positive anatomical changes on spine magnetic resonance imaging (improved disc height, disc water content, Pfirrmann grading) although anatomical findings were inconsistent across studies. However, the overall GRADEscore for this study was very low due to heterogeneity and poor generalizability. There were no serious adverse events reported post intradiscal injection except for a case of discitis.
Keywords: BMAC, discogenic pain, bone marrow mesenchymal stromal cells
Introduction
Low back pain (LBP) is a major cause of worldwide disability, with an estimated point prevalence of 30–50% and a lifetime prevalence as high as 80–85%.1–3 A common etiology of back pain is discogenic pain, seen in 22–42% of LBP cases.4–6 The intravertebral discs (IVDs) are fibrocartilaginous joint-like structures that connect and cushion the vertebrae in the axial skeleton, providing stability while permitting motion between vertebrae.7 Aging, genetic factors, and environmental changes have been hypothesized to reduce IVD cell number and alter their metabolism.8 With increasing catabolic activity and decreasing anabolic activity, there are changes in the expression and structure of collagens and proteoglycans in the extracellular matrix resulting in decreased IVD strength and internal disc disruption (IDD). Also, IVD is generally avascular which contributes to poor healing.9 This above process restricts the regenerative potential of the IVD and limits its ability to uniformly distribute forces causing discogenic pain.10 Associated changes in the surrounding vertebral body and endplate can also contribute to painful stimuli.11
The treatment of discogenic pain is challenging with the most common modalities being conservative or symptom focused. Conservative measures include oral analgesics, physical therapy, and epidural corticosteroid injections. More invasive treatment options include spine surgery, such as discectomy, spinal fusion, and disc replacement.12 Recently, there has been significant research devoted to injectable biological treatment options with the potential to not only improve pain, but also decelerate or restore the structure of the IVD, which may potentially alleviate discogenic pain.
There are a variety of biologics that have been studied for treatment of musculoskeletal conditions, most commonly osteoarthritis or tendinopathy, including platelet rich plasma (PRP) and mesenchymal stromal cells (MSCs) derived from adipose tissue, umbilical cord, peripheral blood, and bone marrow.13,14 MSCs are a population of multipotent cells that can differentiate along the chondrogenic, osteogenic, and adipogenic lineages in vitro.15 Historically, MSCs have been harvested and isolated from bone marrow, known as bone marrow derived MSCs (BM-MSC), commonly by accessing and aspirating from the iliac crest. This bone marrow aspirate primarily contains hematopoietic cells, adipose tissue, and supportive stromal cells with a small amount of BM-MSCs.16 The bone marrow aspirate can be used as an injectate without modification, cultured for cell expansion, or minimally modified and concentrated into bone marrow aspirate concentrate (BMAC) through centrifugation.17 This review will focus on BMAC and BM-MSCs since they are one of the most common biologics used to treat musculoskeletal pain.
The role of BMAC and culture-expanded BM-MSCs in the treatment of discogenic pain from IDD remains unclear. Several non-systematic narrative reviews have been published providing a summary of findings of all biologic agents in the treatment of IDD, although conclusions, quality of evidence, and risk of bias cannot be determined from these broad narrative reviews.18,19 The aim of this systematic review is to appraise the evidence on improvement of pain intensity after intradiscal injection of BMAC and culture-expanded BM-MSCs for treatment of discogenic pain.
Methods
Literature Search Strategy
The study protocol was registered under the PROSPERO International prospective register of systematic reviews (CRD42021282340). A literature search was conducted with the assistance of a medical librarian (L.H.). Embase, PubMed/Ovid MEDLINE, EBM Reviews – Cochrane Central Register of Controlled Trials (CENTRAL), and EBM Reviews – Cochrane Database of Systematic Reviews databases were used to conduct the literature review. Publication date range included the entirety of each database and was completed on October 6th, 2021. Controlled vocabulary supplemented with keywords was used to search for studies describing autologous or allogeneic BMAC or BM-MSCs for discogenic back pain. The actual strategy listing all search terms used and how they were combined is available in the Appendix. In addition, a manual search in PubMed and Google Scholar using the terms “bone marrow aspirate concentrate injection for discogenic pain”, “intervertebral disc injection”, and “stromal cell injection for discogenic pain” was conducted to ensure completeness of the review content.
Study Selection
A total of 764 articles were screened in parallel by 2 independent reviewers (Y.F.H., G.M.A.) (Figure 1). Disagreements were resolved by a third independent reviewer (R.S.D.). Out of 764 articles, 756 studies were identified through database searches and eight studies were identified through a manual search. Inclusion criteria included all human studies in the English language that reported pain intensity after intradiscal injection with BMAC or culture-expanded BM-MSCs to treat discogenic pain. Discogenic pain was defined as predominantly low back pain felt to be originating from degeneration or damage to the intervertebral discs. We did not mandate that studies utilize provocative discography for diagnosis of discogenic pain. Studies were included if they described low back pain attributable to degenerative disc disease (e.g. painful annular fissure) that correlated with physical exam findings, or if they described a concordant response with low-pressure provocative discography. Exclusion criteria comprised the following: review articles, animal studies, and conference proceedings.
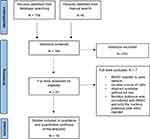 |
Figure 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the literature search and selection process. BMAC, bone marrow aspirate concentrate. Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.36 |
Data Extraction and Outcomes of Interest
The following data were extracted: (1) Study year, (2) Study design, (3) Study funding, (4) Country where the study was performed, (5) Cell/BMAC/BM-MSC source, number of cells and volume of injectate if available, (6) Number of subjects in each arm, (7) Provocative discography inclusion, (8) Age of cohort, and (9) Summary of study findings. The primary outcome of interest was change in pain intensity after intradiscal injection with BMAC or culture-expanded BM-MSCs. Secondary outcomes included correlation of pain intensity based on number or concentration of cells in the intradiscal injectate, change in physical functioning, and change in Magnetic Resonance Imaging (MRI) findings after intradiscal injections with BMAC or culture-expanded BM-MSCs.
Assessment of Risk of Bias
The risk of bias for the studies included was independently evaluated by two reviewers (Y.F.H., E.K.) using guidelines from the Cochrane Collaboration. Risk of bias was assessed in reference to a hypothetical randomized controlled trial (RCT) that randomly selected participants to either receive intradiscal injection of BMAC or culture-expanded BM-MSCs or placebo injection. In reference to this target trial, biases were assessed in random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, attrition bias due to missing data, reporting bias, and other biases. Each domain category was assigned a grade of low risk, high risk, or unclear risk.
If a randomized design was not used, risk of bias was assessed for observational studies based on a hypothetical prospective cohort study that matched participants receiving BMAC or culture-expanded BM-MSCs and would compare the two groups. We used Newcastle-Ottawa quality assessment scale for observational studies that would assess bias based on Selection (Representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, demonstration of outcome of interest does not present at start), comparability (Comparability of cohorts on the basis of the design or analysis) and Outcome (assessment of outcome, was follow-up long enough for outcomes to occur, adequacy of follow-up of cohorts). A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability.
Assessment of Quality of Evidence
The GRADEpro software (Evidence Prime, Inc; http://gradepro.org) was used for GRADE (Grading of Recommendations, Assessment, Development and Evaluations) quality of evidence assessment for each outcome. RCTs are categorized as high-level evidence. This can be downgraded based on risk of bias, inconsistency, indirectness, imprecision, and publication bias. This systematic review contains both RCTs and observational studies. “Observational study” design was chosen as the starting point with the level of evidence categorized as low-level evidence.
Results
Characteristics of Included Studies
Sixteen studies were included in final qualitative analysis without pooling (Figure 1). There were three RCTs,20–22 nine prospective cohort studies,23–31 three case series,32–34 and one retrospective study35 comprising a total of 607 participants. Fifteen studies selected 425 participants with chronic lumbar discogenic pain.20–24,26–35 One study selected 182 participants with chronic cervical discogenic pain.25 The age range of the participants were 18–80 years with the majority of the participants between 35 and 55 years. The follow-up time range was 12–72 months. Extracted variables from each study are reported in Table 1.36
 |
Table 1 Summary of Findings from Included Studies on Use of BMAC and BM-MSC for Intradiscal Disease |
Bone Marrow Aspirate Preparation
Thirteen studies used autologous bone marrow aspirate that were either non-concentrated,34 concentrated,23,25–31,35 or further processed to obtain culture-expanded BM-MSCs24,32,33 for intradiscal injection. Studies23,25–31,35 that used BMAC obtained it from the iliac crest and injected 0.5–6 mL of BMAC per disc with concentrations up to 129.6 million of total nucleated cells (TNC)/mL.23,25–31,35 One study34 used non-concentrated bone narrow aspirate from the iliac crest and injected 1 mL per disc. Six studies20–22,24,32,33 used culture-expanded BM-MSCs and injected 1.73×106 to 4.5×107 cells per disc in one visit.
Three studies used allogeneic culture-expanded BM-MSCs for intradiscal injection.20–22 Amirdelfan et al used bone marrow aspirate, culture-expanded and immunoselected mesenchymal precursor cells from a single healthy donor.20 The participants were injected with either 6×106 or 25×106 culture-expanded BM-MSCs per disc. Noriega et al used bone marrow aspirate cells from five healthy donors.21,22 The participants were injected with 25×106 culture-expanded BM-MSCs per disc.
Provocative Discography
Four studies performed provocative discography to confirm discogenic pain as part of the inclusion criteria.23,24,34,35 Six studies performed provocative discography on some of their participants.20,26,28–30,33 Five studies did not perform provocative discography.21,22,27,31,32 One study did not report whether provocative discography was performed.25 The Dallas Scale Grade for the flow of contrast was not reported.
Outcome Measure Tools
Fourteen studies used an 11-point visual analog scale (VAS) to assess pain intensity.20–31,33,34 Two studies used an 11-point numeric pain scale (NPS)32,35 and one study used the brief pain inventory (BPI).23 Other secondary outcomes assessed by questionnaires included physical functioning assessed by Oswestry disability index (ODI) in nine studies,20–22,24,26,28–30,35 patient health status assessed by the short-form-36 (SF-36) in three studies.20,31,35 One study used a modified single assessment numeric evaluation (SANE) rating scale between 0% and 100% with 0% indicating no improvement and 100% indicating complete pain relief.32 Eight studies used MRI20–24,28,29,31,32 with most assessing for Pfirrmann classification.
Study Funding
Three studies were sponsored by industry.20,23,32 Three studies were funded by institutional or government grants.21,22,24 Eleven studies did not report their funding source.25–31,33–35 However, the co-authors of two studies were employed by industry,33,35 and four studies received bone marrow concentrating devices from industry.26–30
Risk of Bias and Quality Assessment
The Cochrane collaboration risk of bias tool was used to assess the three RCTs. (Figure 2).20–22 In the Noriega et al study, blinding of participants, clinicians, and outcome assessors along with incomplete outcome data, selective reporting, and other biases were graded as low risk for bias. There was a high risk of bias in random sequence generation based on the block randomization sequence. As for the follow-up study,22 there was insufficient information to determine the blinding of participants, clinicians, assessors, incomplete outcome data, and selective reporting.
 |
Figure 2 Risk of bias summary of randomized controlled trials based on authors’ judgements of each item. |
In the Amirdelfan et al study, random sequence generation, incomplete outcome data, and selective reporting were graded as low risk for bias.20 There was a high risk of bias with the lack of blinding of clinicians, associated research staff, and sponsors. Other risk of bias included a non-uniform diagnostic discography as a selection criterion.
The Newcastle-Ottawa scale was used to assess the 13 non-randomized observational studies (Table 2).23–32,34,35 The majority of the studies were given two stars for selecting participants with discogenic back pain and demonstrating that outcome of interest was not present at the start of the study.21–33,35 El Kadiry et al was the only study with control participants for comparison.23 For outcomes, all studies followed their participants for at least one year.
 |
Table 2 Bias Assessment for Observational Studies Using Newcastle-Ottawa Scale |
GRADE Assessment of the Evidence
Based on the GRADE assessment, there is very low-quality evidence that BMAC and culture-expanded BM-MSCs are effective in reducing pain and disability and inducing positive anatomical changes. The GRADE assessment was completed by selecting “observation study” instead of “randomized trial” for the study design, even though there were three RCTs included in this review, to ensure a conservative assessment. The initial “low quality of evidence” was downgraded to “very low quality of evidence” due to high risk of bias, inconsistency, indirectness, and imprecision. A summary of findings with quality of evidence for each outcome and reason for quality assignment is presented in Tables 3 and 4.
 |
Table 3 GRADE Summary of Findings Table |
 |
Table 4 Certainty of Evidence Assessment |
Primary Outcome: Pain Intensity Following Intradiscal Injection with BMAC or Culture-Expanded BM-MSCs
In general, studies highlighted that intradiscal injection with either autologous/allogeneic BMAC or culture-expanded BM-MSCs provided similar efficacy in participants with discogenic pain. All studies using an allogeneic source of BM-MSCs were culture expanded,20–22 and reported a significant improvement in pain scores and physical function after injection with culture-expanded BM-MSCs compared to controls. Studies using an autologous source were either BMAC23,25–31 or culture-expanded BM-MSCs.24,32,33 All studies that administered intradiscal injection with autologous BMAC or culture-expanded BM-MSCs similarly reported significantly improved pain intensity scores and physical function (ODI, BPI, SANE, SF-36) at the latest follow-up23–33 compared to baseline. Mean (or median) improvements in VAS/VPS (Verbal Pain Scale) pain scores at assessed time points were reported to be in 50–71% range in eight studies23,25–31 and below 50% in one study.20 Improvements in functional and quality of life scores ranged from 10% to 90%.23,25–30,32,33,35
One study that administered intradiscal injection with non-concentrated autologous bone marrow aspirate reported no improvement in pain or physical function at 12 months of follow-up compared to baseline.34
Secondary Outcome: Relationship Between Number of Cells Injected and Reported Outcomes
One study found a positive trend with higher number of injected cells and better improvement in pain and physical function. Pettine et al reported that participants’ BMAC with ability to form more than 2000 Colony-forming unit fibroblasts (CFU-F)/mL, regardless of age, had significant VAS and ODI improvement compared to participants receiving BMAC with less than 2000 CFU-F/mL.26,28–30 Elabd et al showed a linear relationship between the total number of culture-expanded BM-MSCs injected and improvement in the overall quality of life.33 Amirdelfan et al reported a significant difference between the four treatment groups (participants receiving 18 million BM-MSCs, 6 million BM-MSCs, Hyaluronic Acid and Saline Control) and time to treatment failure. Additionally, only the cohort receiving 18 million BM-MSCs showed a greater improvement from baseline in the physical component score (SF-36) compared to control participants at 36 months.20
Characterization of BMAC or BM-MSC products was performed in some included studies. Typically, MSCs are characterized by: (1) adherence to plastic under standard culture conditions, (2) expression of CD105, CD73 and CD90, (3) lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR and (4) ability to differentiate to osteoblasts, adipocytes and chondroblasts.37–40 Centeno et al and Elabd et al did not report cell characteristics but described cell harvesting and specified culturing of the cells in a hypoxic environment.33 El Kadiry et al23 reported that BMAC was enriched with mononuclear fraction containing MSCs (CD45−CD44 + CD90+CD105+). Several studies by Pettine et al28–30 reported average BMAC contents of TNC/mL and CFU-F/mL and tested cells for superficial markers such as MSC-specific CD90+, CD105+ and endothelial type CD34+. Amirdelfan et al20 reported using allogeneic stromal precursor antigen-3 (STRO-3) immunoselected MPCs. Noriega et al,21,22 Orozco et al24 and Wolff et al35 did not specify cell characteristics.
Secondary Outcome: Anatomic Changes Based on MRI Assessment
Included studies used MRI20–24,29,32 to assess for structural changes after intradiscal injection with BMAC or culture-expanded BM-MSCs. El-Kadiry et al reported increased disc height and spinal canal space size without worsening disc quality on MRI scans in participants at 8 and 12 months after intradiscal injection.23 Noriega et al demonstrated that Pfirrmann grading favors the culture-expanded BM-MSC-injected group, and this favorable grading was maintained up to 42 months while there were no statistically significant changes (defined by p-value > 0.05) in disc heights and water content between the cohort that received culture-expanded BM-MSCs versus controls.21,22 Orozco et al reported significant elevated water content in the disc even though the disc height did not change.24 Centeno et al and Pettine et al reported regression of posterior disc bulge following intradiscal treatment.29,32 Amirdelfan et al reported no significant modified Pfirrmann score changes in any of the groups post-injection at all follow-up evaluations.20
Discussion
Synthesis of current evidence without pooling revealed that: (1) intradiscal injection with autologous or allogeneic BMAC/culture-expanded BM-MSCs generally provided improved pain intensity and physical functioning in participants with discogenic pain compared to their baseline scores and/or control groups and (2) intradiscal injection with BMAC or culture-expanded BM-MSCs may lead to MRI evidence of improved disc height, disc water content, or improved Pfirrmann grading although these imaging findings were not consistent across all included studies. Intradiscal injection with BMAC/culture-expanded BM-MSCs in participants with discogenic pain was associated with significant improvement in pain intensity and physical function post-injection between one to six years in the included studies. Participants with lumbar intradiscal injection experienced improved pain and function with significantly decreased opioid use.23,31 More than 70% of the participants did not undergo spine surgery throughout follow-up for six years.25,26,28–30 Similarly, participants with cervical intradiscal injection reported improved pain and function at 24 months of follow-up.25 However, the overall level of certainty for the potential associations made in this systematic review is low, because there is very low-quality GRADE evidence to support these.
The analgesic benefits from BMAC and BM-MSCs are likely related to their immunomodulatory profile once injected into the disc. For example, BMAC contains MSCs, platelets, white blood cells, cytokines and growth factors, including Platelet-derived Growth Factor (PDGF), Transforming Growth Factor Beta (TGF-β), and Bone Morphogenetic Protein (BMP)-2 and BMP-7.41,42 BMAC also has clinically relevant concentrations of Interleukin-1 receptor antagonist (IL-1Ra), a natural inhibitor of the pro-inflammatory effects of interleukin 1.43 The potential to reduce pain, decelerate IDD, or restore the IVD is less likely related to the MSC’s capability of self-renewal and differentiation. Instead, it is likely related to MSC ability to secrete trophic and immunomodulatory factors.44–46 While future larger scale RCTs are warranted to confirm this association of positive analgesic and physical functioning benefits, the current evidence suggests very-low quality evidence that intradiscal injection of BMAC and culture-expanded BM-MSCs may improve discogenic pain. Thus, this modality may be offered to patients who fail first-line treatment (physical therapy, epidural steroid injection) and before pursuing more invasive spine surgery.
Additionally, intradiscal injection of other biologic agents have also been studied. Intradiscal injection of PRP for discogenic pain has been assessed in two systematic reviews, which reported a total of five observational studies consisting of 90 participants treated with 1–2mL of PRP.47,48 These reviews evaluating intradiscal injection of PRP for discogenic pain reported an overall reduction in pain scores, reduction in ODI scores at 6 months and improvement in functional score in one RCT which persisted for one year.47–49 There are a limited number of studies that used non-BM-MSCs for intradiscal disease. Three published case series50–52 with a total of 27 participants used autologous adipose-derived MSCs, stromal vascular fraction (SVF), and allogeneic umbilical cord-derived MSCs. They reported improved pain at 6 months and improved functional outcomes. Although the focus of our systematic review was on BMAC and BM-MSCs, future studies should query whether other injectable biologic treatments may offer similar benefits to BMAC and culture-expanded BM-MSCs.
Interestingly, studies assessing participants who received BMAC and culture-expanded BM-MSCs had longer follow-up periods and sustained pain relief up to 72 months while other studies assessing PRP and other sources of MSCs reported follow-up periods of only 6–12 months.
In our systematic review, there was a small subset of participants that experienced decreased efficacy after intra-discal injection of BMAC or culture-expanded BM-MSCs. For these participants, potential factors that may contribute to the lack of response include inadequate number of cells injected,20,29 inadequate quality of cells injected,34 participant’s age,29 other cofounding spine disorders27 or comorbidities, and the severity of the degenerated IVD prior to injection. These factors have been referenced as negative predictive factors in other musculoskeletal disorders treated with MSCs. Mao et al reported that the optimal quantity of stromal cells may be a 108 magnitude to stop or slow disease progression and degeneration.53 Amirdelfan et al suggested that at least 18 million of immunoselected MSCs were required for physical improvement compared to controls in treating discogenic back pain.20 In Haufe et al, participants received non-concentrated bone marrow aspirate, which would be considered a poor quality injectate, and experienced no change in pain relief or functional improvement.34 Furthermore, age can negatively impact the ability of MSCs to rejuvenate and regenerate. In in vitro studies, age-related epigenetic modifications decreased the regenerative potential of MSCs.54,55 In animal studies, increasing donor age impaired bone marrow cell therapy efficacy regardless of disease severity in the recipient.56
Safety
In our review, adverse events included injection related-pain and a case of discitis. Amirdelfan et al reported greater adverse events in the 18 million MPC-treated group compared to the 6 million MPC-treated group, HA group, and normal saline groups. One participant in the 6 million MPC-treated group developed discitis. The overall safety is consistent with reports in the literature regarding MSC injection for various musculoskeletal complaints.57 There have only been rare previous reports of adverse events such as development of hyperplastic gliosis that caused cauda equina syndrome.58,59 However, culture-expanded BM-MSC production requires sophisticated laboratory equipment with experienced staff in current Good Manufacturing Practice facilities to eliminate risks associated with cell manipulation. Hence, development of homogenous “standardized” cell lines such as allogeneic or eg immunoselected allogeneic cells in study by Amirdelfan et al may potentially be safer, more cost effective, and available “off the shelf”. It could also reduce donor dependent variation in ability to produce anti-inflammatory substances.
BMAC may possess seemingly less product heterogeneity, but use of different concentration devices may yield distinct products with distinct regenerative and immunomodulatory profiles.60 In our systematic review, studies have not always reported which devices were used, and therefore we could not compare the safety and efficacy among different production strategies. In addition, bone marrow aspiration technique may also potentially introduce more variability in MSC contents and details of the aspiration technique were not provided.61
Allogeneic vs Autologous BMAC and Culture-Expanded BM-MSCs
Advantages of using autologous BMAC include ease of sample processing since aspiration and injection can be performed in two subsequent procedures on the same day. The risk of graft versus host disease and infection transmission from one person to another is eliminated with autologous products, however, there is always a chance of sample contamination. Autologous BMAC may not undergo as vigorous testing as mass-produced culture-expanded MSCs therefore there is potentially a higher risk of MSC product alteration. Some drawbacks to clinical use of autologous BMAC include the high cost of processing single batches at a time, variability of sample quality and, if culture-expanded MSCs are used, increased time to allow for meaningful doses.62 The treatment with BMAC falls under minimally manipulated product, and therefore, does not require Food and Drug Administration (FDA) approval. If MSCs are isolated from bone marrow aspirate, stored, and expanded, then it is no longer considered a minimally manipulated product and requires an extensive FDA approval process. There are risks associated with the above-described processing such as contamination of cultures or creation of otherwise altered cell lines; but this further processing allows for applications of allogeneic “off the shelf” products, which is similar to the product that was used in the study by Amirdelfan et al. Allogeneic MSC sourcing would also allow for large-scale batch production, facilitate improvement and confirmation of product quality, consistency and safety profile, and allow for easier product availability. Important considerations for treatment efficacy remain as they relate to donor age63 and donor comorbidities,64 and therefore inter-donor MSC variability may impact the quality of cell products.
Cell Dose and Outcomes
One study suggested that higher cell dosing may improve analgesic outcomes29 and two other studies noted some milder improvements in quality of life and SF-36 score with higher doses.20,33 The number of any type of cells injected in one disc ranged markedly from 1.7 million to as much as nearly 390 million cells. Proceduralists may consider increasing cell number in the injectate to optimize therapeutic response. However, future studies are warranted to validate this association and determine if the association is truly dose-dependent versus dose-independent with requirement to meet a minimum threshold of cell number. Furthermore, injected volume and type of medium may also be important given the small anatomical space in the disc ranges on average between 1 and 3 mL.65 This volume may easily approach the typical volume of contrast and saline injected during discography for lumbar discs, however one study also injected up to 6 mL without adverse events.
Imaging Selection
Several studies identified favorable anatomic changes based on MRI and Pfirrmann scores after injection with BMAC or culture-expanded BM-MSCs. In clinical practice, MRI has been the most sensitive imaging method to identify changes in degenerated discs. Suggestion for future studies may include use of more detailed radiological scoring such as Modic changes which frequently correlate with discogenic low back pain, high-intensity zones, and other anatomical changes.66 Spinal X-rays have limited utility given that they can only show decreased disc space, vacuum phenomena and osteophytes and may not capture subtle structural changes.
Provocative Discography
Discography was performed in the majority of studies (10 of 16 studies) but not always for all participants. In clinical practice and patient population with chronic back pain, provocative discography has been an infrequently used method for chronic back pain resistant to treatment, to help decide if there is any indication for surgery, and to determine whether the suspected disc is the true pain generator. Commonly, other diagnostic studies are performed before approaching discograms. While an MRI can be helpful in assessing disc degeneration by looking at Pfirrmann score, Modic changes, and high intensity zones, discography with its mechanical effects can help diagnose if the IVD is the pain generator. Another potential benefit is that it can identify an annular tear and ability to contain biologic injectate within the disc.67 While we are unable to determine whether discogram contributed to participants’ positive outcomes and ability to select participants with true discogenic pain, we hypothesize that it may be a beneficial diagnostic tool to help determine source of pain and demonstrate an intact annulus that can contain and accommodate biologic injectate. However, disadvantages of using provocative discography include procedural disruption of the annulus fibrosus and potential worsening degeneration of the disc associated with disc herniation. This risk was demonstrated in a matched-control prospective study despite the use of 22–25 Gauge needles.68 Therefore, it might be helpful to consider discography with a consistent protocol in future studies as an inclusion criteria alongside other imaging modalities while keeping in mind its potential risks.
Limitations
Several limitations are notable There was a high risk of bias, small sample sizes, and lack of comparator arms in many included studies. This is consistent with prior narrative reviews on injectable biologics for the treatment of intradiscal disease.69–71 Variation both between and within studies was present in design for participants’ selection, cell preparations (volume type and concentration of cells in injectate), levels of disc injection (cervical versus lumbar spine), and follow-up. For example, out of the 10 prospective studies, only one used provocative discography to confirm discogenic pain as an inclusion criterion.24 In the RCT studies, provocative discography was either left to the investigator’s discretion20 or not performed at all.21,32 There are concerns about the lack of blinding of the participants, clinicians, and research staff.20 Lastly, industry funding and influence may add additional bias to the current evidence.20,23,32 In addition, this study is limited by very low-quality GRADE evidence.
Future Directions
Several strategies for future studies should be considered. First, most studies did not report characteristics such as cell surface markers for their BMAC products, though some used a set of MSC-specific markers (eg CD 90, 105, CD166, CD44) to demonstrate that there is a population of MSCs. We encourage future studies to report MSC-specific, hematopoietic, and endothelial cell surface markers. Protein analysis to document contents of anti-inflammatory molecules and cytokines in the BMAC product would also be beneficial to assess capacity to treat discogenic pain. In addition, there are important questions to be raised in future studies to ensure optimal clinical results such as selection of appropriate participants and discs that will benefit from MSCs or BMAC, using clinical symptoms, specific imaging findings (eg Pfirrmann scores) or discography while considering participants’ age and comorbidities. Second, MSC and BMAC have been mostly studied in vitro under ideal external conditions. There remain many questions regarding MSC survival and efficacy in vivo given the relative unavailability of nutrients and oxygen in the IVD environment that may be detrimental.72 Henriksson et al73 reported in their study of four participants that MSCs may be able to survive and proliferate in the IVDs for 6–12 months post-injection but they were undetectable at 28 months. Therefore, further studies assessing the long-term survival of MSCs for at least 12 months should be conducted as restoration of the IVD microenvironment and proteins are unlikely to occur within a shorter period of time.72 To detect improvements, more objective markers such as radiographic evidence from MRI are needed to quantify anatomical changes in the disc after treatment with injectable biologics. Though we are hopeful that MSC/BMAC remain contained within the IVD after injection, in cases when the annulus fibrosus is disrupted, it is possible that MSC leakage occurs. Use of provocative discography suggested better accuracy in determining painful discs and proof of cell containment however, there are risks associated with the procedure that need to be considered.
These data would inform the critical quality attributes needed in the creation of a reproducible, cost-effective and commercially available product. The availability of an approved injectable “off-the-shelf” MSC product would allow for easier incorporation into the real-world clinical practice. To facilitate the efficacy of such a product, the optimal delivery method, including the possibility of using delivery vehicles like hydrogels, needs to be identified to prevent MSC leakage.74 The long-term safety in cases of human leukocyte antigens (HLA) mismatch should also be established.75
In this review we focused on BMAC and MSCs, however other cell products such as nucleus pulposus (NP) and chondrogenic cells or PRP were also commonly investigated as potential biologics for IVD76–80 and comparison with MSC and BMAC would be of interest.
Finally, future high-powered RCTs utilizing homogeneous selection criteria may help inform future systematic reviews where outcomes may be pooled.
Conclusion
This study overall supported modest efficacy in treating discogenic pain and improving functional outcomes with BMAC or MSC injections, however, the level of certainty for the potential associations made in this review is low as documented by very low-quality GRADE evidence to support these conclusions. Generally, improvements in pain and functional scores compared to baseline were found in most studies using either BMAC or BM-MSCs and there were some mild objective improvements noted on spinal MRIs in some studies. Major limitations included significant heterogeneity among participants, limiting generalizability such as areas of pain and variability in types and quantities of treatments, small sample sizes with small or no control groups, high risk of bias, inconsistency in determining painful discs, lack of blinding, and industry funded studies.
Acknowledgments
We would like to acknowledge and thank Leslie Hassett, M.L.S. for assistance with developing the search strategy for this systematic review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Brundtland GH. A WHO scientific group on the burden of musculoskeletal conditions at the start of the new millennium met in Geneva from 13 to 15 january 2000. Who Tech Rep Ser. 2003;919:1–218.
2. Buchbinder R, Blyth FM, March LM, Brooks P, Woolf AD, Hoy DG. Placing the global burden of low back pain in context. Best Pract Res Clin Rheumatol. 2013;27(5):575–589. doi:10.1016/j.berh.2013.10.007
3. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20(17):1878–1883. doi:10.1097/00007632-199509000-00007
4. DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12(2):224–233.
5. Manchikanti L, Singh V, Pampati V, et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001;4(4):308–316.
6. Verrills P, Nowesenitz G, Barnard A. Prevalence and characteristics of discogenic pain in tertiary practice: 223 consecutive cases utilizing lumbar discography. Pain Med. 2015;16(8):1490–1499.
7. Lawson LY, Harfe BD. Developmental mechanisms of intervertebral disc and vertebral column formation. Wiley Interdiscip Rev Dev Biol. 2017;6(6):e283.
8. Oichi T, Taniguchi Y, Oshima Y, Tanaka S, Saito T. Pathomechanism of intervertebral disc degeneration. JOR Spine. 2020;3(1):e1076.
9. Fournier DE, Kiser PK, Shoemaker JK, Battié MC, Séguin CA. Vascularization of the human intervertebral disc: a scoping review. JOR Spine. 2020;3(4):e1123.
10. Dowdell J, Erwin M, Choma T, Vaccaro A, Iatridis J, Cho SK. Intervertebral disk degeneration and repair. Neurosurgery. 2017;80(3S):S46–S54. doi:10.1093/neuros/nyw078
11. Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9(1):20. doi:10.1038/s41413-021-00147-z
12. Zhao L, Manchikanti L, Kaye AD, Abd-Elsayed A. Treatment of discogenic low back pain: current treatment strategies and future options-a literature review. Curr Pain Headache Rep. 2019;23(11):86. doi:10.1007/s11916-019-0821-x
13. Song Y, Zhang J, Xu H, et al. Mesenchymal stem cells in knee osteoarthritis treatment: a systematic review and meta-analysis. J Orthop Translat. 2020;24:121–130. doi:10.1016/j.jot.2020.03.015
14. Kubrova E, D’Souza RS, Hunt CL, Wang Q, van Wijnen AJ, Qu W. Injectable biologics: what is the evidence? Am J Phys Med Rehabil. 2020;99(10):950–960. doi:10.1097/PHM.0000000000001407
15. Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, Razmkhah M. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther. 2020;11(1):492. doi:10.1186/s13287-020-02001-1
16. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi:10.1126/science.284.5411.143
17. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6(2):e441–e445. doi:10.1016/j.eats.2016.10.024
18. Schol J, Sakai D. Cell therapy for intervertebral disc herniation and degenerative disc disease: clinical trials. Int Orthop. 2019;43(4):1011–1025. doi:10.1007/s00264-018-4223-1
19. Vadala G, Ambrosio L, Russo F, Papalia R, Denaro V. Stem cells and intervertebral disc regeneration overview-what they can and can’t do. Int J Spine Surg. 2021;15:S40–S53. doi:10.14444/8054
20. Amirdelfan K, Bae H, McJunkin T, et al. Allogeneic mesenchymal precursor cells treatment for chronic low back pain associated with degenerative disc disease: a prospective randomized, placebo-controlled 36-month study of safety and efficacy. Spine J. 2021;21(2):212–230. doi:10.1016/j.spinee.2020.10.004
21. Noriega DC, Ardura F, Hernandez-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101(8):1945–1951. doi:10.1097/TP.0000000000001484
22. Noriega DC, Ardura F, Hernandez-Ramajo R, et al. Treatment of degenerative disc disease with allogeneic mesenchymal stem cells: long-term follow-up results. Transplantation. 2021;105(2):e25–e27. doi:10.1097/TP.0000000000003471
23. El-Kadiry AE, Lumbao C, Rafei M, Shammaa R. Autologous BMAC therapy improves spinal degenerative joint disease in lower back pain patients. Front Med. 2021;8:622573. doi:10.3389/fmed.2021.622573
24. Orozco L, Soler R, Morera C, Alberca M, Sanchez A, Garcia-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92(7):822–828. doi:10.1097/TP.0b013e3182298a15
25. Pettine K. Two-year follow-up results of treating cervical degenerated discs with bone marrow concentrate to avoid surgery. Stem Cell Res Th. 2017;2(1):5.
26. Pettine K, Dordevic M, Hasz M. Reducing lumbar discogenic back pain and disability with intradiscal injection of bone marrow concentrate: 5-year follow-up. American Journal of Stem Cell Research. 2018;2(1):1–4. doi:10.5923/j.ajscr.20180201.01
27. Pettine K, Santomaso TJ. Treatment of multi-level discogenic low back pain with bone marrow concentrate. Stem Cell Res Th. 2017;2(1):74–79.
28. Pettine K, Suzuki R, Sand T, Murphy M. Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up. Int Orthop. 2016;40(1):135–140. doi:10.1007/s00264-015-2886-4
29. Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015;33(1):146–156. doi:10.1002/stem.1845
30. Pettine KA, Suzuki RK, Sand TT, Murphy MB. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop. 2017;41(10):2097–2103. doi:10.1007/s00264-017-3560-9
31. Navani A, Ambach MA, Navani R, Wei J. Biologics for lumbar discogenic pain: 18 month follow-up for safety and efficacy. IPM Report. 2018;2(3):111–116.
32. Centeno C, Markle J, Dodson E, et al. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med. 2017;15(1):197. doi:10.1186/s12967-017-1300-y
33. Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med. 2016;14:253. doi:10.1186/s12967-016-1015-5
34. Haufe SM, Mork AR. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15(1):136–137.
35. Wolff M, Shillington JM, Rathbone C, Piasecki SK, Barnes B. Injections of concentrated bone marrow aspirate as treatment for Discogenic pain: a retrospective analysis. BMC Musculoskelet Disord. 2020;21(1):135.
36. Liberati A, Altman DG, Tetzlaff J, et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med, 62(10), e1000100 10.1371/journal.pmed.1000100
37. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317.
38. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: international Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019–1024.
39. Camilleri ET, Gustafson MP, Dudakovic A, et al. Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res Ther. 2016;7(1):107.
40. Dudakovic A, Camilleri E, Riester SM, et al. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem. 2014;115(10):1816–1828.
41. Kim GB, Seo MS, Park WT, Lee GW. Bone marrow aspirate concentrate: its uses in osteoarthritis. Int J Mol Sci. 2020;21(9):3224.
42. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033–1042.
43. Cassano JM, Kennedy JG, Ross KA, Fraser EJ, Goodale MB, Fortier LA. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sport Tr A. 2018;26(1):333–342.
44. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cell Transl Med. 2017;6(6):1445–1451.
45. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084.
46. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
47. Chang MC, Park D. The effect of intradiscal platelet-rich plasma injection for management of discogenic lower back pain: a meta-analysis. J Pain Res. 2021;14:505–512.
48. Hirase T, Jack IRA, Sochacki KR, Harris JD, Weiner BK. Systemic review: is an intradiscal injection of platelet-rich plasma for lumbar disc degeneration effective? Cureus. 2020;12(6):e8831.
49. Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, et al. Lumbar intradiskal Platelet-Rich Plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM R. 2016;8(1):1–10.
50. Comella K, Silbert R, Parlo M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J Transl Med. 2017;15(1):12.
51. Kumar H, Ha DH, Lee EJ, et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a Phase I study. Stem Cell Res Ther. 2017;8(1):262.
52. Pang X, Yang H, Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician. 2014;17(4):E525–530.
53. Mao L, Jiang P, Lei X, et al. Efficacy and safety of stem cell therapy for the early-stage osteonecrosis of femoral head: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2020;11(1):445.
54. Cakouros D, Gronthos S. Epigenetic regulation of bone marrow stem cell aging: revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis. 2019;10(1):174–189.
55. Fei DD, Wang YZ, Zhai QM, et al. KAT6A regulates stemness of aging bone marrow-derived mesenchymal stem cells through Nrf2/ARE signaling pathway. Stem Cell Res Ther. 2021;12:1.
56. An S, Wang X, Ruck MA, et al. Age-related impaired efficacy of bone marrow cell therapy for myocardial infarction reflects a decrease in B lymphocytes. Mol Ther. 2018;26(7):1685–1693.
57. Jerome MA, Lutz C, Lutz GE. Risks of intradiscal orthobiologic injections: a review of the literature and case series presentation. Int J Spine Surg. 2021;15(s1):26–39.
58. Aoun SG, Reyes VP, El Ahmadieh TY, et al. Stem cell injections for axial back pain: a systematic review of associated risks and complications with a case illustration of diffuse hyperplastic gliosis resulting in cauda equina syndrome. J Neurosurg-Spine. 2019;31(6):906–913.
59. Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016;40(8):1755–1765.
60. Dragoo JL, Guzman RA. Evaluation of the consistency and composition of commercially available bone marrow aspirate concentrate systems. Orthop J Sports Med. 2020;8(1):2325967119893634.
61. Gronkjaer M, Hasselgren CF, Ostergaard ASL, et al. Bone marrow aspiration: a randomized controlled trial assessing the quality of bone marrow specimens using slow and rapid aspiration techniques and evaluating pain intensity. Acta Haematol Basel. 2016;135(2):81–87.
62. Kim K, Bou-Ghannam S, Kameishi S, Oka M, Grainger DW, Okano T. Allogeneic mesenchymal stem cell sheet therapy: a new frontier in drug delivery systems. J Control Release. 2021;330:696–704.
63. Stolzing A, Jones E, Mcgonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163–173.
64. Cassidy FC, Shortiss C, Murphy CG, et al. Impact of type 2 diabetes mellitus on human bone marrow stromal cell number and phenotypic characteristics. Int J Mol Sci. 2020;21(7):2476.
65. Yang J, Wang X, Fan Y, et al. Tropoelastin improves adhesion and migration of intra-articular injected infrapatellar fat pad MSCs and reduces osteoarthritis progression. Bioact Mater. 2022;10:443–459.
66. Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65(773):361–369.
67. Gruver C, Guthmiller KB. Provocative Discography. In: StatPearls. Treasure Island (FL); 2022.
68. Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. ISSLS Prize Winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine. 2009;34(21):2338–2345.
69. Baroncini A, Eschweiler J, Kobbe P, et al. Mesenchymal stem cell applications in spine disorders: a comprehensive review. Appl Sci Basel. 2021;11:17.
70. Meisel HJ, Agarwal N, Hsieh PC, et al. Cell therapy for treatment of intervertebral disc degeneration: a systematic review. Global Spine J. 2019;9(1 Suppl):39S–52S.
71. Urits I, Capuco A, Sharma M, et al. Stem cell therapies for treatment of discogenic low back pain: a comprehensive review. Curr Pain Headache Rep. 2019;23(9):65.
72. Loibl M, Wuertz-Kozak K, Vadala G, Lang S, Fairbank J, Urban JP. Controversies in regenerative medicine: should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine. 2019;2(1):e1043.
73. Henriksson HB, Papadimitriou N, Hingert D, Baranto A, Lindahl A, Brisby H. The traceability of mesenchymal stromal cells after injection into degenerated discs in patients with low back pain. Stem Cells Dev. 2019;28(17):1203–1211.
74. Smith LJ, Silverman L, Sakai D, et al. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: recommendations of the ORS spine section. JOR Spine. 2018;1(4):e1036.
75. Kot M, Baj-Krzyworzeka M, Szatanek R, Musiał-Wysocka A, Suda-Szczurek M, Majka M. The importance of HLA assessment in “Off-The-Shelf” allogeneic mesenchymal stem cells based-therapies. Int J Mol Sci. 2019;20(22):5680.
76. Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001;389:94–101.
77. Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24(1):5–21.
78. Tschugg A, Diepers M, Simone S, et al. A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: safety results of Phase I-a short report. Neurosurg Rev. 2017;40(1):155–162.
79. Coric D, Pettine K, Sumich A, Boltes MO. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg Spine. 2013;18(1):85–95.
80. Chang Y, Yang M, Ke S, Zhang Y, Xu G, Li Z. Effect of platelet-rich plasma on intervertebral disc degeneration in vivo and in vitro: a critical review. Oxid Med Cell Longev. 2020;2020:8893819.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
