Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
The 1α,25(OH)2D3 Analogs ZK159222 and ZK191784 Show Anti-Inflammatory Properties in Macrophage-Induced Preadipocytes via Modulating the NF-κB and MAPK Signaling
Authors Zhu J, Wilding JPH
Received 10 January 2020
Accepted for publication 3 April 2020
Published 19 May 2020 Volume 2020:13 Pages 1715—1724
DOI https://doi.org/10.2147/DMSO.S245080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Jingjing Zhu,1,2 John PH Wilding2,3
1Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China; 2Institute of Ageing and Chronic Disease, University of Liverpool, Liverpool, Merseyside, UK; 3Clinical Sciences Centre, University Hospital Aintree, Liverpool, Merseyside, UK
Correspondence: John PH Wilding Tel +44 151 529 5885
Fax +44 151 529 5888
Email [email protected]
Purpose: Key research findings suggest that attenuating metaflammation in adipose tissue might be a strategic step to prevent the metabolic syndrome and its associated disease outcomes. The anti-inflammatory effects of 1α,25(OH)2D3 have been confirmed in our previous studies, but adverse effects induced at high concentrations restrict its potential clinical translation. Two synthetic 1α,25(OH)2D3 analogs ZK159222 and ZK191784 have manifested promising tissue-specific immunomodulatory actions, but limited data are available on adipose tissue. Hence, in this study, we investigated whether ZK159222 and ZK191784 act on preadipocytes or macrophages to attenuate metaflammatory responses via modulating inflammatory and metabolic signaling in macrophage-induced preadipocytes.
Methods: Preadipocyte-specific effects of ZK159222 and ZK191784 on macrophage-induced preadipocytes were tested by pre-incubating and incubating preadipocytes with the analogs and MacCM. Separately, macrophage-specific effects of both analogs on macrophage-induced preadipocytes were tested by incubating preadipocytes with analog-MacCM or MacCM. The effects of 1α,25(OH)2D3 were also examined and set as the positive control. Metaflammatory responses were determined as the concentrations and gene expression of major pro-inflammatory cytokines including IL-1β, IL-6, IL-8, MCP-1 and RANTES, measured using ELISA and qPCR. Inflammatory and metabolic signaling including NF-κB and MAPK were probed using Western blotting.
Results: ZK159222 and ZK191784 act on preadipocytes and macrophages to decrease the secretion and gene expression of the major pro-inflammatory cytokines in macrophage-induced preadipocytes. The anti-inflammatory effects were at least as potent as 1α,25(OH)2D3, and no preadipocyte apoptosis was induced at high concentrations. In addition, mostly at high concentrations, both analogs moderately decreased the phosphorylation of relA, p44/42 and p38 MAPK in macrophage-induced preadipocytes.
Conclusion: ZK159222 and ZK191784 act on macrophages and preadipocytes to attenuate metaflammatory responses in macrophage-induced preadipocytes, by decreasing phosphorylation of relA/NF-κB, p44/42 and p38 MAPK.
Keywords: vitamin D, metaflammatory response, relA, p44/42, p38
Introduction
The metabolic syndrome (MetS) is a constellation of interrelated risk factors including central obesity, insulin resistance, dyslipidemia and hypertension, which have been considered to promote the development of type 2 diabetes, cardiovascular disease, and even a plethora of cancers.1,2 A growing body of evidence suggests that metaflammation in adipose tissue contributes to the onset and progress of MetS.3,4 Metaflammation is mainly characterized as adipose tissue expression of various pro-inflammatory cytokines stimulated by M1 macrophage infiltration.5 Therefore, attenuating adipose tissue metaflammation might be a strategic step to prevent MetS and its associated disease outcomes.
The immunomodulatory properties of 1α,25(OH)2D3 have suggested it may have a role in treating inflammatory diseases.6 1α,25(OH)2D3 has also long been known to influence metabolic and immune functions of adipose tissue.7 Moreover, the anti-inflammatory properties of 1α,25(OH)2D3 in adipose tissue metaflammation were confirmed in our previous studies.8,9 However, adverse effects, i.e. calcium release-activated apoptosis, potentially restrict its clinical translation.8,10 Thus, during recent decades, attempts have been made to synthesize 1α,25(OH)2D3 analogs with preservation of immunomodulatory properties but elimination of calcium homeostasis disruption.11
In this study, we primarily investigated whether two synthetic 1α,25(OH)2D3 analogs (namely ZK159222 and ZK191784) act on preadipocytes or macrophages to attenuate metaflammatory responses in macrophage-induced preadipocytes. In addition, both inflammatory and metabolic signaling including NF-κB and mitogen-activated protein kinase (MAPK) were probed to elucidate the underlying anti-inflammatory mechanisms.
Materials and Methods
Reconstitution of ZK159222, ZK191784 and 1α,25(OH)2D3
ZK159222, ZK191784 (kindly provided by Bayer AG, Germany) and 1α,25(OH)2D3 (ENZO Life Sciences, USA) were reconstituted in dimethyl sulfoxide to a concentration of 2.4×10−5 M and then diluted in media (indicated as below) to final concentrations of 10 nM and 1 μM.
Macrophage Culture and Stimulation
The human THP-1 monocytic cell line was kindly provided by Professor Helen R Griffiths (Aston University, UK) and cultured in RPMI-1640 medium (Sigma-Aldrich, UK) with 10% fetal bovine serum at 37°C with 5% CO2. Upon reaching a cell density of 1×106cells/mL, supernatants were replaced with 100 nM phorbol 12-myristate 13-acetate (Sigma-Aldrich, UK) in RPMI-1640 medium with 10% fetal bovine serum to differentiate the monocytes to macrophages for 48 h. Subsequently, supernatants were aspirated and 1 μg/mL lipopolysaccharide in RPMI-1640 medium was added to polarize the macrophages to pro-inflammatory M1 dominant phenotype.12 The M1 dominant macrophages were cultured in RPMI-1640 medium for macrophage-conditioned medium (MacCM), or incubated with ZK159222 (10 nM and 1 μM), ZK191784 (10 nM and 1 μM) or 1α,25(OH)2D3(10 nM) in RPMI-1640 medium for VD-MacCM. All the supernatants were collected after 24 h, filtered through 0.22 μm filters and stored at −80°C (the THP-1 cell line was originally purchased by Professor Helen Griffiths in Birmingham from the UK Government laboratory in Porton Down that has ethical approval for the development of such cell lines. For specific details, please refer to Metabolic memory effect of the saturated fatty acid, palmitate, in monocytes (DOI: 10.1016/j.freeradbiomed.2012.05.026), which was published in 2009 by Professor Griffiths’ research group, firstly described the THP-1 cell line as having been purchased from Health Protection Agency Culture Collections (Porton Down, Salisbury, UK); all RPMI-1640 media used in culture or stimulation were supplemented with 1% penicillin/streptomycin).
Preadipocyte Culture and Stimulation
Commercially available human white preadipocytes derived from subcutaneous adipose tissue of a 44 years old female Caucasian subject with a body mass index of 21 kg/m2 (PromoCell, Germany), were cultured to confluence as previously described.9 Following this, supernatants were aspirated and the preadipocytes were pre-incubated in preadipocyte growth medium (PromoCell, Germany) or with ZK159222 (10 nM and 1 μM), ZK191784 (10 nM and 1 μM) or 1α,25(OH)2D3 (10 nM) in preadipocyte growth medium for 48 h. Subsequently, supernatants were aspirated and the preadipocytes were incubated with 25% RPMI-1640 medium (the control) or 25% MacCM or 25% MacCM along with ZK159222 (10 nM and 1 μM), ZK191784 (10 nM and 1 μM) or 1α,25(OH)2D3 (10 nM) in preadipocyte growth medium for 24 h. Preadipocyte and supernatant collection was conducted after the incubation. Separately, when confluence was reached, supernatants were aspirated and the preadipocytes were incubated with 25% RPMI-1640 medium (the control), 25% MacCM or VD-MacCM (indicated as above) in preadipocyte growth medium for 24 h before preadipocyte and supernatant collection (all preadipocyte growth media used in culture or stimulation were supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B).
Measurement of Metaflammatory Responses
Proteins were extracted from the preadipocytes, and content measured as previously described.13 The concentrations of interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein (MCP)-1 and regulated on activation, normal T cell expressed and secreted (RANTES) in the supernatant were measured independently in duplicate using human ELISA kits following the manufacturer’s instructions (R&D Systems, UK) and SPECTROstar Nano Microplate Reader (BMG LABTECH, Germany), normalized to the total cell protein and presented as ng(cytokine)/mg(cell protein).
RNA was extracted from Trizol-lysed preadipocytes and converted to cDNA using cDNA synthesis kit (Bio-Rad, UK). The relative gene expression of IL-1β, IL-6, IL-8, MCP-1 and RANTES were measured as Ct value independently in duplicate using TaqMan gene expression assays (Applied Biosystems, UK), qPCR core kit following the manufacturer’s instructions (Eurogentec, Belgium) and Stratagene Mx3005P instrument system, normalized to the internal reference PPIA,14 and presented as fold change relative to control using the 2−ΔΔct formula.15
Western Blotting
Proteins were extracted from the preadipocytes and measured as previously described.13 The intracellular densities of relA, phosphorylated relA, p44/42 MAPK, phosphorylated p44/42 MAPK, p38 MAPK and phosphorylated p38 MAPK were measured using the method previously described,13 and normalized to the internal control vinculin. All the antibodies used (New England BioLabs; Abcam, UK) were diluted according to the manufacturer’s instructions. The phosphorylation level of relA, p44/42 MAPK and p38 were calculated as the ratio of phosphorylated relA to relA, phosphorylated p44/42 MAPK and phosphorylated p38 MAPK to p38 MAPK, respectively, and presented as fold change relative to control.
Statistical Analysis
Data were analyzed using one-way ANOVA and followed by Tukey’s test for individual comparison (GraphPad Prism 5, USA). A value of P<0.05 was regarded as statistically significant. The results were confirmed by three independent experiments and shown as mean ± SEM.
Results
ZK159222 and ZK191784 Act on Preadipocytes to Reduce the Major Pro-Inflammatory Cytokines Secreted from Macrophage-Induced Preadipocytes
Metaflammatory responses were stimulated by inducing preadipocytes with 25% MacCM. IL-1β, IL-6, IL-8, MCP-1 and RANTES were selected as major pro-inflammatory cytokines expressed in macrophage/MacCM-induced preadipocytes in keeping with published studies.16–21 The ELISA results show that secretion of the major pro-inflammatory cytokines was increased by 13 ~ 387-fold from MacCM-induced preadipocytes (Figure 1A).
 |
Figure 1 Continued. |
To test whether ZK159222, ZK191784 or 1α,25(OH)2D3 act on preadipocytes to attenuate the metaflammatory responses in macrophage-induced preadipocytes, preadipocytes were pre-incubated with ZK159222, ZK191784 and 1α,25(OH)2D3 to boost their efficacy as formally established,13 and then incubated with ZK159222, ZK191784 and 1α,25(OH)2D3 along with induction of MacCM.
In accordance with our previous study,9 10 nM of 1α,25(OH)2D3 generally reduced the major pro-inflammatory cytokines secreted from MacCM-induced preadipocytes by 43 ~ 81%. 10 nM of ZK159222 and ZK191784 also exhibited significant anti-inflammatory effects as the overall pro-inflammatory secretions from MacCM-induced preadipocytes were decreased by 28 ~ 69% and 27 ~ 68%, respectively. Tukey’s test indicates that both synthetic analogs reduced the secretion of the major pro-inflammatory cytokines as effectively as 1α,25(OH)2D3 (Figure 1A).
Preadipocytes started to undergo apoptosis in 2–4 h when pre-incubated with 1 μM of 1α,25(OH)2D3 (data not shown). However, 1 μM of ZK159222 and ZK191784 significantly and similarly reduced the overall pro-inflammatory secretion from MacCM-induced preadipocytes by 23 ~ 67% and 29 ~ 69% (Figure 1A).
ZK159222 and ZK191784 Act on Preadipocytes to Inhibit the Major Pro-Inflammatory Cytokines Expressed in Macrophage-Induced Preadipocytes
The qPCR results show that gene expression of the major pro-inflammatory cytokines was increased by 13 ~ 1933-fold in MacCM-induced preadipocytes. Consistent with our previous study,9 10 nM of 1α,25(OH)2D3 universally inhibited the major pro-inflammatory cytokines expressed in MacCM-induced preadipocytes by 61 ~ 88%. Likewise, 10 nM of ZK159222 and ZK191784 decreased the overall pro-inflammatory gene expression in MacCM-induced preadipocytes by 31 ~ 68% and 67 ~ 89%, though ZK159222 appeared less potent. Moreover, 1 μM of ZK159222 and ZK191784 similarly inhibited the overall pro-inflammatory gene expression by 47 ~ 74% and 57 ~ 76% (Figure 1B).
ZK159222 and ZK191784 Act on Preadipocytes to Decrease Phosphorylation of Inflammatory and Metabolic Signaling in Macrophage-Induced Preadipocytes
During adipose tissue metaflammation, the NF-κB signaling is activated via phosphorylation of the transcription factor relA in the nucleus.22,23 The Western blotting results show that intracellular levels of relA were not affected, but the phosphorylation was increased by 1.2-fold in MacCM-induced preadipocytes. In accordance with our previous study,9 10 nM of 1α,25(OH)2D3 decreased relA phosphorylation by 39% in MacCM-induced preadipocytes. Although relA phosphorylation was not affected by 10 nM of ZK159222 or ZK191784, 1 μM of both synthetic analogs significantly and consistently decreased the phosphorylation by 17% and 17%, respectively (Figure 2A and B) (for MacCM-induced preadipocytes co-incubated with 1α,25(OH)2D3, ZK159222 and ZK191784, the quantifications were calculated as the percentages of 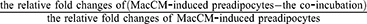 ).
).
The MAPK signaling are required for physiological metabolic adaptation, but inappropriate activation has been associated with the development of MetS.24 As conventional MAPKs, p44/42 and p38 are activated via phosphorylation in the cytoplasm.25 In this study, though intracellular levels of p44/42 and p38 MAPK were not affected, the phosphorylation of both MAPKs in MacCM-induced preadipocytes were increased by 0.2-fold and 1.9-fold, whereas 10 nM of 1α,25(OH)2D3 decreased p44/42 and p38 MAPK phosphorylation by 30% and 76%. For the analogs, only 1 μM of ZK159222 de-phosphorylated p44/42 MAPK (by 31%) in MacCM-induced preadipocytes. By contrast, (10 nM and 1 μM) both analogs universally decreased p38 MAPK phosphorylation by 27% to 58%, though ZK159222 appeared least effective (Figure 2A, C and D).
ZK159222 and ZK191784 Act on Macrophages to Reduce the Major Pro-Inflammatory Cytokines Secreted and Expressed in Macrophage-Induced Preadipocytes
To test whether ZK159222, ZK191784 or 1α,25(OH)2D3 (VD) also act on macrophages to attenuate the metaflammatory responses in macrophage-induced preadipocytes, preadipocytes were incubated with VD-MacCM. VD-MacCM were produced by incubating macrophages with ZK159222, ZK191784 and 1α,25(OH)2D3.
Compared with MacCM-induced preadipocytes, (10 nM) 1α,25(OH)2D3-MacCM decreased secretion of the major pro-inflammatory cytokines by 25 ~ 50%, with the exception of IL-1β. Likewise, (10 nM) ZK159222-MacCM and ZK191784-MacCM similarly reduced the major pro-inflammatory secretion by 33 ~ 54% and 23 ~ 51%. Furthermore, (1 μM) ZK159222-MacCM and ZK191784-MacCM indistinguishably decreased the overall pro-inflammatory secretion by 30 ~ 63% and 24 ~ 44%, respectively (Figure 3A).
 |
Figure 3 Continued. |
In parallel, (10 nM and 1 μM) ZK159222-MacCM, (10 nM and 1 μM) ZK191784-MacCM and (10 nM) 1α,25(OH)2D3-MacCM inhibited the overall pro-inflammatory gene expression by 25 ~ 52% and 33 ~ 59%, 34 ~ 78% and 41 ~ 67%, 39 ~ 76%, respectively. The overall anti-inflammatory effects achieved were largely similar, though (10 nM) ZK191784-MacCM appeared most potent in inhibiting the gene expression of IL-8 and RANTES, while least effective on IL-1β expression (Figure 3B).
Discussion
Physiologically, macrophage–preadipocyte interactions influence especially adipogenesis, lipolysis and apoptosis, which are fundamental metabolic processes to maintain adipose tissue homeostasis.26,27 During metaflammation, infiltration of excess M1 macrophages mobilize preadipocytes in paracrine/autocrine manners to disrupt adipose tissue homeostasis, which might make a critical contribution to MetS.3,4,27,28 Hence, in this study, the metaflammatory responses were stimulated in macrophage-induced preadipocytes and measured only in supernatant/lysate extracted from the preadipocytes, since they are the progenitor of adipose tissue, rather than macrophages.27 The current results revealed that secretion and gene expression of the major pro-inflammatory cytokines were significantly enhanced in macrophage-induced preadipocytes.
1α,25(OH)2D3 has been shown to improve inflammatory as well as metabolic biomarkers of MetS,29 but there still is a long way to its clinical translation, which may be dependent upon the development of advantageous synthetic analogs and research to unravel their immunometabolic effects and mechanism of action.30 The 1α,25(OH)2D3 analogs ZK159222 and ZK191784 have manifested a number of tissue-specific immunomodulatory actions,31–33 but limited data are available on adipose tissue. In this study, preadipocyte and macrophage-specific effects of ZK159222 and ZK191784 on metaflammatory responses in macrophage-induced preadipocytes were thoroughly explored. 1α,25(OH)2D3 served as the positive control since we previously demonstrated that it acts on preadipocytes to attenuate the metaflammatory responses in macrophage-induced preadipocytes.9 The present results show that ZK159222 and ZK191784 act on preadipocytes and macrophages to attenuate the metaflammatory responses in macrophage-induced preadipocytes (Figure 4). Although some differences in effects were obvious, both synthetic analogs not only decreased secretion and gene expression of the major pro-inflammatory cytokines in macrophage-induced preadipocytes as effectively as 1α,25(OH)2D3, but also displayed distinct anti-inflammatory advantages at high concentrations, for preadipocyte apoptosis was not induced. Limitations of this study include the fact that we focused our assessment of the metaflammatory response on pro-inflammatory cytokines. The cytokine array results revealed that 1α,25(OH)2D3 increased serpin-E1 secreted from macrophage-induced preadipocytes. Overexpression of serpin-E1 has been suggested to show some beneficial effects on adipose tissue in metabolic syndrome.34 However, IL-1RA, which acts as the inhibitor of IL-1β,35 was paradoxically decreased (Supplementary Figure). Therefore, the effects of the synthetic analogs on anti-inflammatory cytokines during adipose tissue metaflammation should be considered in the future.
As a crucial inflammatory signaling activated during metaflammation, the NF-κB/relA integrates with metabolism to disrupt adipose tissue homeostasis and contribute to MetS.22,36 Moreover, MAPK has been suggested to be an important metabolic signaling to provoke MetS when inappropriately activated.24 Specifically, p44/42 and p38 MAPK function in adipose tissue to regulate adipogenesis and lipolysis, which are characteristically disordered in MetS.37–40 Only preadipocyte-specific modulating effects on NF-κB and MAPK signaling in macrophage-induced preadipocytes were measured in this study, since not only preadipocytes are the progenitor of adipose tissue, but also were directly pre/incubated with the synthetic analogs.27 The results revealed that the phosphorylation of relA, p44/42 and p38 MAPK were increased in macrophage-induced preadipocytes. 1α,25(OH)2D3 acted on preadipocytes to decrease the phosphorylation of relA, p44/42 and p38 MAPK in macrophage-induced preadipocytes. ZK159222 and ZK191784 also de-phosphorylated these inflammatory and metabolic signaling, but most effectively when used at high concentrations. Last but not least, it would be interesting in future studies to investigate the effects of these analogs on those fundamental metabolic processes in adipose tissue (Figure 4).
Conclusion
The 1α,25(OH)2D3 analogs ZK159222 and ZK191784 act on macrophages and preadipocytes to attenuate secretion and gene expression of the major pro-inflammatory cytokines in macrophage-induced preadipocytes, via decreasing phosphorylation of NF-κB/relA, p44/42 and p38 MAPK.
Abbreviations
IL, interleukin; MacCM, macrophage-conditioned medium; MAPK, mitogen-activated protein kinase; MCP, monocyte chemoattractant protein; MetS, metabolic syndrome (MetS); RANTES, regulated on activation, normal T cell expressed and secreted.
Acknowledgments
Dr Chen Bing (formerly of the Institute of Ageing and Chronic Disease, University of Liverpool – now retired) co-conceived and co-designed the study, and provided ongoing advice during its conduct.
Author Contributions
JZ made substantial contributions to conception and design, acquisition of data. JW supervised the major findings. JW and JZ analyzed and interpreted the data. JZ drafted the article. JW revised it critically for important intellectual content. All the authors gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the China Scholarship Council, with a grant awarded to Jingjing Zhu (No. 201306920002).
Disclosure
Professor John PH Wilding reports grants, personal fees and consultancy fees paid to the University of Liverpool from Astra Zeneca and Novo Nordisk; personal fees and consultancy fees paid to the University of Liverpool from Boehringer Ingelheim, Janssen, Mumdipharma, Napp and Sanofi; and consultancy fees paid to the University of Liverpool from Astellas, Eli Lilly, Rhythm Pharma and Wilmington Healthcare, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43(1):1–23. doi:10.1016/j.ecl.2013.09.009
2. O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. doi:10.1111/obr.12229
3. Torres S, Fabersani E, Marquez A, Gauffin-Cano P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur J Nutr. 2019;58(1):27–43. doi:10.1007/s00394-018-1790-2
4. Grundy SM. Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Invest. 2015;45(11):1209–1217. doi:10.1111/eci.12519
5. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi:10.1038/nature21363
6. Lin Z, Li W. The roles of Vitamin D and its analogs in inflammatory diseases. Curr Top Med Chem. 2016;16(11):1242–1261. doi:10.2174/1568026615666150915111557
7. Abbas MA. Physiological functions of Vitamin D in adipose tissue. J Steroid Biochem Mol Biol. 2017;165(Pt B):369–381. doi:10.1016/j.jsbmb.2016.08.004
8. Zhu J, Bing C, Wilding JPH. Vitamin D receptor ligands attenuate the inflammatory profile of IL-1beta-stimulated human white preadipocytes via modulating the NF-kappaB and unfolded protein response pathways. Biochem Biophys Res Commun. 2018;503(2):1049–1056. doi:10.1016/j.bbrc.2018.06.115
9. Zhu J, Bing C, Wilding JPH. 1alpha,25(OH)2D3 attenuates IL-6 and IL-1beta-mediated inflammatory responses in macrophage conditioned medium-stimulated human white preadipocytes by modulating p44/42 MAPK and NF-kappaB signaling pathways. Diabetol Metab Syndr. 2019;11:9. doi:10.1186/s13098-019-0405-2
10. Berridge MJ. Vitamin D reactive oxygen species and calcium signalling in ageing and disease. Philos Trans R Soc Lond B Biol Sci. 2016;371:1700. doi:10.1098/rstb.2015.0434
11. Jones G, Kaufmann M. Update on pharmacologically-relevant vitamin D analogues. Br J Clin Pharmacol. 2019;85(6):1095–1102. doi:10.1111/bcp.13781
12. Verreck FA, de Boer T, Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101(13):4560–4565. doi:10.1073/pnas.0400983101
13. Ding C, Wilding JP, Bing C. Vitamin D3 analogues ZK159222 and Zk191784 have anti-inflammatory properties in human adipocytes. Endocrinol Metab Genet. 2016;1:1–8. doi:10.15761/EMG.1000001
14. Feroze-Merzoug F, Berquin IM, Dey J, Chen YQ. Peptidylprolyl isomerase A (PPIA) as a preferred internal control over GAPDH and beta-actin in quantitative RNA analyses. BioTechniques. 2002;32(4):776–778, 780, 782. doi:10.2144/02324st03
15. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi:10.1038/nprot.2008.73
16. Koenen TB, Stienstra R, van Tits LJ, et al. The inflammasome and caspase-1 activation: a new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology. 2011;152(10):3769–3778. doi:10.1210/en.2010-1480
17. Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflam Res. 2009;58(11):727–736. doi:10.1007/s00011-009-0060-4
18. Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49(9):1894–1903. doi:10.1194/jlr.M800132-JLR200
19. Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30(9):1347–1355. doi:10.1038/sj.ijo.0803259
20. Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. doi:10.1172/JCI24335
21. Kitade H, Sawamoto K, Nagashimada M, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61(7):1680–1690. doi:10.2337/db11-1506
22. Catrysse L, van Loo G. Inflammation and the metabolic syndrome: the tissue-specific functions of NF-kappaB. Trends Cell Biol. 2017;27(6):417–429. doi:10.1016/j.tcb.2017.01.006
23. Leon-Pedroza JI, Gonzalez-Tapia LA, Del Olmo-Gil E, Castellanos-Rodriguez D, Escobedo G, Gonzalez-Chavez A. [Low-grade systemic inflammation and the development of metabolic diseases: from the molecular evidence to the clinical practice]. Cir Cir. 2015;83(6):543–551. Spanish. doi:10.1016/j.circir.2015.05.041
24. Gehart H, Kumpf S, Ittner A, Ricci R. MAPK signalling in cellular metabolism: stress or wellness? EMBO Rep. 2010;11(11):834–840. doi:10.1038/embor.2010.160
25. Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi:10.1128/MMBR.00031-10
26. Bertheuil N, Chaput B, Menard C, et al. Adipose mesenchymal stromal cells: definition, immunomodulatory properties, mechanical isolation and interest for plastic surgery. Ann Chir Plast Esthet. 2019;64(1):1–10. doi:10.1016/j.anplas.2018.07.005
27. Sorisky A, Molgat AS, Gagnon A. Macrophage-induced adipose tissue dysfunction and the preadipocyte: should I stay (and differentiate) or should I go? Advan Nutri. 2013;4(1):67–75. doi:10.3945/an.112.003020
28. Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne). 2016;7:30. doi:10.3389/fendo.2016.00030
29. Slusher AL, McAllister MJ, Huang CJ. A therapeutic role for vitamin D on obesity-associated inflammation and weight-loss intervention. Inflam Res. 2015;64(8):565–575. doi:10.1007/s00011-015-0847-4
30. Leyssens C, Verlinden L, Verstuyf A. The future of vitamin D analogs. Front Physiol. 2014;5:122. doi:10.3389/fphys.2014.00122
31. Toell A, Gonzalez MM, Ruf D, Steinmeyer A, Ishizuka S, Carlberg C. Different molecular mechanisms of vitamin D(3) receptor antagonists. Mol Pharmacol. 2001;59(6):1478–1485. doi:10.1124/mol.59.6.1478
32. Nijenhuis T, van der Eerden BC, Zugel U, et al. The novel vitamin D analog ZK191784 as an intestine-specific vitamin D antagonist. FASEB J. 2006;20(12):2171–2173. doi:10.1096/fj.05-5155fje
33. van der Eerden BC, Fratzl-Zelman N, Nijenhuis T, et al. The vitamin D analog ZK191784 normalizes decreased bone matrix mineralization in mice lacking the calcium channel TRPV5. J Cell Physiol. 2013;228(2):402–407. doi:10.1002/jcp.24144
34. Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol. 2012;157(3):291–298. doi:10.1111/j.1365-2141.2012.09074.x
35. Boraschi D, Italiani P, Weil S, Martin MU. The family of the interleukin-1 receptors. Immunol Rev. 2018;281(1):197–232.
36. Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22(11):557–566. doi:10.1016/j.tcb.2012.08.001
37. Hong S, Song W, Zushin PH, et al. Phosphorylation of Beta-3 adrenergic receptor at serine 247 by ERK MAP kinase drives lipolysis in obese adipocytes. Mol Metab. 2018;12:25–38. doi:10.1016/j.molmet.2018.03.012
38. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–604. doi:10.3109/10799893.2015.1030412
39. Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. doi:10.1042/BJ20100323
40. Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8(3):1031–1063.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




