Back to Journals » Drug Design, Development and Therapy » Volume 14
The Advantages of Levodopa-Carbidopa Intestinal Gel for Patients with Advanced Parkinson’s Disease: A Systematic Review
Authors Zhang XR, Jiang ZY, Zhang ZR, Chen HJ, Wu K, He JC, Xie CL
Received 2 September 2019
Accepted for publication 24 January 2020
Published 26 February 2020 Volume 2020:14 Pages 845—854
DOI https://doi.org/10.2147/DDDT.S229621
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Xing-Ru Zhang, 1,* Zhi-Yu Jiang, 2,* Zeng-Rui Zhang, 3 Hui-Jun Chen, 1 Ke Wu, 4 Jin-Cai He, 1 Cheng-Long Xie 1
1The Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, People’s Republic of China; 2The Department of General Surgery, The Sir Run Run Shaw Hospital, Medical College of Zhejiang University, Hangzhou 310000, People’s Republic of China; 3Department of Neurology, Huzhou Central Hospital, Huzhou 313000, People’s Republic of China; 4Zhejiang Provincial Key Laboratory of Aging and Neurological Disorder Research, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cheng-Long Xie; Jin-Cai He Tel/Fax +86 577 88 83 2693
Email [email protected]; [email protected]
Background: Levodopa-carbidopa intestinal gel (LCIG) is a new type of administration that results in steadier levodopa plasma concentrations in advanced Parkinson’s disease (PD) patients and effectively reduces poor mobility and dyskinesia.
Methods: Electronic databases were searched up to January 1, 2018. The inclusion criteria for this review were as follows: LCIG vs oral medication in advanced PD patients.
Results: Five trials, with a total of 198 patients, met all the inclusion criteria. The quality score of these studies ranged from 3 to 5. Two clinical trials showed that compared with oral medication, LCIG had a better treatment effect on on-time with troublesome dyskinesia (TSD) (p = 0.02) and on-time without TSD (p < 0.00001) in advanced PD patients. In addition, four of the 5 studies showed that the LCIG may have better efficacy than oral medication for improving the scores of the UPDRS, and two studies found that LCIG demonstrated better efficacy for improving the PDQ-39 scores. The video recording results indicated a potential decline in both dyskinesia and the “off” state in LCIG-treated patients. The incidence of adverse events was not significantly different between the LCIG and oral medication groups.
Conclusion: Compared with oral treatment, LCIG exerts its effectiveness, mostly by reducing the time of on-time with TSD, increasing the time of on-time without TSD and scores of UPDRS and PDQ-39. It is suggesting that LCIG was likely to be a new type of administration used in clinical applications. However, due to methodological flaws, these findings should be viewed with caution, and more RCTs are needed in the field to complement our findings.
Keywords: Levodopa-carbidopa intestinal gel, Parkinson’s disease, oral medication, clinical trials, systematic review
Introduction
Parkinson’s disease (PD) is a progressive, neurodegenerative and disabling disorder characterized by effects on dopaminergic neurotransmission and movement disorders.1 Using dopamine replacement agents can alleviate motor symptoms effectively, and the administration of levodopa is the standard therapy for the treatment of PD.2 PD symptoms are often well controlled with oral levodopa at an early disease stage. With PD progression, a number of patients report disabling motor complications, including daily motor fluctuations and dyskinesia, and these complications are closely associated with chronic levodopa treatment.3 Moreover, unstable levodopa plasma concentrations resulting from factors such as pulsatile delivery of oral levodopa, labile gastric emptying, and narrowing therapeutic windows, may contribute to these complications.4
Levodopa-carbidopa intestinal gel (LCIG) is a newly device-aided therapy that in Japan is named ABT-SLV187, and in the United States is referred to as carbidopa-levodopa enteral suspension (CLES).5 With a portable pump that is connected to a PEG-J, a percutaneous endoscopic gastrostomy with a thinner inner J-tube, LCIG is continuously administered to the upper intestine.6 According to pharmacokinetic studies, direct intestinal levodopa administration can effectively lead to steady state concentrations of levodopa in plasma. LCIG may also reduce poor mobility and dyskinesia.7 However, adverse events associated with the pump or the tube are common.8
To date, a detailed comparison between the treatment of advanced PD patients with LCIG and oral medication has not been performed. Furthermore, it is unclear whether LCIG is a more suitable treatment for avoiding motor symptoms and dyskinesia in patients with advanced PD. Therefore, the objective of this review was to systematically evaluate the efficacy and advantages of LCIG, by comparing the two therapeutic methods (LCIG vs oral medication). Thus, we hope to describe the therapeutic potential of LCIG in advanced PD patients.
Methods
Search Strategy
A comprehensive literature search was performed to identify related publications evaluating LCIG vs oral medication in the treatment of patients with advanced Parkinson’s disease from the following databases: PubMed, Google Scholar and the Cochrane Library, up to January 1, 2018. An experienced reviewer (ZYJ) independently filtered the titles, abstracts and references based on the eligibility criteria. The following search strategy was used for each database:
- Levodopa-carbidopa intestinal gel
- Levodopa-carbidopa intestinal infusion
- LCIG
- or/1–3
- Oral treatment
- Oral medication
- or/5–6
- Parkinson disease
- Parkinson’s disease
- PD
- or/8–10
- 4 and 7 and 11
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) clinical trials comparing LCIG with oral medication to treat advanced PD; (2) participants (aged ≥30 years) with advanced PD who signed an informed consent form; (3) trials depicting advanced PD patients; (4) studies describing advanced PD patients with certain degrees of responses such as motor fluctuations, dyskinesia, painful dystonia, or bradykinesia despite optimal pharmacological treatment; and (5) studies that were published in English.
The exclusion criteria were as follows: (1) case reports, abstracts, reviews, editorials, letters comments or end game; (2) nonhuman subjects; and (3) no tests evaluating the efficacy of treatment of LCIG vs oral medication.
Outcome measurements The primary outcome was the UPDRS scores. The secondary outcomes were the results of the video recording, PDQ-39 scores, time of the on-time with troublesome dyskinesia (TSD) and the on-time without TSD.
Data Extraction and Quality Assessment
The detailed information from each trial was carefully extracted by two authors and was recorded as follows: (1) name of the first author, year of publication and follow-up time; (2) study design, study population and treatment types; (3) individual data obtained from each treatment group, including sample size, sex, mean age, race and duration of PD; and (3) treatment regimen, baseline indexes, outcome measures and adverse events. Based on our previous study, we used a six–item modified scale9 to assess the risk of bias of the incorporated clinical trials. If the outcome data of the meta-analysis were only expressed graphically or missing, we attempted to contact the authors for further information. When no response was received, we used digital ruler software to measure the data from the graphs. We made an attempt to extract data of the mean value and standard deviation for each comparison from every study.
Statistical Analysis
All statistical analyses were performed with Cochrane’s Review Manager version 5.3. We considered all the frequencies of adverse events as dichotomous data, and expressed the 95% confidence intervals (CIs). Moreover, we considered the outcomes of on-time with TSD and on-time without TSD as continuous data. The weighted mean difference (WMD) was applied as a standard statistic to measure the absolute difference between the mean values in the two groups. The mean effect was shown as the WMD with its 95% CI.10 We used a fixed effects model, and the statistical heterogeneity among studies was assessed using the Q statistic and the I2 index. I2 values <50% indicate an acceptable degree of heterogeneity among studies.11 A probability value of P<0.05 was considered significant. Ethical approval was not required for this type of literature research.
Results
Results of the Search
Our initial search of three databases identified 1352 publications. After removing duplicates, a total of 1052 references remained. After screening the titles and abstracts, we removed 454 references for at least one of the following reasons: (1) case reports, abstracts, reviews, editorials, letters comments or end games; (2) basic experiment trials; and (3) other disease. Finally, after screening the full texts of 153 remaining studies, which explored the efficacy of LCIG vs oral medication, 5 studies were identified for the final analysis12–16 (Figure 1).
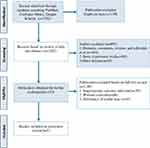 |
Figure 1 Algorithm of study selection and inclusion in the review. |
Study Characteristics
In this review, five clinical trials (2 crossover trials, 3 RCTs), with a total of 198 advanced PD patients who met the inclusion criteria, were included in the analysis. Of the patients in the crossover trials, 18 were randomized to begin with oral tablets, followed with crossing over to LCIG, and 18 were randomized to begin therapy in the opposite order. Of the patients in the RCTs, 93 were randomized to the LCIG group, and 69 were randomized to the oral medication group. The sample size included in this review ranged from 12 to 71. The follow-up duration ranged from 7 to 54 weeks. Moreover, in this review, the baseline indexes of H&Y (Hoehn & Yahr stage) were recorded in 2 trials, UPDRS (Unified Parkinson’s Disease Rating Scale) in 4 trials, PDQ-39 (39-item Parkinson’s Disease Questionnaire) in 2 trials, “off” time in 2 trials, on-time with troublesome dyskinesia (TSD) in 2 trials, and on-time without TSD in 2 trials. Regarding outcome measures, UPDRS was recorded in 4 trials; video recording was shown in 2 trials, PDQ-39 was observed in 2 trials, on-time with TSD and on-time without TSD were reported in 2 trials. The basic characteristics collected from five studies are shown in Tables 1 and 2.
 |
Table 1 Basic Characteristics of the Included Studies |
 |
Table 2 Main Outcome Measures of Included Studies Baseline Indexs Outcome Measures |
Effectiveness
Outcome Measures of UPDRS
The UPDRS is currently the gold standard for the assessment of disease state in Parkinson’s disease, and it includes a comprehensive assessment of motor symptoms and reliable clinimetric properties.17 In Nyholm et al, the total score of the UPDRS sections I, II and IV was 34.9±7.6 at baseline. At the end of the trial, there was no significant change in the score between the treatment groups, but in section IV, LCIG-treated patients had fewer complications.12 In Nyholm et al, the baseline score of the UPDRS sections I, II, III and IV are shown in Table 2. At the end of the trial, the LCIG-treated patients reported lower median total scores of the UPDRS than the oral medication group (p<0.05), especially in all sections of the UPDRS in the LCIG group.13 In Olanow et al, the UPDRS sections I, II and III scores at the beginning of the trial are shown in Table 2. After the short-term endpoint of the study, LCIG-treated patients showed significant improvement in the UPDRS section II score and quality of life measures compared with the oral medication group. However, no significant difference was found between the treatment groups (p=0.502) in the UPDRS section III.14 In Antonini et al, the score of the UPDRS section IV dyskinesia questions (#32-34) at baseline is reported in Table 2. Finally, there was no significant difference between the treatment groups in the UPDRS section IV dyskinesia questions (#32-34) (p>0.05). However, the oral-treated patients showed significant worsening, while LCIG-treated patients showed no change from baseline to the end of the study.16
Outcome Measures of Video Recording
The video recording comprised four different examinations, such as rising from a chair, walking, alternating hand movements, and “piano playing”.12 In Nyholm et al, the video recording showed a significant decline in both dyskinesia and the off-state during infusion. The mean-variance scores were 2.0 for oral treatment and 1.1 for LCIG.12 In Nyholm et al, the video evaluations showed a marked reduction in moderate to severe “off” state in LCIG-treated patients. The “no” or “mild symptoms” categories of the five UPDRS items were all significantly increased in both treatment arms. Regarding the video evaluation of dyskinesia, there were no significant differences between the treatment groups.13
Outcome Measures of PDQ-39
PDQ-39 is a questionnaire that reflects the quality of life of PD patients and has been proven to have test-retest reliability and satisfactory internal validity.18 In Nyholm et al, the summary index of the median PDQ-39 was 25 (range 10 to 42) with infusion and 35 (range 16 to 55) with oral medication, which is consistent with a higher of quality of life with infusion (p<0.01).13 In Olanow et al, compared to the baseline, the summary index of PDQ-39 was −10.9±3.3 with infusion and −3.9±3.2 with oral medication (p=0.0155).14
Outcome Measures of on-Time with TSD
For the 5 studies evaluating the time of on-time with TSD, 1 study provided clear data,14 and 1 study provided graphical data.16 In Olanow et al, the time of on-time with TSD was 1.0±1.6 h per day in LCIG-treated patients and 1.2±1.7 h per day in the oral medication group at baseline. After the short-term study, the time of on-time with TSD was −0.11±0.52 h per day with infusion and −0.03±0.52 h per day with oral (p=0.8574).14 In Antonini et al, compared to the baseline, the hours of on-time with TSD had a significant mean decrease in the LCIG-treated patients (p<0.05).16 A meta-analysis of 2 studies revealed that the LCIG group showed a significantly decreased time of on-time with TSD (n = 94, WMD = −0.30, 95% CI: −0.54 to −0.06, p = 0.02; heterogeneity: Chi2 = 1.07, p = 0.30, I2 = 6%, Figure 2A).
Outcome Measures of on-Time Without TSD
Of the 5 studies evaluating the time of on-time without TSD, 1 study provided clear data,14 and 1 study provided graphical data.16 In Olanow et al, the time of on-time without TSD was 8.7±2.0 h per day in LCIG-treated patients and 7.8±2.5 h per day in the oral medication group at baseline. After week 12, the time of on-time without TSD was 4.11±0.75 h per day with infusion and 2.24±0.76 h per day with oral medication (p=0.0059).14 In Antonini et al, a significant improvement was shown in both groups. Beginning as early as week 2, the hours of on-time without TSD improved from baseline to every time point in the LCIG-treated patients, but when compared with the oral-treated patients, these improvements were not significant (p=0.491).16 A meta-analysis of 2 studies showed that the LCIG group significantly improved the time of on-time without TSD compared with the oral-treated group (n = 94, WMD = 2.77, 95% CI: 2.42 to 3.12, p< 0.00001; heterogeneity: Chi2 = 0.00, p = 0.96, I2 = 0%, Figure 2B).
Risk of Bias
Table 3 shows the risk of bias of the included trials. The total score of the six–item modified scale is 6 points. The risk of bias of the included trials ranged from 3 to 5 points. Two trials received 3 points (40%), and three trials received 5 points (60%).
 |
Table 3 Risk of Bias of Included Studies |
Adverse Events
In the three trials, the incidence of adverse events (AEs) was not significantly different between the LCIG and oral medication groups. There were a variety of AEs that involved the digestive, nervous, and psychiatric systems. Among these, the most common AEs included abdominal pain (33.3% in the levodopa-carbidopa intestinal gel group vs 20.9% in the oral medication group, p = 0.07), procedural pain (22.2% vs 26.9%, p = 0.60), constipation (15.3% vs 22.4%, p = 0.37), nausea (22.2% vs 11.9%, p = 0.23), dyskinesia/hyperkinesia (15.3% vs 19.4%, p = 0.49), wound infection (8.3 vs 17.9, p = 0.11), insomnia (9.7% vs 16.4%, p = 0.44), depression (12.5% vs 10.4%, p = 0.75). No deaths were reported in either treatment groups in any of the trials. The more details are shown in Table 4.
 |
Table 4 Summary of Most Common Adverse Events |
Discussion
Summary of the Main Result
Five clinical trials, with a total of 198 advanced PD patients, were included in the analysis. The quality score of these studies ranged from 3 to 5. First, our results showed that LCIG had a greater treatment effect on on-time with TSD (p = 0.02) and on-time without TSD (p < 0.00001) than oral medication did. In addition, compared with oral medication, four of the 5 studies showed that the LCIG may have a better effect on improving the scores of the UPDRS, and two studies suggested that LCIG improved PDQ-39 scores. The results of video recording indicated a potential decline in both dyskinesia and the “off” state in LCIG-treated patients. Second, three trials analyzed the incidence of AEs, and no deaths were found. There was no significant difference between the LCIG and oral medication groups; the most common AEs included abdominal pain, procedural pain, constipation and nausea.
Interpretation of the Results
Dyskinesia is usually referred to as levodopa-induced dyskinesia (LID) and often occurs as a motor complication in patients with advanced PD. Chronic levodopa treatment is a major contributor to the development of dyskinesia, especially in a dose-dependent fashion. However, chronic levodopa treatment is insufficient to generate dyskinesia.16 Several conditions such as presynaptic nigrostriatal degeneration, correspondingly preserved postsynaptic nigrostriatal system and a short half-life (pulsatile delivery) are required to generate dyskinesia. In most atypical parkinsonism, levodopa hardly induces LID, such as a postsynaptic system disease and progressive supranuclear palsy.19 When levodopa is administered orally, there is a dose-dependent increase in peak-dose dyskinesia,20 except when it is delivered continuously, such as when LCIG is administered. Furthermore, LCIG, which involves a higher dose than that used orally, still typically reduces pre-existent LID. Indeed, this result may be due to the ability of LCIG to effectively maintain steady state levodopa plasma concentrations.7 Our review consistently found that LCIG demonstrated a better treatment effect compared with oral administration on the time of the on-time with TSD and on-time without TSD in advanced PD patients. Nevertheless, the sample size of these trials was limited, and the trials had different designs. Therefore, we were not able to perform a systematic analysis to confirm whether LCIG treatment had a greater therapeutic effect on the scores of the UPDRS and PDQ-39 when compared with oral treatment. Thus, the results of this review require additional multicenter RCTs to evaluate the whole potential of LCIG in the treatment of advanced PD patients.
In our review, four studies used UPDRS to evaluate the disease state of PD patients. UPDRS has been in development since 1987 and is widely used in the assessment of PD patients.17 The advantages of UPDRS include its almost comprehensive coverage of PD motor symptoms and its effectiveness and reliability. However, it also has some shortcomings, including some ambiguity in the text content, insufficient indication of the scorer, some defects in the metrics, and problems in the screening of some important non-motor symptoms in PD patients.21 In 2001, the Movement Disorder Society (MDS) revised UPDRS and produced a new version, called MDS-sponsored UPDRS revision (MDS-UPDRS).22 Compared to the original UPDRS 55 options, MDS-UPDRS has 65 rating options. The evaluation of MDS-UPDRS is more comprehensive than UPDRS, especially the evaluation of several important non-motor symptoms of PD. According to the original UPDRS, there are still 5 grades for each question: (0) normal, (1) slight, (2) mild, (3) moderate, (4) severe, but MDS-UPDRS is more focused on the degree of impairment and disabilities in slight and mild patients. Another important point is that MDS-UPDRS provides detailed guidance for evaluators, and MDS-UPDRS requires patients and caregivers to participate in the assessment of some non-motor and motor symptoms in daily life.23 Therefore, in some future studies, we should try to use the MDS-UPDRS scale to make our results more reliable.
Limitation
Some limitations of this review are summarized as follows. First, reversely small sample size was included from five clinical trials. In addition, fragmentary data from a few trials may contribute to the unclear risk of bias. Second, 52 weeks was the longest duration of treatment in the five included trials. Therefore, a key challenge was that the benefits over time and the cost-effectiveness of LCIG cannot be evaluated at present.
The Implication for Further Studies
The findings from this review revealed a better treatment effect on the hours of on-time with TSD or on-time without TSD in LCIG-treated patients. However, because of the limited number of included trials, we are not certain whether LCIG treatment has a notable treatment effect on the UPDRS, PDQ-39 scores and the results from video recordings. Therefore, more multicenter RCTs in the future are indispensable, especially with longer follow-up times. AEs such as abdominal pain and procedural pain were more likely to occur with greater frequency with PEG-J tubes, because the PEG could move back and forth, gliding along the PEG tube. During the first days postprocedure when the stoma is maturing, the movement of the PEG may predispose the patients to develop peritonitis. Moreover, with the leaking gastric fluids, the skin around the peristomal area may become irritated.6,8,24 Therefore, we should explore advanced technology to solve these issues. In summary, no deaths occurred in either treatment group. Finally, patients with dementia were excluded from some trials, because patients could participate only if the MMSE was 28 to 29.24 Therefore, we require more rigorous RCTs to assess whether LCIG is suitable for patients with mild to severe dementia.
Conclusions
In general, compared with oral treatment, LCIG exerts its effectiveness, mostly by reducing the time of on-time with TSD, increasing the time of on-time without TSD and scores of UPDRS and PDQ-39. It is suggesting that LCIG was likely to be a new type of administration used in clinical applications. However, due to methodological flaws, these findings should be viewed with caution, and more RCTs are needed in the field to complement our findings.
Author Contributions
Z-RZ, Z-YJ, K-W, H-JC, X-RZ, J-CH and C-LX substantially contributed to conception and design, acquisition of data, or analysis and interpretation of data. All authors drafted the article or revised it critically for important intellectual content, approved final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The study was supported by the Projects of National Science Foundation of China (No. 81600977) and the Projects of Natural Science Foundation of Zhejiang Province (Y19H090059).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Vidailhet M. Movement disorders in 2010: Parkinson disease-symptoms and treatments. Nat Rev Neurol. 2011;7:70–72. doi:10.1038/nrneurol.2010.216
2. LeWitt PA, Fahn S. Levodopa therapy for Parkinson disease: a look backward and forward. Neurology. 2016;86:S3–12. doi:10.1212/WNL.0000000000002509
3. Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol. 2012;8:435–442. doi:10.1038/nrneurol.2012.126
4. Antonini A, Chaudhuri KR, Martinez-Martin P, Odin P. Oral and infusion levodopa-based strategies for managing motor complications in patients with Parkinson’s disease. CNS Drugs. 2010;24:119–129. doi:10.2165/11310940-000000000-00000
5. Murata M, Mihara M, Hasegawa K, et al. Safety and efficacy of levodopa-carbidopa intestinal gel: results from an open-label extension study in Japanese, Korean and Taiwanese patients with advanced Parkinson’s disease. Ther Adv Neurol Disord. 2018;11:1756286418759315. doi:10.1177/1756286418759315
6. Udd M, Lyytinen J, Eerola-Rautio J, et al. Problems related to levodopa-carbidopa intestinal gel treatment in advanced Parkinson’s disease. Brain Behav. 2017;7:e00737. doi:10.1002/brb3.2017.7.issue-7
7. Nyholm D. Enteral levodopa/carbidopa gel infusion for the treatment of motor fluctuations and dyskinesias in advanced Parkinson’s disease. Expert Rev Neurother. 2006;6:1403–1411. doi:10.1586/14737175.6.10.1403
8. Epstein M, Johnson DA, Hawes R, et al. Long-term PEG-J tube safety in patients with advanced Parkinson’s disease. Clin Transl Gastroenterol. 2016;7:e159. doi:10.1038/ctg.2016.19
9. Macleod MR, O’Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi:10.1161/01.STR.0000125719.25853.20
10. Vesterinen HM, Sena ES, Egan KJ, et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods. 2014;221:92–102. doi:10.1016/j.jneumeth.2013.09.010
11. Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951–957. doi:10.1111/j.1365-2753.2008.00986.x
12. Nyholm D, Askmark H, Gomes-Trolin C, et al. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol. 2003;26:156–163. doi:10.1097/00002826-200305000-00010
13. Nyholm D, Nilsson Remahl AI, Dizdar N, et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology. 2005;64:216–223. doi:10.1212/01.WNL.0000149637.70961.4C
14. Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13:141–149. doi:10.1016/S1474-4422(13)70293-X
15. Othman AA, Dutta S. Population pharmacokinetics of levodopa in subjects with advanced Parkinson’s disease: levodopa-carbidopa intestinal gel infusion vs. oral tablets. Br J Clin Pharmacol. 2014;78:94–105. doi:10.1111/bcp.2014.78.issue-1
16. Antonini A, Fung VS, Boyd JT, et al. Effect of levodopa-carbidopa intestinal gel on dyskinesia in advanced Parkinson’s disease patients. Mov Disord. 2016;31:530–537. doi:10.1002/mds.26528
17. Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–750. doi:10.1002/(ISSN)1531-8257
18. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4:241–248. doi:10.1007/BF02260863
19. Espay AJ, Morgante F, Merola A, et al. Levodopa-induced dyskinesia in Parkinson disease: current and Evolving Concepts. Ann Neurol. 2018;84:797–811. doi:10.1002/ana.25364
20. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–2508.
21. Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ. Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Mov Disord. 2002;17:867–876. doi:10.1002/(ISSN)1531-8257
22. Goetz CG, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–47. doi:10.1002/(ISSN)1531-8257
23. Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi:10.1002/mds.v23:15
24. Fernandez HH, Standaert DG, Hauser RA, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord. 2015;30:500–509. doi:10.1002/mds.26123
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

