Back to Journals » Clinical Ophthalmology » Volume 16
Ten-Year Outcomes of LASIK for Pediatric Myopic Anisometropia
Received 24 August 2022
Accepted for publication 6 December 2022
Published 22 December 2022 Volume 2022:16 Pages 4293—4301
DOI https://doi.org/10.2147/OPTH.S387302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Omar Hashem,1 Hosam Sheha2,3
1Cornea and Refractive Department, Research Institute of Ophthalmology, Giza, Cairo, Egypt; 2Department of Ophthalmology, Florida International University, Herbert Wertheim College of Medicine & Glaucoma Research Organization, Miami, FL, USA; 3Department of Ophthalmology, Manhattan Eye, Ear and Throat Hospital, Hofstra Northwell School of Medicine, New York, NY, USA
Correspondence: Omar Hashem, Research Institute of Ophthalmology, 2 Al Ahram Street, Giza, Cairo, Egypt, Tel +201222422032, Email [email protected]
Purpose: To evaluate long-term safety, effectiveness, and stability of unilateral LASIK in pediatric myopic anisometropic amblyopia.
Methods: This retrospective study included children who received unilateral LASIK for myopic anisometropia of > 6 D, after mandatory 6-month occlusion/penalization therapy. They were evaluated at 6 months, 1 year, 2 years and biannually until 10 years. Outcome measures included visual acuity, refraction, ocular alignment, stereopsis, corneal clarity, and corneal topography.
Results: 32 patients (16 girls) with mean age of 8.6 ± 2.3 years completed 10 years of follow up after unilateral LASIK. Mean preoperative spherical equivalent refraction (SER) was − 10.3D ± 2.0D in the affected eye, with anisometropic difference of − 9.5D ± 1.7D. Mean post-LASIK SER was − 1.3D± 0.8D (p< 0.001). Anisometropia significantly decreased to 0.3D± 0.8D, 0.4D± 1.0D, and 1.0± 2.5D at 6 months, 1 year and 10 years respectively (p< 0.001). 11 patients (34%) who had preoperative intermittent exotropia (< 15°) regained orthophoria in all gazes, while 5 of 10 who had constant exotropia with large angle (> 30°) required strabismus surgery for ocular alignment. BCVA improved from 0.04± 0.6 Decimal at baseline to 0.6 ± 0.2 after LASIK and occlusion therapy (p< 0.001). Despite insignificant refractive regression in both eyes, patients have maintained orthophoria, improved stereopsis, clear cornea, and the topography showed no evidence of post-LASIK ectasia.
Conclusion: LASIK appears safe, effective, and stable for correcting refractory pediatric myopic anisometropia, in which conventional measures fail or endanger normal visual development. Eliminating anisometropic aniseikonia consequently restores binocular vision and stereopsis which, along with amblyopia therapy, would reverse amblyopia and prevent recurrence.
Keywords: amblyopia, aniseikonia, LASIK, pediatric myopic anisometropia
Introduction
Anisometropia is a condition in which there is a considerable difference in the refractive power between the two eyes.1 The prevalence of anisometropia increases between the age of 5 and 15 years, when one eye grows shorter or longer than the other, resulting in hyperopic or myopic anisometropia respectively.2–5 Myopic anisometropia of ≥ 4 diopters (D) is a frequent cause of amblyopia due to inequality of the retinal images of both eyes which is known as aniseikonia. When foveal fusion fails to correct aniseikonia, patients suffer from altered binocular vision and decreased stereoacuity.6–12 The defocused image from the amblyopic eye is usually suppressed,6,13,14 and the severity of amblyopia directly correlates with the magnitude of anisometropia.6 Anisometropic amblyopia is frequently associated with sensory strabismus and diplopia that increase the depth of amblyopia and further interfere with education, sports, self-esteem, and future career choice.15–17
The basic treatment of pediatric anisometropic amblyopia relies on correcting the refractive error along with modulated occlusion or penalization of the dominant eye.18 Children may tolerate full binocular glasses correction up to 6 D difference, however, they may not sustain binocular vision due to the associated aniseikonia.19 If they cannot tolerate full binocular glasses correction, contact lenses can be tried with the advantage of larger visual field and better quality of vision.18,20 In the case of contact lense intolerance and poor compliance, refractive surgery should be considered to preserve the visual functions, eliminating aniseikonia, restoring stereopsis and binocular fusion.21,22 Previous studies have demonstrated the short-term and intermediate-term safety and efficacy of refractive surgery in treating pediatric myopic anisometropic amblyopia.23 This includes photorefractive keratectomy (PRK),24–27 laser assisted in situ keratomileusis (LASIK), laser-assisted subepithelial keratectomy (LASEK),28–30 implantation of phakic intraocular lenses,31,32 and refractive lens exchange.33
The purpose of this study was to evaluate the long-term safety, effectiveness, and stability of unilateral LASIK in pediatric refractory myopic anisometropic amblyopia.
Methods
This retrospective study was approved by the Ethical Committee at the Research Institute of Ophthalmology, Cairo, Egypt and was conducted in adherence with the Declaration of Helsinki.
Participants
Children who underwent unilateral LASIK for high myopic refractory anisometropic amblyopia with and without strabismus and completed 10 years of follow up were enrolled in this study. The preoperative baseline difference in spherical equivalent refraction (SER) between eyes was set to ≥ 6.00 D. Before LASIK, all participants received mandatory 6-month occlusion or penalization therapy, and spectacle correction until the linear optotype visual acuity was equal to the single E acuity (crowding phenomenon). Our goal was to achieve emmetropia in all cases within the treatment limit of LASIK as the corneal thickness permits. Otherwise, we aimed to reduce anisometropia to ≤ 2.00 D.
Exclusion criteria included previous intraocular surgery, corneal scarring, active inflammation, pachymetry value less than 480 µm, keratoconus, a Schirmer test of less than 5.0 mm, narrow palpebral fissure, intraocular pressure greater than 21 mmHg, associated posterior segment pathology, and patients who were not able to complete the treatment plan and follow-up.
Preoperative and Postoperative Examinations
Distance visual acuity (uncorrected visual acuity and best corrected visual acuity) (UCVA and BCVA), orthoptic evaluation, stereoscopic vision using the Lang stereo-test I & II, cycloplegic and manifest refraction (retinoscopy optometry and subjective trial of lens), Schirmer test, slit-lamp examination of the anterior segment, palpebral fissure width, fundus examination by indirect ophthalmoscope, tonometry, ultrasound pachymetry, and corneal topography were measured at baseline and each of the follow-up visits. In addition, postoperative corneal haze was graded based on a 5-point scale (with 0 as no haze and 4 as maximal haze). At the last follow-up, Titmus butterfly stereogram and preschool random-dot stereogram were used to measure near stereoacuity while a synoptophore with the Yan′s random-dot stereogram was used to measure far stereoacuity.
Procedure
Parents/legal guardians were given a detailed description of the procedure and its related risk and potential benefits. We further explained the concept of reducing the difference in SER between the two eyes and that our goal was not to just get rid of spectacles. On the contrary, the child may need to wear glasses and maintain occlusion therapy to improve the lazy eye after LASIK. We also emphasized that the procedure would not stop possible post LASIK progression of refractive error. All parents took ample time to read and sign written informed consent stating they understood and approved the treatment plan and the procedure with its potential benefits and risks. We asked all participants who wear contact lenses to stop wearing them at least 2 weeks prior to surgery.
The surgery was performed under general anesthesia in all patients except for one, a 10-year-old girl, who was cooperative and received topical anesthesia.34 General anesthesia was induced by intravenous ketamine hydrochloride (2mg/kg) and atropine (0.01mg/kg) and maintained during the procedure by propofol (Diprivan) infusion drip (6–8mg/kg/hour) together with continuous flow of oxygen. Nidek EC-5000 CX II excimer laser was calibrated and used to perform the procedure. After washing the eye with povidone-iodine 10% solution, the head was draped with sterile towels, and a wire speculum was inserted. To avoid lack of fixation under general anesthesia, centration was achieved by good head positioning with the plane of the iris perpendicular to the laser beam and was maintained by laser eye-tracking during ablation.
The pupillary center was marked with a gentian-violet marker. The Moria M2 microkeratome (Moria, Antony, France) was adjusted to create a 130 µm thick corneal lamellar flap. Suction was activated after being centered on the corneal marking, and the intraocular pressure was confirmed to be above 60 mmHg. The created flap was gently lifted with a blunt spatula and reflected on its hinge. The laser was carefully centered, with the diameter of the optical ablation zone set between 5.5 mm and 6.5 mm according to the desired correction and the predetermined ablation limits. The ablation depth did not exceed 120 µm of the stroma, to leave at least 290 µm of residual stromal bed to yield a total central corneal thickness (CCT) of ≥420 µm. After ablation, the flap was repositioned, and the interface thoroughly washed to remove any cellular debris. The suction ring was gently removed, and the flap was allowed to settle down. A suitable therapeutic contact lens (Acuvue, Johnson & Johnson, Jacksonville, FL, USA) was placed and an overnight eye patch and shield were also applied to guard against rubbing or accidental trauma that could displace the corneal flap.
Postoperative Management
After surgery, antibiotics and steroid eyedrops were administered four times daily for one week, and then steroids were tapered over 1 month. Artificial tears eye drops were also used four times daily for 3 months. Therapeutic contact lens was removed after 1 week. Patients were followed-up and data were recorded at 6 months, 1 year, 2 years and then biannually until 10 years.
Amblyopia therapy was resumed 1 month after surgery by correcting any significant residual error of refraction along with occlusion or penalization of the dominant eye. Occlusion was performed for 4 hours daily and weaned over ~18 months or until BCVA in the amblyopic eye approached that of the dominant eye, ie, within 2 lines difference. At this point the training on the synoptophore was considered to improve binocular vision.
Statistical Analysis
Data were collected and analyzed using the Statistical Program for the Social Sciences (SPSS) version 22. Categorical variables were expressed as numbers and percentages. The quantitative variables were expressed in mean ± standard deviation. Paired t-test was used to compare the quantitative data between the operated and the fellow eyes. Additionally, repeated-measures ANOVA followed by pairwise comparison using Paired t-test P was applied to compare the quantitative data throughout the follow-up times in each of the operated and the fellow eyes. A P-value less than 0.05 was considered significant.
Results
Table 1 summarizes patients’ demographics and baseline parameters. A total of 32 children with high anisometropic amblyopia underwent unilateral LASIK between January 2004 and July 2008 at the Research Institute of Ophthalmology and completed 10 years of follow-up. They were 16 boys and 16 girls with a mean age of 8.7 ± 2.4 years (range 4–12). The mean preoperative SER in the affected eye was −10.3±2.1D versus −0.9±1.1D in the fellow eye (p<0.001), resulting in a mean anisometropic difference of −9.5±1.7D. There was no effect of age or gender on the degree of anisometropia. The mean baseline BCVA in the affected eyes was 0.04±0.6 Decimal versus 0.9±0.1 Decimal in the fellow eyes (p <0.001). Also, 11 children had associated sensory intermittent exotropia (<15°) and 10 had constant sensory exotropia with large angle (>30°).
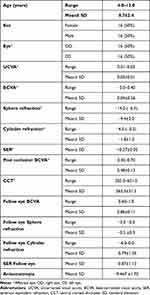 |
Table 1 Patients’ Demographic and Baseline Parameters |
Before LASIK, all children received mandatory 6-month occlusion or penalization therapy in the sound eye, and full spectacle correction of the affected eye. Mean post occlusion BCVA in the affected eyes significantly improved to 0.5±0.1 Decimal (range: 0.3–0.7) (p<0.001). Mean preoperative CCT was 564±31µm (range: 505–621µm) which allowed targeting of emmetropia in the affected eyes during LASIK. The procedures were uneventful in all cases except one (a 7-year-old girl) who had an incomplete corneal flap due to halted microkeratome, and the procedure was successfully performed 6 months later with a deeper flap cut (160 microns) and a larger diameter flap. This child reached a fairly good post LASIK UCVA (0.4), compared to preoperative BCVA of 0.05 Decimal.
Mean post-LASIK SER was markedly reduced from −10.3±2.0D at baseline to −1.3±0.8D (range: −0.3D to −3.5D) at 6 months (p<0.001), resulting in significant decrease in the anisometropic difference from 9.5±1.7D to 0.4±1.2D (p<0.001). Gradual insignificant increase in mean SER, due to myopia regression, occurred in the treated eyes at 1 year (−1.5±.9D), 2 years (−1.8±1.1D), 4 years (−2.20±1.28), 6 years (−2.36±1.67), 8 years (−2.64±1.88), and 10 years (−3.22±1.92). Similar slight increase in SER occurred in the fellow eyes as well, resulting in stable anisometropic difference between eyes as shown in Table 2 and illustrated in Figure 1.
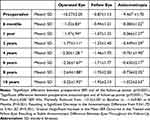 |
Table 2 Spherical Equivalent Refraction and Anisometropia at Baseline and Throughout the Follow-Up. |
After 2 years, mean regression value was −1.58D ± 1.1D (range: −0.7 to −3.2D). However, 12 eyes (38%) remained within ±1.0D and 20 eyes (63%) were within ±2.0D of the fellow eye, achieving the targeted refractive balance between the two eyes. The overall myopic regression was associated with slight changes in the corneal curvature suggesting an increase in the axial length with age. Post-LASIK, children continued the amblyopia therapy and maintained a mean BCVA of 0.6±0.1 Decimal throughout follow-up (Figure 2).
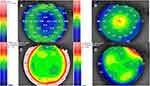
Keratometry values were used to evaluate corneal curvature changes and to determine the corneal cylindrical changes. There were significant differences in mean keratometric values between preoperative and 6 months postoperative (P < 0.05). Overall, mean keratometry was 44.2 D±2.3 preoperatively and 37.3D ± 2.1 at 6 months. Mean keratometric astigmatism also decreased from 1.71 ± 0.84 cyl D (range 0.25 to 3.50 cyl D) preoperatively to 0.49 ± 0.21 cyl D (range 0.25 to 1.25 cyl D) at 6 months. Stability of corneal refractive change, based on keratometry and pachymetry readings, was reached at 6 months in all eyes and remained stable throughout follow-up (Figure 3). This indicates that the myopic shift after 2 years was merely caused by increasing axial length in both eyes. In addition, anterior and posterior corneal elevation maps (Figure 3) were within normal values indicating no signs of post-LASIK ectasia. Moreover, 12 patients received ocular response analyzer (ORA) at the 10-year visit which revealed good corneal hysteresis (CH) and a good corneal resistant factor (CRF).
Regarding the strabismus, 11 patients (34%) who had preoperative sensory intermittent exotropia (< 15°), regained orthophoria in all gazes after LASIK, while 5 of 10 who had sensory constant exotropia with large angle (>30°) required surgical correction 6–12 months after LASIK for ocular alignment in order to regain binocular vision. Preoperative examination with full binocular correction (contact lenses alone or combined with spectacles) at the beginning of the study showed that 8 eyes (25%) had extra foveal fusion, 4 eyes (12.5%) had foveal fusion, and none had stereopsis. Binocular vision had improved significantly at the 2 years follow-up visit and the occlusion treatment was reduced. Stereo-acuity developed in 24 patients (75%), and 19 patients (59%) achieved good stereopsis with stereo-acuity disparity ranging from 400 to 200 seconds of arc in the Lang stereo-test II, and from 25 to 50 arc seconds in Titmus stereo-test.
Discussion
To the best of our knowledge, this is the largest series (n=32) with the longest follow-up period (10 years) that demonstrated long-term safety, effectiveness, and stability of unilateral LASIK in children with high myopic anisometropia (SER −9.5D ±1.7D) associated with deep amblyopia. Anisometropia was significantly reduced, and amblyopia markedly improved after strict pre- and post-LASIK occlusion/penalization therapy. The CCT of 564±31µm enabled full myopic refractive correction in some patients with emmetropic fellow eyes. The remaining patients had post LASIK refraction within the targeted ±2.0D of the fellow eyes and tolerated small prescription glasses. Along with good compliance to amblyopia therapy, children maintained a mean BCVA of 0.6±0.1 Decimal throughout the follow- up.
Our results verified the previously published studies that demonstrated the short-term (1 year) and intermediate-term (2–4 years) safety and efficacy of LASIK in achieving the targeted refractive balance between the two eyes. Agarwal et al35 performed LASIK on 16 children and markedly reduced the SER from −14.88 D to −1.44 D which remained stable for 1 year. 12 eyes maintained their preoperative BCVA, 2 gained 1 row, and 2 lost 1 row. 3 eyes developed grade 2 corneal haze. Ghanem et al30 also reported reduction of SER from −9.0D to −1.0D after LASIK. Within 2 years of follow up ~50% of the operated eyes remained within ±1.0D of the fellow eye. The mean BCVA improved significantly with 20% of eyes gaining 3 or more lines and no patient had lost vision due to haze.
After 2 years, there were insignificant changes in SER refraction in the operated eyes suggesting an ongoing trend of myopic shift. The mean post-LASIK regression value at 10 years was −1.58D ± 1.1D (range: −0.7 to −3.2D). With simultaneous myopic regression in the fellow eyes, no anisometropia was elicited throughout the follow-up. 12 eyes (38%) remained within ±1.0D and 20 eyes (63%) were within ±2.0D of the fellow eye, maintaining the targeted refractive balance between the two eyes. These results are comparatively better than the previously reported myopic shift in children after corneal refractive surgery, perhaps due to our strict measures to ensure optimum results. Ghanem et al30 observed −2.25 D myopic shift at 2 years postoperatively. Lin et al36 reported −2.74 D mean myopic regression in 12 children with myopic anisometropia 2 years after LASIK. Regardless, myopic shift cannot be avoided in this age group, and it also occurs without refractive surgery. Goss et al37 reported myopic shift of 0.5D per year in normal phakic pediatric eyes, and Gordon et al38 suggested that myopic shift may continue until the age of 18 years. Therefore, the anticipated myopic shift was not considered a complication or contraindication for LASIK in this age group.
The children maintained a mean BCVA of 0.6±0.1 Decimal throughout the follow-up. Vision gain was primarily attributed to reduced refractive error in the amblyopic eye and elimination of aniseikonia.39 Post-LASIK sustained amblyopia therapy was also crucial in attaining better binocular sensory outcomes and to prevent the recurrence of amblyopia. Visual outcomes were mostly better in younger children with better baseline BCVA and vice versa. It is well known that children within the critical period of visual development are more sensitive to amblyopia treatment, and this sensitivity gradually decreases thereafter when the visual maturation is complete and the visual centers become resistant to visual input.40
Correction of anisometropia amblyopia also helped to restore ocular alignment and good binocular vision. Post LASIK, small angle sensory exotropia had resolved in children with intermittent exotropia of <20 prism diopters, and good binocular vision was gained and confirmed by Lang Stereopsis test. Children with residual strabismus had undergone strabismus surgery for ocular alignment 6–12 months post-LASIK, resulting in orthophoria that remained solid due to the development of binocular vision. At the last follow-up visit, patients maintained balanced refraction, stable corrected visual acuity, improved stereopsis, orthophoria, and stable refraction. Despite the reported incomplete corneal flap cut in only one patient, no other postoperative complications were elicited throughout the follow-up period. In addition, corneal topography, and ORA showed long-term stability of corneal curvature and biomechanics.
Conclusion
In conclusion, LASIK procedure appears safe, effective, and stable for correcting refractory pediatric myopic anisometropia. It resulted in improvement of refractive error, visual acuity and consequently binocular vision and stereopsis. However, there are many special considerations in performing pediatric LASIK regarding preoperative assessment, amblyopia therapy, anesthesia, fixation, microkeratome selection, size of the globe, and refractive endpoint that make the surgery different from adult LASIK. Therefore, pediatric candidates for LASIK refractive surgery should be carefully selected and LASIK procedure should be considered only when conventional measures have been exhausted, or chronic non-compliance or intolerance of conventional treatments endanger normal visual development.
Data Sharing Statement
No further data will be shared.
Acknowlegemnts
This study was partially presented at the American Society of Cataract and Refractive Surgery (ASCRS) virtual meeting, May 2020. It was also presented at the American Academy of Ophthalmology (AAO) Virtual meeting, November 2020. Dr. Ahmed Alebiary assisted in the statistical analysis. Adam El Sheha and Ryan El Sheha assisted in manuscript writing and editing. The content is the responsibility of the authors and does not represent the views of the acknowledged individuals.
Funding
No funding to report.
Disclosure
No relevant financial or non-financial competing interests to report.
References
1. Freeman MI. Spectacles vs contact lenses in the correction of unilateral axial myopia. Arch Ophthalmol. 1992;110(2):180. doi:10.1001/archopht.1992.01080140036019
2. Deng L, Gwiazda JE. Anisometropia in children from infancy to 15 years. Invest Ophthalmol Vis Sci. 2012;53(7):3782–3787. doi:10.1167/iovs.11-8727
3. O’Donoghue L, McClelland JF, Logan NS, Rudnicka AR, Owen CG, Saunders KJ. Profile of anisometropia and aniso-astigmatism in children: prevalence and association with age, ocular biometric measures, and refractive status. Invest Ophthalmol Vis Sci. 2013;54(1):602–608. doi:10.1167/iovs.12-11066
4. Hu YY, Wu JF, Lu TL, et al. Prevalence and associations of anisometropia in children. Invest Ophthalmol Vis Sci. 2016;57(3):979–988. doi:10.1167/iovs.15-18647
5. Nunes AF, Batista M, Monteiro P. Prevalence of anisometropia in children and adolescents. F1000Res. 2021;10:1101. doi:10.12688/f1000research.73657.4
6. Townshend AM, Holmes JM, Evans LS. Depth of anisometropic amblyopia and difference in refraction. Am J Ophthalmol. 1993;116(4):431–436. doi:10.1016/S0002-9394(14)71400-X
7. Tomac S, Birdal E. Effects of anisometropia on binocularity. J Pediatr Ophthalmol Strabismus. 2001;38(1):27–33. doi:10.3928/0191-3913-20010101-09
8. Shippman S, Heiser L, Hall LS, Cohen KR. Development of primary axial myopic anisometropia. Am Orthopt J. 2003;53:109–114. doi:10.3368/aoj.53.1.109
9. Donahue SP. The relationship between anisometropia, patient age, and the development of amblyopia. Trans Am Ophthalmol Soc. 2005;103:313–336.
10. Afsari S, Rose KA, Gole GA, et al. Prevalence of anisometropia and its association with refractive error and amblyopia in preschool children. Br J Ophthalmol. 2013;97(9):1095–1099. doi:10.1136/bjophthalmol-2012-302637
11. Shih MH, Chen WJ, Huang FC. Refractive changes in amblyopic children with high anisometropia. Optom Vis Sci. 2015;92(10):1012–1015. doi:10.1097/OPX.0000000000000691
12. Barrett BT, Bradley A, Candy TR. The relationship between anisometropia and amblyopia. Prog Retin Eye Res. 2013;36:120–158. doi:10.1016/j.preteyeres.2013.05.001
13. Vincent SJ, Collins MJ, Read SA, Carney LG, Yap MK. Interocular symmetry in myopic anisometropia. Optom Vis Sci. 2011;88(12):1454–1462. doi:10.1097/OPX.0b013e318233ee5f
14. Levi DM, McKee SP, Movshon JA. Visual deficits in anisometropia. Vision Res. 2011;51(1):48–57. doi:10.1016/j.visres.2010.09.029
15. Lubkin V, Kramer P, Meininger D, Shippman S, Bennett G, Visintainer P. Aniseikonia in relation to strabismus, anisometropia and amblyopia. Binocul Vis Strabismus Q. 1999;14(3):203–207.
16. Adams GG, Karas MP. Effect of amblyopia on employment prospects. Br J Ophthalmol. 1999;83(3):380. doi:10.1136/bjo.83.3.378c
17. Smith EL
18. Roberts CJ, Adams GG. Contact lenses in the management of high anisometropic amblyopia. Eye. 2002;16(5):577–579. doi:10.1038/sj.eye.6700159
19. Weakley DR
20. Autrata R, Krejcirova I, Griscikova L, Dolezel Z. Refrakční chirurgie při myopické anizometropické amblyopii u dětí a srovnání s konzervativní léčbou kontaktními čočkami [Refractive surgery in children with myopic anisometropia and amblyopia in comparison with conventional treatment by contact lenses]. Cesk Slov Oftalmol. 2016;72(2):12–19. Czech.
21. Astle WF, Huang PT, Ingram AD, Farran RP. Laser-assisted subepithelial keratectomy in children. J Cataract Refract Surg. 2004;30(12):2529–2535. doi:10.1016/j.jcrs.2004.06.025
22. Paysse EA, Hamill MB, Hussein MA, Koch DD. Photorefractive keratectomy for pediatric anisometropia: safety and impact on refractive error, visual acuity, and stereopsis. Am J Ophthalmol. 2004;138(1):70–78. doi:10.1016/j.ajo.2004.01.044
23. Al Ammari HM, Al Shamlan FT. Amblyopia treatment efficacy in anisometropia. Clin Ophthalmol. 2019;13:2395–2402. doi:10.2147/OPTH.S224463
24. Alio JL, Artola A, Claramonte P, Ayala MJ, Chipont E. Photorefractive keratectomy for pediatric myopic anisometropia. J Cataract Refract Surg. 1998;24(3):327–330. doi:10.1016/S0886-3350(98)80319-2
25. Autrata R, Rehurek J. Clinical results of excimer laser photorefractive keratectomy for high myopic anisometropia in children: four-year follow-up. J Cataract Refract Surg. 2003;29(4):694–702. doi:10.1016/S0886-3350(02)01896-5
26. Cotter SA. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113(6):895–903. doi:10.1016/j.ophtha.2006.01.068
27. Paysse EA, Coats DK, Hussein MA, Hamill MB, Koch DD. Long-term outcomes of photorefractive keratectomy for anisometropic amblyopia in children. Ophthalmology. 2006;113(2):169–176. doi:10.1016/j.ophtha.2005.06.010
28. Autrata R, Rehurek J. Laser-assisted subepithelial keratectomy and photorefractive keratectomy versus conventional treatment of myopic anisometropic amblyopia in children. J Cataract Refract Surg. 2004;30(1):74–84. doi:10.1016/S0886-3350(03)00417-6
29. Astle WF, Fawcett SL, Huang PT, Alewenah O, Ingram A. Long-term outcomes of photorefractive keratectomy and laser-assisted subepithelial keratectomy in children. J Cataract Refract Surg. 2008;34(3):411–416. doi:10.1016/j.jcrs.2007.10.027
30. Ghanem AA, Moad AI, Nematallah EH, El-Adawy IT, Anwar GM. Laser in situ keratomileusis for treated myopic anisometropic amblyopia in children. Saudi J Ophthalmol. 2010;24(1):3–8. doi:10.1016/j.sjopt.2009.12.001
31. BenEzra D, Cohen E, Karshai I. Phakic posterior chamber intraocular lens for the correction of anisometropia and treatment of amblyopia. Am J Ophthalmol. 2000;130(3):292–296. doi:10.1016/S0002-9394(00)00492-X
32. Alio JL, Toffaha BT, Laria C, Pinero DP. Phakic intraocular lens implantation for treatment of anisometropia and amblyopia in children: 5-year follow-up. J Refract Surg. 2011;27(7):494–501. doi:10.3928/1081597X-20110120-01
33. Tychsen L, Packwood E, Hoekel J, Lueder G. Refractive surgery for high bilateral myopia in children with neurobehavioral disorders: 1. Clear lens extraction and refractive lens exchange. J AAPOS. 2006;10(4):357–363. doi:10.1016/j.jaapos.2006.04.003
34. Phillips CB, Prager TC, McClellan G, Mintz-Hittner HA. Laser in situ keratomileusis for treated anisometropic amblyopia in awake, autofixating pediatric and adolescent patients. J Cataract Refract Surg. 2004;30(12):2522–2528. doi:10.1016/j.jcrs.2004.02.020
35. Agarwal A, Agarwal A, Agarwal T, Siraj AA, Narang P, Narang S. Results of pediatric laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(5):684–689. doi:10.1016/S0886-3350(00)00299-6
36. Lin XM, Yan XH, Wang Z, et al. Long-term efficacy of excimer laser in situ keratomileusis in the management of children with high anisometropic amblyopia. Chin Med J. 2009;122(7):813–817.
37. Goss DA. Variables related to the rate of childhood myopia progression. Optom Vis Sci. 1990;67(8):631–636. doi:10.1097/00006324-199008000-00014
38. Gordon RA, Donzis PB. Myopia associated with retinopathy of prematurity. Ophthalmology. 1986;93(12):1593–1598. doi:10.1016/S0161-6420(86)33523-1
39. Jampolsky A, Flom BC, Weymouth FW, Moses LE. Unequal corrected visual acuity as related to anisometropia. AMA Arch Ophthalmol. 1955;54(6):893–905. doi:10.1001/archopht.1955.00930020899013
40. Elston JS, Timms C. Clinical evidence for the onset of the sensitive period in infancy. Br J Ophthalmol. 1992;76(6):327–328. doi:10.1136/bjo.76.6.327
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.