Back to Journals » Journal of Experimental Pharmacology » Volume 14
Testicular Toxicity of Chloroxylenol in Rats: Biochemical, Pathological and Flow Cytometric Study
Authors El-Naggar DA, El-Zalabany LMA, Shahin DA, Attia AM, El-Mosallamy SA
Received 15 January 2022
Accepted for publication 27 June 2022
Published 13 July 2022 Volume 2022:14 Pages 213—220
DOI https://doi.org/10.2147/JEP.S358571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Paola Rogliani
Doaa Abdallah El-Naggar,1 Laila Mohammed Ahmad El-Zalabany,1 Doaa Abdelhalim Shahin,2 Afaf Mahmoud Attia,1 Shaaban Abdelfattah El-Mosallamy1
1Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Mansoura University, Dakahliya, Egypt; 2Department of Clinical Pathology, Faculty of Medicine, Mansoura University, Dakahliya, Egypt
Correspondence: Doaa Abdallah El-Naggar, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Mansoura University, Tel +20 1063433525, Dakahliya, Egypt, Email [email protected]
Background: Chloroxylenol (para-chloro-meta-xylenol, PCMX) is claimed to be highly harmful both to humans and the environment. Toxic effects of PCMX on testicular functions are scarcely discussed in the literature.
Aim of Study: To study testicular toxic effects of PCMX on male Sprague-Dawley rats.
Materials and Methods: Forty animals were randomly distributed into three groups: negative control (G I), vehicle group (G II) and PCMX group (G III). PCMX group was subdivided into three subgroups: GIIIa: received PCMX 100 mg/kg, GIIIb: received PCMX 200 mg/kg and G IIIc: received PCMX 500 mg/kg. Hormonal assay included assessment of serum testosterone and estradiol levels. Histopathological examination of testicular tissue, analysis of cellular viability, necrosis and apoptosis in testicular tissue by flow cytometry, analysis of cellular DNA content and phases of cell cycle analysis by flow cytometry were also performed.
Results: Rats in the groups exposed to PCMX (G IIIa, G IIIb and G IIIc) had significantly lower estradiol and testosterone levels in comparison to control groups (G I and GII). Histopathological examination of testicular tissue of PCMX-exposed rats showed irregular crossly sectioned seminiferous tubules with their lumina containing scanty spermatids and spermatozoa. G IIIc animals showed eosinophilic proteinaceous material and vacuolated and necrotic interstitial cells of Leydig. Rats in PCMX-exposed groups (G IIIa, G IIIb and G IIIc) showed significantly lower testicular tissue viability in comparison to control groups (G I and G II). Rats in PCMX-exposed groups (G IIIa, G IIIb and G IIIc) showed significantly lower percentage of cells in the G0/G1 phase in comparison to control groups (G I and G II).
Conclusion: Rats exposed to PCMX had significant reduction in testosterone and estradiol levels with marked histopathological alterations affecting testicular tissues. These effects are dose-dependent.
Keywords: chloroxylenol toxicity, testicular toxicity, cell cycle, semen analysis
Introduction
Chloroxylenol (para-chloro-meta-xylenol, PCMX) is one of the oldest antimicrobial chemicals. It is a halogenated phenolic compound largely utilized as an antiseptic. It is also used as a preservative in some cosmetic products and medications.1 The United States Food and Drug Administration (FDA) reopened the organizational record on some currently used antimicrobial agents for over-the-counter human use including PCMX and recommended that further safety data are required to support the extensive use of these chemicals.2
Data on the spectrum of PCMX toxicity are inconsistent. Some studies assessing health risks of PCMX-containing products indicated a lack of genotoxicity and insignificant systemic toxicity.1,3 Other studies, however, found that PCMX could alter cellular blood components and produce oxidative DNA damage after repeated exposure.4,5
Testicular toxicity is a major cause of infertility. Various factors including, hormonal, environmental, behavioral and nutritional imbalances are responsible for testicular toxicity.6 In vivo studies suggested that PCMX has too weak hormonal activity on the androgenic receptors. However, these studies are claimed to be insufficient to assess its potential reproductive and developmental toxicity to mammals.7 Further research on PCMX toxicity was strongly recommended.8
The aim of the current study is to study the toxic effects of PCMX on the testicular functions of male Sprague-Dawley rats.
Materials and Methods
Animals and Treatments
The current study was conducted on a total of 40 adult male 150–200 grams Sprague-Dawley rats. The study protocol was approved by the Ethical committee, Faculty of Medicine, Mansoura University (code MD.19.05.30). For experimental animals’ welfare, we respected the Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research of the American Psychological Association.
Considering the exploratory nature of the study, we used the “resource equation” approach for sample size calculation. All animals were housed under general standard circumstances with a 12 h light dark cycle with food and water supply. Rats were fed on standard pellet animal diet (El-Gomhorya Company, Egypt). Each pellet contained carbohydrates, protein and fat in ratios of 67.0%, 23.0% and 10.0%, respectively. Animals’ weight gain and food consumption were weekly monitored.
Rats were haphazardly distributed into three subgroups:
- Group I (GI) (n = 8): Negative control group (untreated rats).
- Group II (GII) (n = 8): Vehicle group: rats were treated with oral corn oil (cat. no. 8001–30-7, Sigma-Aldrich, Saint Louis, USA) for 8 weeks.
- Group III (GIII) (n = 24): PCMX group: rats within this group were divided into three subgroups (n = 8/subgroup):
- GIIIa: Rats received PCMX (cat. no. 88–04-0, Sigma-Aldrich, Saint Louis, USA) dissolved in corn oil by oral ingestion at a dosage of 100 mg/kg daily for 8 weeks.
- GIIIb: Rats received PCMX dissolved in corn oil by oral ingestion at a dosage of 200 mg/kg daily for 8 weeks.
- GIIIc: Rats received PCMX dissolved in corn oil by oral ingestion at a dosage of 500 mg/kg daily for 8 weeks.
Before sacrificing, rats were anesthetized through an intraperitoneal injection of 0.5 mL sodium thiopental (Eipico, Egypt). Blood was collected via orbital puncture. Cauda epididymis and testes were excised following standard approved procedures for histopathology and flow cytometry.
Hormonal Assay
Serum testosterone9 and estrogen levels10 were determined using iFlash 1800 chemiluminescence immunoassay analyzer (YHLO, Chennai, India) after twenty-four hours from the last dose.
Semen Analysis11
The cauda epididymis of one testis was removed and placed in 2 mL of sodium chloride 0.9% solution in a sterilized Petri dish at 37°C. Then, a sterilized scissor was used to get the epididymal contents out into the solution to form a suspension. The sperm motility was examined under high power 40x of light microscope. The sperm count was performed under a light microscope at 100x. The number of sperms in 1 mL of the fluid was obtained using the following formula:
Mean number of sperm in each chamber × 10,000 × dilution factor
Morphological changes were examined under a microscope with a magnification of 400. About one hundred spermatozoa were randomly observed under oil immersion lens in several fields to measure the percentage of abnormal spermatozoa.11
Histopathological Examination
Serial paraffin sections (5 µm) of right testis were sliced and set for hematoxylin and eosin (H & E) staining for examination under a light microscope.12
Analysis of Cellular Viability, Necrosis and Apoptosis in Testicular Tissue by Flow Cytometry
The left testicle was left in a glass Petri dish containing phosphate-buffered saline (PBS). The tunica albuginea was excised and the de-capsulated testis was chopped into very tiny pieces that were processed in a Medi-machine system (Dako, Italy) for automated tissue dissection. Tissue lumps were removed through a 50 μm sterilized mesh filter. After that, the outcome was centrifuged at 300 g for 5 minutes to obtain the pellet.13
Cells were washed two times with cold PBS and suspended in 1X Binding Buffer (BD Biosciences, New Jersey, USA). One hundred µL of the solution were moved to a sterile tube. Five µL of annexin V (BD Biosciences, New Jersey, USA) and 5 µL Propidium Iodide (BD Biosciences, New Jersey, USA) were added. Then, cells were carefully mixed by utilizing vortex, incubated for 15 minutes at 25°C in dark room and 1X Binding Buffer (200 µL) was superadded. Using flow cytometry, cells were assessed for viability, necrosis and apoptosis within one hour.14
Analysis of Cellular DNA Content and Phases of Cell Cycle Analysis by Flow Cytometry15
Ten mL of 1X ammonium chloride lyse was added to 1 mL of heparinized blood in a conical centrifuge tube and incubated for 15 min at 25°C. Then, the sample was centrifuged at 300–400 g for 5 minutes at room temperature and the supernatant was discarded.
A drop of cold 70% ethyl alcohol was added to the pellet and vortexing was done to confirm fixation and reduce clattering. The solution was then incubated at 4°C for thirty minutes. The sample was centrifuged at 850 g and the supernatant was discarded. Fifty µL of ribonuclease (100µg/mL stock) were added to make sure that only DNA is stained and 200 µL propidium iodide (50 µg/mL stock solution) was also added. Using a flow cytometer, analysis of cellular DNA content and cellular phases was done.15
Statistical Analysis
Data were analyzed by utilizing SPSS Version 22.0 (IBM, Armonk, USA). Quantitative data were expressed as mean and standard deviation (SD) and compared using one-way ANOVA with post-hoc Tukey’s test. P value <0.05 was considered statistically significant.
Results
Hormonal Assay
Rats in the groups exposed to PCMX (G IIIa, G IIIb and G IIIc) had significantly lower estradiol and testosterone levels in comparison to control groups (G I and GII). No significant differences were noted between the PCMX-exposed groups regarding estradiol levels (Table 1).
 |
Table 1 Hormonal Blood Levels in Studied Groups |
Semen Analysis
Rats in the PCMX-exposed groups (G IIIa, G IIIb and G IIIc) had significantly worse sperm count, motility and abnormal morphology when compared with control groups (G I and GII). Moreover, G IIIa animals showed significantly better sperm count, motility and abnormal morphology when compared with G IIIb and G IIIc animals. In addition, G IIIb animals had significantly better sperm motility and abnormal morphology when compared with G IIIc animals (Table-2).
 |
Table 2 Sperm Analysis Among Different Studied Groups |
Histopathological Examination
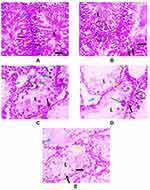
Histopathological examination of testicular tissue of PCMX-exposed rats showed irregular crossly sectioned seminiferous tubules with their lumina containing scanty spermatids and spermatozoa. Moreover, the number of spermatocytes lining the tubules was decreased with increasing dose. Also, several spermatocytic giant cells were observed and the interstitial space increased in a dose-dependent manner. G IIIc animals showed eosinophilic proteinaceous material and vacuolated and necrotic interstitial cells of Leydig (Figure 1).
Analysis of Testicular Tissue by Flow Cytometry
Figure 2 shows that rats in PCMX-exposed groups (G IIIa, G IIIb and G IIIc) showed significantly lower testicular tissue viability in comparison to control groups (G I and G II). Also, animals in PCMX-exposed groups showed significantly marked testicular tissue necrosis in comparison to controls. Moreover, G IIIc animals demonstrated significantly higher early and late apoptosis when compared with control groups.
Cell Cycle Analysis by Flow Cytometry
Figure 3 and Table 3 demonstrate the percentage of mononuclear cells in different cell cycle phases in the peripheral blood. Rats in PCMX-exposed groups (G IIIa, G IIIb and G IIIc) showed significantly lower percentage of cells in the G0/G1 phase in comparison to control groups (G I and G II). Moreover, G IIIb and G IIIc rats showed significantly higher percentage of cells in the sub-G1 phase in comparison to control groups.
 |
Table 3 Comparison Between Cell Cycle Phases in the Mononuclear Cells in Peripheral Blood of Different Tested Groups of Rats |
Discussion
Previous animal and human studies have shown that oral, dermal or inhalation exposure to PCMX can be associated with serious health hazards.3 Its effects range from enhancement of oxidative stress to carcinogenic effects.16 However, testicular toxicity of PCMX is a rarely discussed issue.17
In the current study, we utilized the previously declared concentrations of PCMX to simulate the non-observed-adverse-effect level (NOAEL) which was suggested to be 100 mg/kg per day.1
In our experiment, rats exposed to PCMX had significantly lower estradiol and testosterone levels in comparison to controls. To the best of our knowledge, these effects were not previously reported. However, the endocrine disrupting effects of other chlorophenols (CPs) on testosterone and estradiol functions were identified by experimental and human studies.18–21 Being a chlorinated phenol, it is logic for PCMX to induce the hormonal changes detected in the present work.
Moreover, the present study noted that rats in the PCMX-exposed groups had significantly worse sperm count, motility and abnormal morphology when compared with control groups. These detrimental properties of PCMX appeared to be dose dependent. Again, this novel finding was not previously known for PCMX but for other CPs.21–23 The depressed levels of androgens noted in this study can affect physiological development of sperm resulting in reduced sperm parameters.24
In the present work, testicular tissue of rats exposed to PCMX (100 and 200 mg/kg) revealed altered structure, lining and contents of the seminiferous tubules. Moreover, specimens from higher exposure animals (500 mg/kg) showed eosinophilic proteinaceous material and the interstitial cells of Leydig were vacuolated and necrotic. These findings can also be explained by the effects of PCMX on androgen levels. It was documented that changes in testosterone level or decrease in the activity of the androgenic receptors could alter the histological structure of testicles, epididymis as well as mammary glands. This can occur due to a central depression of GnRH, direct depression of Leydig cell function or through increased elimination of testosterone. The morphologic changes usually depend on the severity of exposure, the period of follow-up, as well as the species under study.25
While PCMX-related testicular changes were not previously recognized, previous studies reported that PCMX could induce histopathological changes affecting gills, spleen, liver and kidneys. These changes included hyperplasia, degeneration, necrosis and nuclear pyknosis.4
In order to demonstrate the effects of PCMX on cellular viability and death, analysis of the testicular tissue with annexin V and propidium iodide (PI) was conducted in the present study. It was observed that the viable cells (Annexin V as well as PI negative) were significantly decreased in PCMX-treated rats in comparison with the controls. These results are supported by the conclusions of Newby et al26 who demonstrated that the intact keratinocytes and dermal fibroblasts in humans exposed to toxic concentrations of PCMX revealed a significant reduction in cellular viability. They attributed these findings to increased release of tumor necrosis factor-alpha and interleukin-8.
In the current work, the groups of rats exposed to PCMX at different concentrations (100, 200 and 500 mg/kg) showed significant reduction in the ratio of the peripheral blood mononuclear cells in the G0/G1 phase, whereas rats exposed to PCMX (200 and 500 mg/kg) showed significant increase in the ratio of the cells in the sub-G1 phase (dead cells).
In agreement with our results, Capkin et al,4 who evaluated the genotoxic effect of PCMX on blood cells of the fish, observed a mutagenic effect on blood DNA of rainbow trout on exposure to 4.2 ± 0.9 µg/l of PCMX for nearly 6 weeks with significant DNA damage depending on the comet assay technique.
It was proved that the DNA structure checkpoint mechanisms arrest the cells at the G2/M phase owing to DNA damage and prevent anaphase until whole chromosomes have acquired their normal bipolar attachment.27 However, this arrest did not occur in our study.
Conclusion
Rats exposed to PCMX had significant reduction in testosterone and estradiol levels with marked histopathological alterations affecting testicular tissues. These effects are dose-dependent. Further studies are recommended to confirm these conclusions and to uncover other PCMX-related toxicities.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yost LJ, Rodricks JD, Turnbull D, et al. Human health risk assessment of chloroxylenol in liquid hand soap and dishwashing soap used by consumers and health-care professionals. Regul Toxicol Pharmacol. 2016;80:116–124. doi:10.1016/j.yrtph.2016.06.003
2. Kim S, Rhee M. Microbicidal effects of plain soap vs triclocarban-based antibacterial soap. J Hosp Infect. 2016;94(3):276–280. doi:10.1016/j.jhin.2016.07.010
3. Tan J, Kuang H, Wang C, et al. Human exposure and health risk assessment of an increasingly used antibacterial alternative in personal care products: chloroxylenol. Sci Total Environ. 2021;786:147524. doi:10.1016/j.scitotenv.2021.147524
4. Capkin E, Ozcelep T, Kayis S, Altinok I. Antimicrobial agents, triclosan, chloroxylenol, methylisothiazolinone and borax, used in cleaning had genotoxic and histopathologic effects on rainbow trout. Chemosphere. 2017;182:720–729. doi:10.1016/j.chemosphere.2017.05.093
5. Guo Y, Gao J, Cui Y, et al. Chloroxylenol at environmental concentrations can promote conjugative transfer of antibiotic resistance genes by multiple mechanisms. Sci Total Environ. 2022;816:151599. doi:10.1016/j.scitotenv.2021.151599
6. Dubey S, Shri M, Gupta A, Rani V, Chakrabarty D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ Chem Lett. 2018;16(4):1169–1192.
7. FDA Consumer Antiseptics Rule. DA request for data on safety and efficacy of chloroxylenol prepared for American cleaning institute 1331 L street, NW, suite 650 Washington, DC 20005 prepared by exponent 2595 canyon blvd, suite 440 boulder; 2014.
8. Pernoncini KV, Montagnini BG, de Góes MLM, Garcia PC, Gerardin DCC. Evaluation of reproductive toxicity in rats treated with triclosan. Reprod Toxicol. 2018;75:65–72. doi:10.1016/j.reprotox.2017.11.010
9. Wheeler M. The determination of bio-available testosterone. Ann Clin Biochem. 1995;32(4):345–357. doi:10.1177/000456329503200401
10. Carr B, Bradshaw K. Disorders of the ovary and female reproductive tract. Harrison’s Princ Inter Med. 2005;16(2):2198.
11. Divya S, Madhuri D, Lakshman M, Reddy AG. Epididymal semen analysis in testicular toxicity of doxorubicin in male albino Wistar rats and its amelioration with quercetin; 2018.
12. Hussien NA, Hamdi H. Genotoxic and hypogonadism effect of triclosan treatment and the mitigating effect of vitamin e in male albino mice. J Basic Clin Pha. 2017;8(4):200–204.
13. Rodríguez-Casuriaga R, Folle GA, Santiñaque F, López-Carro B, Geisinger A. Simple and efficient technique for the preparation of testicular cell suspensions. JoVE. 2013;78:e50102.
14. Wlodkowic D, Skommer J, Darzynkiewicz Z. Cytometry in cell necrobiology revisited. Recent advances and new vistas. Cytometry Part A. 2010;77(7):591–606. doi:10.1002/cyto.a.20889
15. Kim KH, Sederstrom JM. Assaying cell cycle status using flow cytometry. Curr Protoc Mol Biol. 2015;111(1):
16. Scibior-Bentkowska D, Czeczot H. Cancer cells and oxidative stress. Postepy Hig Med Dosw. 2009;63:58–72.
17. Sreevidya VS, Lenz KA, Svoboda KR, Ma H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol-Three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ Pollut. 2018;235:814–824. doi:10.1016/j.envpol.2017.12.108
18. Hu Y, Li D, Ma X, et al. Effects of 2,4-dichlorophenol exposure on zebrafish: implications for the sex hormone synthesis. Aquat Toxicol. 2021;236:105868. doi:10.1016/j.aquatox.2021.105868
19. Pollack AZ, Mumford SL, Krall JR, et al. Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive hormones in healthy women: a chemical mixture approach. Environ Int. 2018;120:137–144. doi:10.1016/j.envint.2018.07.028
20. Sun W, Jia Y, Ding X, et al. Combined effects of pentachlorophenol and its byproduct hexachlorobenzene on endocrine and reproduction in zebrafish. Chemosphere. 2019;220:216–226. doi:10.1016/j.chemosphere.2018.12.100
21. Zhang H, Liu W, Chen B, et al. Differences in reproductive toxicity of TBBPA and TCBPA exposure in male Rana nigromaculata. Environ Pollut. 2018;243(Pt A):394–403. doi:10.1016/j.envpol.2018.08.086
22. Gravance CG, Garner DL, Miller MG, Berger T. Flow cytometric assessment of changes in rat sperm mitochondrial function after treatment with pentachlorophenol. Toxicol In Vitro. 2003;17(3):253–257. doi:10.1016/s0887-2333(03)00039-0
23. Zhang X, Kang H, Peng L, et al. Pentachlorophenol inhibits CatSper function to compromise progesterone’s action on human sperm. Chemosphere. 2020;259:127493. doi:10.1016/j.chemosphere.2020.127493
24. Ibtisham F, Nawab A, Zhao Y, Li G, Xiao M, An L. Pharmaceutica Analytica Acta; 2016.
25. Chapin RE, Creasy DM. Assessment of circulating hormones in regulatory toxicity studies II. Male reproductive hormones. Toxicol Pathol. 2012;40(7):1063–1078. doi:10.1177/0192623312443321
26. Newby CS, Barr RM, Greaves MW, Mallet AI. Cytokine release and cytotoxicity in human keratinocytes and fibroblasts induced by phenols and sodium dodecyl sulfate. J Invest Dermatol. 2000;115(2):292–298. doi:10.1046/j.1523-1747.2000.00056.x
27. Chen M, Tang R, Fu G, et al. Association of exposure to phenols and idiopathic male infertility. J Hazard Mater. 2013;250:115–121. doi:10.1016/j.jhazmat.2013.01.061
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.