Back to Journals » Journal of Blood Medicine » Volume 10
Thalassemia and hepatocellular carcinoma: links and risks
Authors Marsella M, Ricchi P
Received 29 June 2019
Accepted for publication 22 August 2019
Published 17 September 2019 Volume 2019:10 Pages 323—334
DOI https://doi.org/10.2147/JBM.S186362
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Maria Marsella,1 Paolo Ricchi2
1Department of Woman and Child, Pediatric Unit, San Giuseppe Moscati Hospital, Avellino, Italy; 2Unità Operativa Semplice Dipartimentale (UOSD) Malattie Rare Del Globulo Rosso, Dipartimento di oncoematologia, Azienda Ospedaliera Di Rilievo Nazionale “A. Cardarelli”, Napoli, Italy
Correspondence: Paolo Ricchi
Unità Operativa Semplice Dipartimentale (UOSD), Malattie Rare Del Globulo Rosso, Dipartimento di Oncoematologia, Azienda Ospedaliera Di Rilievo Nazionale “A. Cardarelli”, Via A. Cardarelli 9, Napoli 80131, Italy
Tel +39 081 747 2256
Fax +39 081 747 2250
Email [email protected]
Abstract: The increased survival and lifespan of thalassemia patients, in the setting of better iron overload monitoring and chelation, have also however increased the incidence of diseases and complications, which were less likely to develop. Among these, one of the most worrying in recent years is hepatocellular carcinoma (HCC). Due to blood transfusions, many patients with thalassemia are or have been infected with hepatitis C virus (HCV) or hepatitis B virus (HBV), especially those born before the 1990s or in countries in which universal HBV vaccination and safe blood programs are still not completely implemented. However, HCC has also been described in nontransfused patients and in those who are HCV- and HBV-negative. Therefore, other risk factors are involved in hepatocarcinogenesis in thalassemia. The following review analyzes recent literature on the role of different risk factors in the progression of liver disease in thalassemia as well as the importance of surveillance. Treatment of HCC in thalassemia is still highly debated and requires further studies.
Keywords: hepatocellular carcinoma, thalassemia, risk factor
Introduction
Thalassemias are genetic anemias which result from the reduced synthesis of one or more of the globin subunits of normal hemoglobins. This results in an imbalanced alpha/beta-globin chain ratio, ineffective erythropoiesis and reduced red blood cell survival, which lead to variable degrees of chronic anemia and transfusion requirement. Currently, thalassemia care providers classify patients as transfusion-dependent thalassemia (TDT) or nontransfusion-dependent thalassemia (NTDT) based on the established role of transfusion therapy in defining phenotypes.1 In patients with TDT, peripheral hemolysis and ineffective erythropoiesis are less prominent than in NTDT, because of the requirement of lifelong regular transfusions for survival; therefore, they are prone to have all complications linked to this practice, such as iron overload, virus transmitted diseases and organ damage like heart failure, cirrhosis and various endocrinopathies.2
In patients with NTDT, the level of anemia is lower than that observed in TDT; on the contrary, ineffective and expanded erythropoiesis are higher and this not only determines complications specific to NTDT but also contributes to decreased hepcidin levels and increased iron absorption from the gut, with high liver iron accumulation.3,4 These conditions together with the occasional need for transfusions to ameliorate anemia increase the probability of developing further complications, including endocrinopathies. Thus, NTDT and TDT patients, despite having specific clinical complication profiles, share in any case variable degrees of iron overload and, due to regular or occasional blood transfusions, are often infected with either hepatitis C virus (HCV) or hepatitis B virus (HBV).
Until 2000, mortality was mainly linked to high prevalence and incidence of cardiac disease, which caused reduced survival and high morbidity.5 Heart damage ranged from the presence of pulmonary hypertension to severe and decompensated left cardiomyopathy and ventricular or supraventricular arrhythmias, respectively, in patients with NTDT and TDT. Because of the wider and more correct use of chelating agents, including combination therapy,6,7 together with MRI organ iron overload assessment, individuals with severe heart iron overload are less frequently encountered.8 On the contrary, current data show that most transfusion-dependent thalassemia patients have normal cardiac iron, but also a significant proportion of them still have liver iron overload with concomitant liver fibrosis.9 The increased lifespan of patients with thalassemia could in long term reveal complications requiring multistep, multifactorial and long-acting processes such as cancer.
Based on current data, patients with thalassemia have several established risk factors for the development of hepatocarcinoma (HCC), which include liver iron overload and high prevalence of viral hepatitis with or without cirrhosis. But, recent evidence suggest the presence of other factors, previously less documented and explored, potentially involved in the pathogenesis of HCC and probably further increasing the incidence among patients with thalassemia.
HCC incidence
Since 2000, in the general population, the incidence of HCC not only is on the rise but is also expected to further increase in most countries.10 The incidence of HCC rises progressively with advancing age showing a higher prevalence among males.11 In Europe, in 2012, the estimated incidence rate was 10.0 in men and 3.3 in women per 100,000, respectively.12 Obviously, the incidence is higher when considering populations at higher risk, because of the presence of the most important causes of chronic liver disease, such HBV and HCV, and therefore is highest in Africa and Asia, approaching almost 80/100,000.11
The first case of HCC in thalassemia was reported in 1986 in a 22-year-old man with transfusional iron overload. At postmortem autopsy, liver iron concentration was 50 times normal. The patient resulted in HBV-negative, but at the time HCV was not identifiable.13 Recently, the incidence of HCC has been accurately reviewed by Taher and Moukadder and Finianos et al.14,15 Starting from their analysis, until 2002, only two cases of HCC in thalassemia major patients were reported by the literature,13,16 but by 2018, around 93 cases of HCC in TDT or NTDT have been described.17–26 The majority of HCC cases are from the survey of Borgna-Pignatti who, collecting data from most of the Italian centers in 2004 and in 2014, described 22 and later 60 new cases, respectively (Figure 1).17,19 In the last survey, the evaluation of the total number of the screened patients allowed extrapolation of a cumulative incidence. Thirty-two cases out of 4248 patients with TDT and 28 cases out of 1607 patients with NTDT, which corresponded to a cumulative incidence of 1.02% and 1.74%, respectively. Such a high number of NTDT patients affected by HCC was timely confirmed also by further case reports or mini-series.20–22,26,27 Therefore, despite no data available on specific thalassemic genotype involved in hepatocarcinogenesis, it could be concluded that NTDT patients seem to be at a higher risk of developing HCC. Similarly, Mancuso et al in a prospective study based on ultrasound screening of 105 adults with TDT found a 2% incidence of HCC during a 1-year observation period, an incidence comparable to that observed among HCV positive subjects. In Italy, HCC incidence results higher in thalassemia than in the general population, in which it affects 3.5 women and 12.8 men out of 100.000 each year.28
Interestingly, in thalassemia, the incidence among females and males is comparable, while in the general population the greater prevalence of HCC among males is sustained by the higher incidence of cirrhosis, higher levels of smoking and alcohol intake. Experimental findings in animals also highlight the role of male sex hormones and/or hormone receptors in HCC development.29 It is worth to note that in the series by Borgna-Pignatti et al, 55% of the patients were hypogonadic and it is plausible that this may account to limit the differences in incidence between genders. Furthermore, compared to the general population, HCC appears at a younger age in thalassemia patients, suggesting the presence of specific, but also multiple, risk factors for this population.
The role of HCV infection
Globally, HCV-related liver diseases account for approximately 399,000 deaths per year.30 HCV infection is correlated with the development of cirrhosis, HCC and other liver complications, especially if left untreated. Patients with hemoglobinopathies, transfused before screening of blood donors was introduced in 1992, are at higher risk of bloodborne HCV infection. In the early 1990s, immediately after HCV screening became available, the prevalence of chronic HCV infection among patients with thalassemia was reported to range from 4.4% in Turkey to 85.4% in Italy.31 In the last 20 years, the prevalence has progressively decreased, but remains higher than the general population. The most frequent HCV genotype identified in Italian patients is 1b, with a prevalence exceeding 60%.32 Overall, 88% of thalassemia patients with HCC are infected with HCV (either as HCV Ab or HCV RNA). In the initial Italian registry by Borgna-Pignatti, 86% of the 22 patients with HCC and thalassemia, were HCV+; similarly, in the updated data, 87% of the 62 patients with HCC were HCV+ (Figure 1).17,19 It has also been demonstrated that in thalassemic patients, HCC can occur even in the absence of significant cirrhosis.33
The role of HCV in the pathogenesis of HCC is complex. HCV-mediated carcinogenesis is both directly related to the virus through molecular mechanisms and indirectly due to HCV complications like fibrosis and cirrhosis. The HCV core protein can modulate several signaling pathways involved in cell cycle regulation, cell growth promotion, cell proliferation, apoptosis, oxidative stress and lipid metabolism. The dysregulation of specific pathways such as TGF-β, vascular endothelial growth factor, Wnt/β-catenin, cyclooxygenase-2 and peroxisome proliferator-activated receptor α, by the HCV core protein is implicated in the development of HCC. In particular, HCV induces the production of TGF- β, which activates hepatic stellate cells that are responsible for fibrosis.30,34
In patients with chronic HCV hepatitis, iron overload potentiates and accelerates the progression of fibrosis.35,36 In HCV-positive patients, liver iron is often increased, due to a direct negative effect of the virus on hepcidin expression.37 A Japanese study demonstrated that patients with chronic hepatitis C and moderate/severe liver iron overload who underwent weekly phlebotomy until they reached mild iron deficiency were significantly less likely to progress to HCC compared to a control group (odds ratio 0.57; P=0.0337).38
Of course, prevention of bloodborne infections is the key to significantly reduce HCC in thalassemia patients. However, for those who have already contracted the disease, effective chelation therapy and treatment of the HCV infection are needed to prevent liver complications and reduce morbidity and mortality.
The role of HBV infection
HBV is the most common cause of HCC worldwide, accounting for 54% of the cases, with global mortality rates in 2010 estimated in 786,000 deaths (341,400 deaths for HCC and 312,400 for cirrhosis).39 The role of HBV in hepatocarcinogenesis was first reported in the 1970s when chronic HBV infection was associated with an increased incidence of HCC in the general population.40 Currently, the incidence is highest in endemic hepatitis B areas, such as East Asia and sub-Saharan Africa, with 85% of the total number of cases, while in most Western countries the incidence is much lower, and most cases are secondary to HCV and more recently nonalcoholic fatty liver disease. HBV-related HCC is theoretically a preventable disease, as proven by the reduction of cases with the introduction of HBV vaccination in children in some endemic countries.41
Annual occurrence rates of HCC in patients with HBV-related cirrhosis are about 2.3%.42 Risk factors for the development of HCC in these patients are higher HBV DNA levels, HBeAg positivity, higher HBV surface antigen levels, HBV genotype C, basal core promoter mutations, older age, male gender, chronic active hepatitis, higher ALT levels and higher alfa-fetoprotein (aFP). Anti-HBV treatment with nucleoside analogs has proven to decrease the occurrence of HCC in patients with HBV-related cirrhosis.43 Coinfection with HCV, along with other risk factors (alcohol abuse, nonalcoholic fatty liver disease, iron overload, other underlying liver diseases), further increases the risk of developing HCC in HBsAg-positive patients with liver cirrhosis.
The pathogenetic mechanisms underlying HBV carcinogenesis appear complex and the following processes have been demonstrated in playing a role: HBV genome integration, chronic inflammation, epigenetic mechanisms, host immune response and cytokine production promoted by the HBV core protein.44
HBV infection is also reported in thalassemia patients. Worldwide, 0.3%–5.7% of thalassemia patients are hepatitis B surface antigen (HBsAg)-positive.39 In particular, according to the updated Italian registry, 5% of the patients were HBsAg-positive and 58% had evidence of past HBV infection.17 The pathogenesis of chronic hepatitis B in hepatic carcinogenesis in patients with thalassemia is still unclear. In the first Italian study, Borgna-Pignatti et al reported two HCC patients with HBs Ag+, one with associated HCV RNA+ and the other with HVC AB+, but negative HCV RNA status. Both patients had significant iron overload at diagnosis.19 To date, there seem to be no reported cases in the literature of HCC in HBV-infected thalassemia patients in the absence of HCV-coinfection or significant iron overload.14
Current treatment guidelines for patients with chronic hepatitis B in the general population include the use of interferon and oral nucleoside/nucleotide analogs. However, there are no data in the literature regarding treatment in thalassemia patients with HBV infection. Therefore, the role of these agents in preventing or reducing the risk of HCC in thalassemia requires investigation.
Advances in serology and viral nucleic acid testing (NAT) of blood donors have significantly reduced the risk of transfusion-transmitted hepatitis B. However, a specific residual risk remains associated with extremely low viral DNA levels in blood donors with occult HBV infection (OBI) that are intermittently or not detectable by the current available assays. OBI is a clinical form of hepatitis B, defined as the presence of replication competent HBV DNA in the liver and/or blood of subjects testing negative for HBsAg. Patients who undergo regular transfusion therapy remain therefore at risk of transmission. Studies have shown that OBI can be related to liver disease including HCC, fibrosis and cirrhosis.45,46
The prevalence of OBI varies greatly worldwide and among specific patient populations, being higher in Asia. Studies conducted among blood donors demonstrated the presence of HBV DNA in 0%–4.6% of HBsAg-negative and anti-HBc positive subjects, with or without anti-HBs, with a median prevalence of 1.0%.47–54 In an Italian study, HBV DNA was detected in 16% of the subjects with normal liver histology who underwent abdominal surgery from 2002 to 2006.45 Worldwide the incidence of transfusion-related transmission of HBV from OBI donors might be underestimated. The role of OBI in the progression toward cirrhosis and HCC in patients with chronic liver disease due to other causes is still debated. Further molecular and onco-pathogenetic studies are required. It is, however, important to keep in mind that patients with OBI can experience reactivation of HBV replication if receiving cancer chemotherapy or other immunosuppressive therapies. Currently, antiviral therapy is not recommended for persons with OBI.55
The role of iron overload
Iron overload is clearly implicated in the development of HCC. The incidence of HCC in patients with hereditary hemochromatosis (HH) is around 5–10%, increasing to 18% in the presence of cirrhosis. The risk of carcinogenesis in patients with HH and African dietary iron overload syndrome is from 10 to 200 times higher compared to the general population.56,57
Several studies have reported cases of HCC in NTDT patients with high iron overload. In the Italian registry update, 4 of the 62 patients with HCC had no evidence of exposure to HBV and HCV and were all affected by thalassemia intermedia. In three of these, ferritin levels at the time of diagnosis were higher than in other thalassemia intermedia patients.17,19,26 In a Greek study, HCC was reported in 3 of 9 patients with thalassemia intermedia. All patients had liver fibrosis and hepatic iron overload and had received iron chelation late in life. None had a history of viral hepatitis.20 A study from Lebanon reported 2 additional cases in thalassemia intermedia patients negative for previous HBV and HCV infection.21
Iron certainly plays a role in the development of HCC through the acceleration of fibrosis to cirrhosis. However, both animal and human in vitro studies have demonstrated that excess iron has direct and indirect effects on liver carcinogenesis through complex and multifactorial mechanisms, even in the absence of cirrhosis.58–63 In NTDT, ineffective erythropoiesis and hypoxia increase expression of growth differentiation factor 15 and hypoxia-inducible transcription factors, which downregulate hepcidin. This results in increased iron absorption from the gut and recycling of iron from the reticuloendothelial system, leading to preferential hepatocyte iron loading.64,65 Oxidative stress is known to play an important role in carcinogenesis. Iron overload has been proven to favor the formation of reactive oxygen species (ROS) through the Fenton reaction, causing lipid peroxidation and subsequent formation of toxic byproducts that impair protein synthesis and disrupt DNA, leading to mutations in tumor suppressor genes (such as p53) and DNA repair genes.21,56,57
Furthermore, it has been suggested that iron overload also interferes with immune surveillance. There is growing evidence that iron and ferritin have a negative effect on the tumoricidal function of macrophages in mice, decreasing antibody-mediated and mitogen-stimulated phagocytosis.66,67 Iron determines inhibition of lymphocyte proliferation, alteration of T lymphocyte subsets and modification of lymphocyte distribution in different compartments of the immune system. In vitro studies found that non-transferrin bound iron inhibits CD4 lymphocytes. This may explain the presence of low CD4 to CD8 ratio seen in thalassemic and genetic hemochromatosis patients described in some series. The consequent unbalance of immunoregulation may favor tumor cell growth.15,56,67–71
Delayed chelation and iron overload monitoring, along with poor compliance, are major issues in NTDT patients, who are at higher risk for developing HCC even in the absence of hepatitis infection or cirrhosis. Recent NTDT management guidelines recommend initiation of chelation therapy in patients with either ferritin levels higher than 800 ng/L or liver iron concentration (LIC) above 5 mg/g dw. However, serum ferritin has been shown to underestimate iron overload in NDTD, with an irregular correlation between ferritin level and LIC, as measured by R2 MRI or superconducting quantum imaging device. Therefore, a decision-making algorithm has also been developed to aid iron monitoring and initiation of chelation therapy.2,72
The potential role of nonalcoholic fatty liver (NAFL) as part of the metabolic syndrome and diabetes mellitus (DM)
Recently, using liver ultrasound imaging and deranged aminotransferase levels, a never assessed before prevalence of liver steatosis 35.5% was found in a cohort of patients with NTDT. It is worth to note that only 4.5% of the patients of the cohort were HCV-RNA-positive.73 A previous study has directly linked liver steatosis to HCV infection as shown by its wide presence (about 50%) in liver specimens taken from subjects affected by chronic hepatitis.74 However, the study had several limitations. First of all, the diagnosis was only ultrasound-based. Furthermore, liver steatosis in non-alcoholic fatty liver disease is considered to be a hepatic manifestation of the metabolic syndrome, the evaluation of which was limited to BMI assessment. In fact, hepatic steatosis or “fatty liver” is a trait for pathologic conditions ranging from NAFL to nonalcoholic steatohepatitis (NASH) as part of the metabolic syndrome, whose prevalence was not assessed in the study. Overall, these findings, despite unexpected, were not surprising, because in the general population of Western countries, an increasing prevalence of liver steatosis, obesity, diabetes and metabolic syndrome has been detected and is becoming the leading cause of chronic liver disease. In most of the patients with NAFLD/NASH where HCC develops, there is no histological evidence of cirrhosis.75
NAFLD represents a new emerging risk factor for the onset of liver and extra-hepatic carcinogenesis, characterized by impaired production of adipocytokines which, in turn, favor an increase in pro-inflammatory cytokines, further contributing to the microenvironment favorable to the development of cancer.76 Data from the Surveillance, Epidemiology and End Results-Medicare linked database between 2004 and 2009 showed that NAFLD represented the third most common cause of HCC, after hepatitis C and alcohol-related disease diagnosed in 14.1% of the patients with HCC.77 However, to date, the prevalence of NAFLD in the general population is increasing especially in developed countries. At the same time, the prevalence of NAFLD has increased in patients with HCC, with several studies showing that steatosis at present has surpassed HCV and HBV as the first cause of HCC, ranging from 25% to 35% of all cases.78
Interestingly, in the general population with hyperferritinemia, the coexistence of hepatic iron and fat is common. Fat plays an interactive and aggressive role in the progression of diseases (fibrosis, cirrhosis and hepatocellular carcinomas) to the point that new studies and methodologies have been recently assessed to quantify liver fat and liver iron content by measurement of confounder-corrected proton density fat fraction and R2.79 Thus, further data are needed to correctly determine the prevalence of NAFLD and metabolic syndrome among wider cohorts of NTDT and TDT patients, particularly in Western countries. The assessment of the presence of fat in the liver should be extensively performed during the radiologic evaluation of liver iron overload.
While currently the presence of liver fat is unpredictably associated with iron in the liver and with metabolic syndrome, TDT and NTDT patients, as a direct consequence of iron overload, frequently suffer from endocrinopathies and DM whose prevalence has been historically more accurately quantified. Studies from different cohorts report a prevalence of DM in thalassemia which varies from 4.9 to 20%.19,80–84 However, again there are possible complex interactions between risk factors, which should be discussed. In fact, it is worth to note also that HCV infection may induce insulin resistance by a direct effect on insulin signaling pathways and may cause a direct cytopathic effect at the islet cell level, reducing insulin release.84 On the other hand, epidemiologic and pathogenetic data seem to suggest the existence of a direct relationship between type 2 diabetes mellitus (T2DM) with cancer risk, being also irrespective of chronic viral hepatitis and hemochromatosis. Patients with a history of T2DM at baseline had a hazard ratio of 2.14 (95% confidence interval, 1.69–2.71) to develop HCC with respect to those without.85 Biological mechanisms linking T2DM and HCC are complex and difficult to elucidate, but given the previous recognized close inter-play between T2DM, obesity and NAFLD, the consequent activation of a pro-inflammatory and pro-oxidant status is again potentially implicated in the development and progression of HCC.86 It is interesting to note that in the 2014 HCC update by Borgna, the incidence of DM was 31%. Unfortunately, the diabetic status prevalence was not reported by other major series available in the literature.17
The treatment
To date, very little evidence has been published to support specific treatment guidelines for thalassemia patients with HCC. The management is mainly based on data extrapolated from experience in the general population.87–96 Several treatment options have been reported to be safe and effective: surgical resection, chemoembolization, simultaneous percutaneous radioablation and ethanol injection.23,24,33,89 In the Italian survey by Borgna, 22 patients were treated with chemoembolization alone, 13 with thermoablation with radiofrequency, 16 underwent surgical resection, preceded or followed by chemoembolization or thermoablation, 3 patients underwent liver transplant, 18 received palliative therapy and 3 cases kinase inhibitor, sorafenib. Two of the transplanted patients died for causes independent of thalassemia (cirrhotic liver failure and meningococcal sepsis).17,83 Sorafenib has proven to significantly improve the prognosis of early-stage HCC.89,90 However, its role in thalassemia patients requires further studies, as it produced unclear outcomes in the 3 Italian patients reported by Borgna.17,26
Unfortunately, there are little data available on liver transplantation in thalassemia, as only a few patients have undergone this procedure.83 Outcomes from published cases show promising results.17,26,33 In an Italian study, survival of the only transplanted patient was 69 months, whereas the survival of the 8 untransplanted patients was 25.25±23.65 months (range, 3–64 months).26 Two other patients from a different Italian study underwent successful liver transplantation with satisfactory outcomes in both cases after 6 months and 2 years of follow-up evaluations, respectively.33 There has been only one report of combined liver and heart transplantation for end-stage iron-induced cardiac and liver failure in an adult with thalassemia major. At a 2-year follow-up, the patient’s hepatic and cardiac functions were normal.97 For years, thalassemia has been considered a contraindication to liver transplantation, mainly due to cardiac comorbidities.83,98,99 However, given the encouraging results reported above, this issue should be reconsidered. Today, thalassemia patients survive longer with fewer cardiac comorbidities, thanks to adequate chelation therapy and iron overload monitoring. Selected patients, without significant heart disease or severe pulmonary hypertension, should be considered eligible for liver transplantation.
To date, the best choice of treatment remains controversial, because the evidence is mainly based on a series of case reports. Considering the rarity of the condition, multicenter studies are recommended.
Surveillance and prevention
HCC is a growing cause of premature morbidity and mortality among patients with thalassemia. As discussed before, current data suggest the presence of multiple and long-acting risk factors, in the development of HCC in thalassemia patients. One could hypothesize that the longer is the exposure and the more numerous are the players in game, the higher is the risk of HCC occurrence. Generally, those who develop HCC show no signs of illness for a long time after exposure; it can take several years or more for symptoms to appear. On the other hand, we cannot assume that cancer risk disappears as soon as the carcinogenetic stimulus is removed. The ideal intervention (primary prevention) to reduce HCC occurrence should be the early correction or elimination of all risk factors. The lack of tools or biomarkers able to stratify the risk of HCC in the differently exposed population suggests that all patients with presence and/or history of exposure to one or more risk factors (HCV infection, HBV infection, liver iron overload, etc) should be monitored carefully and started to the HCC screening early.100 It should also be considered that, while HCV infection is probably the main and more frequently observed risk factor and that cirrhosis is generally a pre-cancerous stage, several cases of HCC have been reported in patients with NTDT with a non-viral and non-cirrhotic liver. For these reasons, surveillance for the tumor is strongly needed probably in most thalassemia patients in the presence of just one risk factor even in the absence of cirrhosis. These patients should follow the same recommendations currently used for the general population affected by cirrhosis or HCV chronic hepatitis, with an abdominal ultrasound performed every 6 months.96 The American Association for the Study of Liver Diseases recommends using abdominal ultrasound for surveillance of patients with cirrhosis without aFP, because this biomarker lacks adequate sensitivity and specificity, as confirmed by the updated study of Borgna Pignatti et al, where aFP levels were normal in 44% of the patients.17,101 By contrast, a recent meta-analysis reports that addition of aFP to abdominal ultrasound significantly increases the sensitivity of early-stage HCC in clinical practice.102
Concerning liver iron overload and the associated risk, there are no studies addressing this condition specifically. In all patients, LIC should be evaluated using modern, noninvasive and currently widely available T2* or R2* MRI methodology. As per consequence of the seminal study of Musallam, in never chelated patients, probably a cutoff of 5 mg Fe/g dry weight could be considered to start screening patients for HCC and this evaluation should be carried out yearly, as suggested.14,103 However, it should be considered that probably there is no safe level of iron, especially considering long-term exposure.104 Therefore, also individuals with a history of previous moderate-to-severe liver iron overload, who are currently well chelated, will need to be strictly followed by an abdominal ultrasound to screen the long-term health consequences of their previous exposure. Obviously, in the current management of thalassemia, any attempt should be carried out to maintain long-term low levels of hepatic iron, but avoiding the risk of overchelation. Iron chelation and iron monitoring in thalassemia patients, especially if HCV- or HBV-positive, appear to be crucial in order to prevent the development of HCC.
The role of iron-induced oxidative stress in carcinogenesis has been thoroughly described in literature. More recently, treatment with combinations of antioxidants has been proposed to reduce and/or neutralize the effects of labile iron and ROS (vitamin E, polyphenols, selenium, zinc, N-acetylcysteine, etc).105 However, the potential anti-carcinogenetic effects in thalassemia require further studies.
More unpredictable and less quantified is the risk linked to liver steatosis, DM and metabolic syndrome. A part the still unmet need to accurately diagnose these morbidities in thalassemia, to what extent, these factors alone or in combination with those previously described could represent a productive ground for liver cancerogenesis, even in the absence of cirrhosis, should yet be accurately assessed. However, particularly in Western countries, it is reasonable to recommend, as for the general population, the prevention of obesity and its related complications encompassing DM, assuming that the benefit will be similar.
Given that the advent of donor blood screening with NAT technology has dramatically reduced the new cases of transfusion-transmitted hepatitis B or C, by analogy with the general population, any effort should be made to remove any existing HCV infection. Most of our cohorts of thalassemic patients were infected with HCV before the 1990s. Chronic infection with HCV remains one of the major and well-recognized risk factors for developing HCC. Older regimens for the treatment of HCV infection, based on interferon alone or in combination with ribavirin, achieved limited response rates (sustained virological response (SVR) rates of 25–64% in thalassemia) and were associated with morbidity, adverse drug–drug interactions and hemolysis with consequent iron overload.3,6,15,73,106–115 Therefore, in patients with thalassemia, the 2016 European Association for the Study of the Liver guidelines recommend interferon‐free regimens for the treatment of HCV infection. Direct-acting antiviral agents (DAAs) have demonstrated to be safe and effective in patients with chronic HCV infection.32,116–119 To date, data on DAAs are limited in patients with hemoglobinopathies. However, in a large multicenter observational study, these agents demonstrated to be safe and effective in patients with hemoglobinopathies and cirrhosis/chronic hepatitis due to HCV. Of the 139 patients included in the study, 136 patients (97.8%) achieved a response at end of treatment, and 130 (93.5%) achieved a SVR with no significant treatment-related adverse events reported.32 In a more recent study, in 99 patients from one large thalassemia center in Italy, the final SVR was 97% (96/99) in the intention-to-treat analysis. At the time of publication, the mean follow-up observation period was 668 days (range 87–1159 days) from starting on DAAs, and none of the patients had developed HCC.120
Several studies in the general population have shown that the risk of HCC is significantly lower in patients who achieved SVR following antiviral treatment compared to untreated patients.30,121–123 However, in literature, cases of HCC are still reported in patients who have become HCV negative after treatment with different regimens (peg-IFN alone or peg-IFN interferon plus RBV or DAAs).124 The eradication of the HCV virus may extremely improve the quality of life of the adult individual with thalassemia, but the advantage in terms of reduction of liver complications, HCC occurrence and overall survival requires long-term observation. In fact, among the general population, there has been a large debate whether the achievement of SVR may have a positive impact in HCC incidence or not.
Therefore, definite conclusions cannot be currently made and longer follow-up periods will be necessary to understand the effects of anti-HCV treatment on the incidence of HCC in thalassemia patients. While treatment with DAAs has proven to be effective in obtaining SVR, it has been hypothesized that this treatment alone is not sufficient to block the progression of liver disease in the context of inflammation, oxidative stress, severe liver injury and metabolic dysregulation. Patients with advanced fibrosis, who achieve SVR after DAA therapy, are still at a long-term risk for progression to liver cirrhosis and HCC and therefore should continue to receive routine HCC surveillance and screening. These considerations could be particularly reasonable for patients with thalassemia, where other risk factors are frequently concomitant.125
Recently, anti-inflammatory/hepatoprotective drugs have been proposed in combination or after DAA treatment, to reduce liver disease progression and HCV recurrence risk.126
Conclusion
Apart from case reports or series with limited numbers of patients, data on hepatic carcinogenesis and HCC incidence in patients with thalassemia are poor and related to a few registers or collections. To better understand its development and pathogenesis, new updates and larger studies from different populations are mandatory. HCC, as most cancers, occurs as a result of a multistep process requiring the accumulation of numerous genetic lesions, frequently caused by several actors that may operate synergistically. Patients with thalassemia are/have been frequently affected by one or more well-recognized predisposing conditions to liver carcinogenesis since a young age (ie, HCV and/or HBV infection, iron overload, or advanced cirrhosis). Less specific, but probably of not secondary importance, is the contribution of the increased prevalence of type 2 diabetes, consequence of HCV infection, iron overload or the obesity pandemic, which seems to have not spared thalassemia patients (Figure 2).
In this population, given the increase in age, we are recording a parallel rise in the HCC occurrence, as expected. In the absence of studies that could help to exactly stratify individual risk, any action should be performed to remove as soon as possible all discussed risk factors. Conventional ultrasound surveillance of cirrhotic and non-cirrhotic patients should be a priority in the management of patients with at least one HCC risk factor. Further studies are needed to find biomarkers that could help to exactly stratify the individual risk and tailor screening programs and treatment.
 |
Figure 1 Number of cases of HCC associated with HBV infection (HBsAg-positive or evidence of past infection), HCV infection and in absence of viral infections in thalassemia patients in the update by Borgna-Pignatti et al.17 |
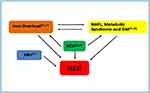 |
Figure 2 Complex interplay between risk factors for HCC in thalassemia patients. |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vitrano A, Calvaruso G, Lai E, et al. The era of comparable life expectancy between thalassaemia major and intermedia: is it time to revisit the major-intermedia dichotomy? Br J Haematol. 2017;176(1):124–130. doi:10.1111/bjh.14381
2. Taher AT, Mussalam K, Cappellini MD. Guidelines for the Management of Non Transfusion Dependent Editors of the 2nd Edition. TIF; 2018.
3. Ricchi P, Ammirabile M, Costantini S, et al. Splenectomy is a risk factor for developing hyperuricemia and nephrolithiasis in patients with thalassemia intermedia: a retrospective study. Blood Cells Mol Dis. 2012;49(3–4):133–135. doi:10.1016/j.bcmd.2012.05.012
4. Ricchi P, Ammirabile M, Costantini S, et al. A useful relationship between the presence of extramedullary erythropoeisis and the level of the soluble form of the transferrin receptor in a large cohort of adult patients with thalassemia intermedia: a prospective study. Ann Hematol. 2012;91(6):905–909. doi:10.1007/s00277-011-1385-y
5. Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10(1):1–8. doi:10.1186/1532-429X-10-42
6. Ricchi P, Ammirabile M, Spasiano A, et al. Combined chelation therapy in thalassemia major with deferiprone and desferrioxamine: a retrospective study. Eur J Haematol. 2010;85(1):36–42.
7. Pepe A, Meloni A, Rossi G, et al. Cardiac and hepatic iron and ejection fraction in thalassemia major: multicentre prospective comparison of combined deferiprone and deferoxamine therapy against deferiprone or deferoxamine monotherapy. J Cardiovasc Magn Reson. 2013;15(1):1–11. doi:10.1186/1532-429X-15-1
8. Dessi C, Leoni G, Moi P, et al. Thalassemia major between liver and heart: where we are now. Blood Cells Mol Dis. 2015;55(1):82–88.
9. Aydinok Y, Porter JB, Piga A, et al. Prevalence and distribution of iron overload in patients with transfusion-dependent anemias differs across geographic regions: results from the CORDELIA study. Eur J Haematol. 2015;95(3):244–253.
10. Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34(15):1787–1794. doi:10.1200/JCO.2015.64.7412
11. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
12. Globocan. Liver fact sheet. Available from: http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf.
13. Borgna-Pignatti C, De Stefano P, Sessa F, Avato F. Hepatocellular carcinoma in thalassemia major. Med Pediatr Oncol. 1986;14(6):327–328.
14. Moukhadder HM, Halawi R, Cappellini MD, Taher AT. Hepatocellular carcinoma as an emerging morbidity in the thalassemia syndromes: a comprehensive review. Cancer. 2017;123(5):751–758. doi:10.1002/cncr.30462
15. Finianos A, Matar CF, Taher A. Hepatocellular carcinoma in β-thalassemia patients : review of the literature with molecular insight into liver carcinogenesis. Int J Mol Sci. 2018;19(12):4070. doi:10.3390/ijms19124070.
16. Zurlo MG, De Stefano P, Borgna-Pignatti C, et al. Survival and causes of death in thalassaemia major. Lancet (London, England). 1989;2(8653):27–30. doi:10.1016/s0140-6736(89)90264-x
17. Borgna-Pignatti C, Garani MC, Forni GL, et al. Hepatocellular carcinoma in thalassaemia: an update of the Italian registry. Br J Haematol. 2014;167(1):121–126. doi:10.1111/bjh.13009
18. Ansari S, Azarkivan A, Halagi F. Incidence of hepatocellular carcinoma in patients with thalassemia who had hepatitis C. Acta Med Iran. 2013;51(6):404–407.
19. Borgna-Pignatti C, Vergine G, Lombardo T, et al. Hepatocellular carcinoma in the thalassaemia syndromes. Br J Haematol. 2004;124(1):114–117. doi:10.1046/j.1365-2141.2003.04732.x
20. Fragatou S, Tsourveloudis I, Manesis G. Incidence of hepatocellular carcinoma in a thalassemia unit. Hemoglobin. 2010;34(3):221–226. doi:10.3109/03630269.2010.485071
21. Maakaron JE, Cappellini MD, Graziadei G, Ayache JB, Taher AT. Hepatocellular Carcinoma in Hepatitis-Negative Patients with Thalassemia Intermedia: A Closer Look at the Ann Hepatol. 2013;12(1):142–146.
22. Maakaron JE, Musallam KM, Ayache JB, Jabbour M, Tawil AN, Taher AT. A liver mass in an iron-overloaded thalassaemia intermedia patient. Br J Haematol. 2013;161(1):1. doi:10.1111/bjh.12195
23. Mancuso A, Rigano P, Renda D, et al. Hepatocellular carcinoma on cirrhosis-free liver in a HCV-infected thalassemic. Am J Hematol. 2005;78(2):158–159. doi:10.1002/ajh.20289
24. Mancuso A, Sciarrino E, Renda MC, Maggio A. A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin. 2006;30(1):119–124. doi:10.1080/03630260500455565
25. Moukhadder HM, Roumi JE, Bou-Fakhredin R, Taher AT. Hepatocellular carcinoma in a beta-thalassemia intermedia patient: yet another case in the expanding epidemic. Hemoglobin. 2018;42(1):58–60. doi:10.1080/03630269.2018.1434197
26. Restivo PG, Renda D, Valenza F, et al. Hepatocellular carcinoma in patients with thalassaemia syndromes: clinical characteristics and outcome in a long term single centre experience. Br J Haematol. 2010;150:226–248. doi:10.1111/j.1365-2141.2010.08179.x
27. Musallam KM, Cappellini MD, Wood JC, Taher AT. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev. 2012;26(Suppl 1):S16–S19. doi:10.1016/S0268-960X(12)70006-1
28. Globocan. Estimated age-standardized incidence and mortality rates for liver cancer (Southern Europe) in 2018, all ages. Available from: http://gco.iarc.fr/today/online-analysis-dual-bars-2. Published 2018.
29. Leong TY-M, Leong AS-Y. Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB (Oxford). 2005;7(1):5–15. doi:10.1080/13651820410024021
30. Mahmoudvand S, Shokri S, Taherkhani R, Farshadpour F. Hepatitis c virus core protein modulates several signaling pathways involved in hepatocellular carcinoma. World J Gastroenterol. 2019;25(1):42–58. doi:10.3748/wjg.v25.i1.42
31. Wonke B, Hoffbrand AV, Brown D, Dusheiko G. Antibody to hepatitis C virus in multiply transfused patients with thalassaemia major. J Clin Pathol. 1990;43(8):638–640. doi:10.1136/jcp.43.8.638
32. Origa R, Ponti ML, Filosa A, et al. Treatment of hepatitis C virus infection with direct-acting antiviral drugs is safe and effective in patients with hemoglobinopathies. Am J Hematol. 2017;92(12):1349–1355. doi:10.1002/ajh.24911
33. Mancuso A. Hepatocellular carcinoma in thalassemia: a critical review. World J Hepatol. 2010;2(5):171–174. doi:10.4254/wjh.v2.i5.171
34. Schulze-Krebs A, Preimel D, Popov Y, et al. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129(1):246–258. doi:10.1053/j.gastro.2005.03.089
35. Angelucci E, Muretto P, Nicolucci A, et al. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood. 2002;100(1):17–21. doi:10.1182/blood.v100.1.17
36. Zou DM, Sun WL. Relationship between hepatitis C virus infection and iron overload. Chin Med J (Engl). 2017;130(7):866–871. doi:10.4103/0366-6999.202737
37. Girelli D, Pasino M, Goodnough JB, et al. Reduced serum hepcidin levels in patients with chronic hepatitis C. J Hepatol. 2009;51(5):845–852. doi:10.1016/j.jhep.2009.06.027
38. Kato J, Miyanishi K, Kobune M, et al. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42(10):830–836. doi:10.1007/s00535-007-2095-z
39. Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int J Mol Sci. 2019;20(6):1358. doi:10.3390/ijms20061358
40. Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69.
41. Petruzziello A. Epidemiology of hepatitis B virus (HBV) and hepatitis C virus (HCV) related hepatocellular carcinoma. Open Virol J. 2018;12(1):26–32. doi:10.2174/1874357901812010026
42. Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21(3):650–655.
43. Tawada A, Kanda T, Imazeki F, Yokosuka O. Prevention of hepatitis B virus-associated liver diseases by antiviral therapy. Hepatol Int. 2016;10(4):574–593. doi:10.1007/s12072-016-9720-y
44. Kanda T, Wu S, Sasaki R, et al. HBV core protein enhances cytokine production. Dis (Basel, Switzerland). 2015;3(3):213–220.
45. Raimondo G, Navarra G, Mondello S, et al. Occult hepatitis B virus in liver tissue of individuals without hepatic disease. J Hepatol. 2008;48(5):743–746. doi:10.1016/j.jhep.2008.01.023
46. Siagris D, Christofidou M, Triga K, et al. Occult hepatitis B virus infection in hemodialysis patients with chronic HCV infection. J Nephrol. 2006;19(3):327–333.
47. Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. 2008;48(5):1001–1026. doi:10.1111/j.1537-2995.2008.01701.x
48. Spreafico M, Berzuini A, Foglieni B, et al. Poor efficacy of nucleic acid testing in identifying occult HBV infection and consequences for safety of blood supply in Italy. J Hepatol. 2015;63(5):1068–1076. doi:10.1016/j.jhep.2015.06.016
49. Mortensen E, Kamali A, Schirmer PL, et al. Are current screening protocols for chronic hepatitis B virus infection adequate? Diagn Microbiol Infect Dis. 2016;85(2):159–167. doi:10.1016/j.diagmicrobio.2015.12.005
50. Cholongitas E, Haidich A-B, Apostolidou-Kiouti F, Chalevas P, Papatheodoridis GV. Hepatitis B virus reactivation in HBsAg-negative, anti-HBc-positive patients receiving immunosuppressive therapy: a systematic review. Ann Gastroenterol. 2018;31(4):480–490. doi:10.20524/aog.2018.0266
51. Di Stefano M, Volpe A, Stallone G, et al. Occult HBV infection in hemodialysis setting is marked by presence of isolated antibodies to HBcAg and HCV. J Nephrol. 2009;22(3):381–386.
52. Hassan ZK, Hafez MM, Mansor TM, Zekri ARN. Occult HBV infection among Egyptian hepatocellular carcinoma patients. Virol J. 2011;8:90. doi:10.1186/1743-422X-8-90
53. Liu C-J, Chen D-S, Chen P-J. Epidemiology of HBV infection in Asian blood donors: emphasis on occult HBV infection and the role of NAT. J Clin Virol. 2006;36(Suppl 1):S33–S44.
54. Candotti D, Grabarczyk P, Ghiazza P, et al. Characterization of occult hepatitis B virus from blood donors carrying genotype A2 or genotype D strains. J Hepatol. 2008;49(4):537–547. doi:10.1016/j.jhep.2008.04.017
55. Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS. Update of the statements on biology and clinical impact of occult hepatitis b virus infection. J Hepatol. 2019. doi:10.1016/j.jhep.2019.03.034
56. Mandishona E, MacPhail AP, Gordeuk VR, et al. Dietary iron overload as a risk factor for hepatocellular carcinoma in Black Africans. Hepatology. 1998;27(6):1563–1566. doi:10.1002/hep.510270614
57. Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S79–S86. doi:10.1016/j.gastro.2004.09.019
58. Blanc JF, De Ledinghen V, Bernard PH, et al. Increased incidence of HFE C282Y mutations in patients with iron overload and hepatocellular carcinoma developed in non-cirrhotic liver. J Hepatol. 2000;32(5):805–811.
59. Bralet MP, Regimbeau JM, Pineau P, et al. Hepatocellular carcinoma occurring in nonfibrotic liver: epidemiologic and histopathologic analysis of 80 French cases. Hepatology. 2000;32(2):200–204. doi:10.1053/jhep.2000.9033
60. Herrinton LJ, Friedman GD, Baer D, Selby JV. Transferrin saturation and risk of cancer. Am J Epidemiol. 1995;142(7):692–698.
61. Ioannou GN, Kowdley KV. Iron, HFE mutations, and hepatocellular carcinoma: is hepatic iron a carcinogen? Clin Gastroenterol Hepatol. 2003;1(4):246–248. doi:10.1016/S1542-3565(03)00126-5
62. Moyo VM, Makunike R, Gangaidzo IT, et al. African iron overload and hepatocellular carcinoma (HA-7-0-080). Eur J Haematol. 1998;60(1):28–34.
63. Turlin B, Juguet F, Moirand R, et al. Increased liver iron stores in patients with hepatocellular carcinoma developed on a noncirrhotic liver. Hepatology. 1995;22(2):446–450.
64. Taher A, Hershko C, Cappellini MD. Iron overload in thalassaemia intermedia: reassessment of iron chelation strategies. Br J Haematol. 2009;147(5):634–640. doi:10.1111/j.1365-2141.2009.07848.x
65. Nemeth E. Hepcidin in beta-thalassemia. Ann N Y Acad Sci. 2010;1202:31–35. doi:10.1111/j.1749-6632.2010.05585.x
66. Matzner Y, Hershko C, Polliack A, Konijn AM, Izak G. Suppressive effect of ferritin on in vitro lymphocyte function. Br J Haematol. 1979;42(3):345–353. doi:10.1111/j.1365-2141.1979.tb01142.x
67. Walker EMJ, Walker SM. Effects of iron overload on the immune system. Ann Clin Lab Sci. 2000;30(4):354–365.
68. Green R, Esparza I, Schreiber R. Iron inhibits the nonspecific tumoricidal activity of macrophages. A possible contributory mechanism for neoplasia in hemochromatosis. Ann N Y Acad Sci. 1988;526:301–309. doi:10.1111/j.1749-6632.1988.tb55514.x
69. Djeha A, Brock JH. Effect of transferrin, lactoferrin and chelated iron on human T-lymphocytes. Br J Haematol. 1992;80(2):235–241. doi:10.1111/j.1365-2141.1992.tb08906.x
70. Deugnier Y, Turlin B. Iron and hepatocellular carcinoma. J Gastroenterol Hepatol. 2001;16(5):491–494.
71. Seligman PA, Kovar J, Gelfand EW. Lymphocyte proliferation is controlled by both iron availability and regulation of iron uptake pathways. Pathobiology. 1992;60(1):19–26. doi:10.1159/000163692
72. Taher AT, Porter JB, Viprakasit V, et al. Defining serum ferritin thresholds to predict clinically relevant liver iron concentrations for guiding deferasirox therapy when MRI is unavailable in patients with non-transfusion-dependent thalassaemia. Br J Haematol. 2015;168(2):284–290.
73. Ricchi P, Meloni A, Spasiano A, et al. The impact of liver steatosis on the ability of serum ferritin levels to be predictive of liver iron concentration in non-transfusion-dependent thalassaemia patients. Br J Haematol. 2018;180(5):721–726.
74. Clouston AD, Powell EE. Interaction of non-alcoholic fatty liver disease with other liver diseases. Best Pract Res Clin Gastroenterol. 2002;16(5):767–781.
75. Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49(3):851–859.
76. Divella R, Mazzocca A, Daniele A, Sabba C, Obesity PA. Nonalcoholic fatty liver disease and adipocytokines network in promotion of cancer. Int J Biol Sci. 2019;15(3):610–616.
77. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–1730.
78. Puoti C. New insights on hepatocellular carcinoma: epidemiology and clinical aspects. Hepatoma Res. 2018;4(9):57.
79. Lin H, Fu C, Kannengiesser S, et al. Quantitative analysis of hepatic iron in patients suspected of coexisting iron overload and steatosis using multi-echo single-voxel magnetic resonance spectroscopy: Comparison with fat-saturated multi-echo gradient echo sequence. J Magn Reson Imaging. 2018;48(1):205–213. doi:10.1002/jmri.25967
80. Gamberini MR, De Sanctis V, Gilli G. Hypogonadism, diabetes mellitus, hypothyroidism, hypoparathyroidism: incidence and prevalence related to iron overload and chelation therapy in patients with thalassaemia major followed from 1980 to 2007 in the Ferrara Centre. Pediatr Endocrinol Rev. 2008;6(Suppl 1):158–169.
81. Multicentre study on prevalence of endocrine complications in thalassaemia major. Italian working group on endocrine complications in non-endocrine diseases. Clin Endocrinol (Oxf). 1995;42(6):581–586.
82. De Sanctis V, Roos M, Gasser T, Fortini M, Raiola G, Galati MC. Impact of long-term iron chelation therapy on growth and endocrine functions in thalassaemia. J Pediatr Endocrinol Metab. 2006;19(4):471–480.
83. Mancuso A, Perricone G. Time to define a new strategy for management of hepatocellular carcinoma in thalassaemia? Br J Haematol. 2015;168(2):304–305.
84. Borgna-Pignatti C, Gamberini MR. Complications of thalassemia major and their treatment. Expert Rev Hematol. 2011;4(3):353–366.
85. Koh W-P, Wang R, Jin A, Yu MC, J-M Y. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese health study. Br J Cancer. 2013;108(5):1182–1188.
86. Mantovani A, Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. 2017;5(13):270.
87. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236.
88. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917.
89. Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15(26):3210–3216.
90. Poon D, Anderson BO, Chen L-T, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10(11):1111–1118.
91. Verslype C, Van Cutsem E, Dicato M, et al. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 10th world congress on gastrointestinal cancer, Barcelona, 2008. Ann Oncol Off J Eur Soc Med Oncol. 2009;20(Suppl 7):vii1–vii6.
92. El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134(6):1752–1763.
93. Tanwar S, Khan SA, Grover VPB, Gwilt C, Smith B, Brown A. Liver transplantation for hepatocellular carcinoma. World J Gastroenterol. 2009;15(44):5511–5516.
94. Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–S37.
95. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the study of the liver. J Hepatol. 2001;35(3):421–430.
96. Llovet JM, Ducreux M, Lencioni R, et al. EASL-EORTC Clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48(5):599–641.
97. Olivieri NF, Liu PP, Sher GD, et al. Brief report: combined liver and heart transplantation for end-stage iron-induced organ failure in an adult with homozygous beta-thalassemia. N Engl J Med. 1994;330(16):1125–1127.
98. Mela M, Mancuso A, Burroughs AK. Review article: hepatocellular carcinoma: indications for liver transplantation. Aliment Pharmacol Ther. 2003;17(Suppl 2):130–137.
99. Menon KV, Hakeem AR, Heaton ND. Review article: liver transplantation for hepatocellular carcinoma - a critical appraisal of the current worldwide listing criteria. Aliment Pharmacol Ther. 2014;40(8):893–902.
100. Ricchi P, Meloni A, Di Matola T, et al. Galectin-3 and myocardial fibrosis by cardiac magnetic resonance in thalassaemia major. Blood. 2016;128(22):4832LP- 4832.
101. Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54(6):1987–1997. doi:10.1002/hep.24545
102. Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1. doi:10.1053/j.gastro.2018.01.064
103. Musallam KM, Cappellini MD, Wood JC, et al. Elevated liver iron concentration is a marker of increased morbidity in patients with beta thalassemia intermedia. Haematologica. 2011;96(11):1605–1612. doi:10.3324/haematol.2011.047852
104. Berdoukas V, Farmaki K, Wood JC, Coates T. Iron chelation in thalassemia: time to reconsider our comfort zones. Expert Rev Hematol. 2011;4(1):17–26. doi:10.1586/ehm.10.74
105. Fibach E, Rachmilewitz EA. The role of antioxidants and iron chelators in the treatment of oxidative stress in thalassemia. Ann N Y Acad Sci. 2010;1202:10–16. doi:10.1111/j.1749-6632.2010.05577.x
106. Di Marco V, Capra M, Angelucci E, et al. Management of chronic viral hepatitis in patients with thalassemia: recommendations from an international panel. Blood. 2010;116(16):2875–2883. doi:10.1182/blood-2009-11-248724
107. Di Marco V, Capra M, Gagliardotto F, et al. Liver disease in chelated transfusion-dependent thalassemics: the role of iron overload and chronic hepatitis C. Haematologica. 2008;93(8):1243–1246. doi:10.3324/haematol.12554
108. Hershko C. Pathogenesis and management of iron toxicity in thalassemia. Ann N Y Acad Sci. 2010;1202:1–9. doi:10.1111/j.1749-6632.2010.05544.x
109. EASL. Recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66(1):153–194. doi:10.1016/j.jhep.2016.09.001.
110. Harmatz P, Jonas MM, Kwiatkowski JL, et al. Safety and efficacy of pegylated interferon alpha-2a and ribavirin for the treatment of hepatitis C in patients with thalassemia. Haematologica. 2008;93(8):1247–1251. doi:10.3324/haematol.12352
111. Inati A, Taher A, Ghorra S, et al. Efficacy and tolerability of peginterferon alpha-2a with or without ribavirin in thalassaemia major patients with chronic hepatitis C virus infection. Br J Haematol. 2005;130(4):644–646. doi:10.1111/j.1365-2141.2005.05645.x
112. Di Marco V, D’Ambrosio R, Bronte F, et al. Dual therapy with peg-interferon and ribavirin in thalassemia major patients with chronic HCV infection: is there still an indication? Dig Liver Dis. 2016;48(6):650–655. doi:10.1016/j.dld.2016.02.004
113. Kamal SM, Fouly AH, Mohamed MK, et al. Peginterferon alpha-2b therapy with and without ribavirin in patients with thalassemia: a randomized study. J Hepatol. 2006;44:S217. doi:10.1016/j.jhep.2005.09.019
114. Ricchi P, Ammirabile M, Costantini S, et al. The impact of previous or concomitant IFN therapy on deferiprone-induced agranulocytosis and neutropenia: a retrospective study. Expert Opin Drug Saf. 2010;9(6):875–881. doi:10.1517/14740338.2010.510831
115. Ricchi P, Lanza AG, Ammirabile M, et al. Hepatitis C virus distribution and clearance following interferon-monotherapy among thalassaemia major and intermedia patients. Br J Haematol. 2011;155(4):524–527. doi:10.1111/j.1365-2141.2011.08717.x
116. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi:10.1056/NEJMoa1402355
117. Prati D, Maggioni M, Milani S, et al. Clinical and histological characterization of liver disease in patients with transfusion-dependent beta-thalassemia. A multicenter study of 117 cases. Haematologica. 2004;89(10):1179–1186.
118. Sulkowski MS, Jacobson IM, Nelson DR. Daclatasvir plus sofosbuvir for HCV infection. N Engl J Med. 2014;370(16):1560–1561. doi:10.1056/NEJMc1401726
119. Sharara AI, Rustom LBO, Marrache M, et al. Sofosbuvir/velpatasvir for chronic hepatitis C infection in patients with transfusion-dependent thalassemia. Am J Hematol. 2019;94(2):E43–E45. doi:10.1002/ajh.25339
120. Ponti ML, Comitini F, Murgia D, et al. Impact of the direct-acting antiviral agents (DAAs) on chronic hepatitis C in Sardinian patients with transfusion-dependent Thalassemia major. Dig Liver Dis. 2019;51(4):561–567. doi:10.1016/j.dld.2018.12.016
121. Aleman S, Rahbin N, Weiland O, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis. 2013;57(2):230–236.
122. van der Meer AJ, Feld JJ, Hofer H, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66(3):485–493. doi:10.1016/j.jhep.2016.10.017
123. El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in veterans with hepatitis C virus infection. Hepatology. 2016;64(1):130–137. doi:10.1002/hep.28535
124. Makiyama A, Itoh Y, Kasahara A, et al. Characteristics of patients with chronic hepatitis C who develop hepatocellular carcinoma after a sustained response to interferon therapy. Cancer. 2004;101(7):1616–1622. doi:10.1002/cncr.20537
125. Su F, Ioannou GN. Hepatocellular carcinoma risk after direct‐acting antiviral therapy. Clin Liver Dis. 2019;13(1):6–12. doi:10.1002/cld.781
126. Li H, Huang M-H, Jiang J-D, Peng Z-G, Hepatitis C. From inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J Gastroenterol. 2018;24(47):5297–5311. doi:10.3748/wjg.v24.i47.5297
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
