Back to Journals » International Journal of Women's Health » Volume 6
Ten-year literature review of global endometrial ablation with the NovaSure® device
Authors Gimpelson R
Received 22 October 2013
Accepted for publication 17 December 2013
Published 11 March 2014 Volume 2014:6 Pages 269—280
DOI https://doi.org/10.2147/IJWH.S56364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Richard J Gimpelson
Mercy Clinic, Minimally Invasive Gynecology, Department of Obstetrics and Gynecology, Mercy Hospital St Louis, St Louis, MO, USA
Abstract: This review examines the peer-reviewed literature describing prospective studies that report amenorrhea rates, patient satisfaction, and surgical reintervention rates following the NovaSure® endometrial ablation procedure. A search of the English-language literature published from 2000 to 2011 was conducted using PubMed. Ten prospective studies, six single-arm NovaSure trials, and four randomized controlled trials comparing the NovaSure procedure with other global endometrial ablation modalities met the inclusion criteria and were reviewed. The follow-up periods ranged from 6 to 60 months. Amenorrhea rates for the NovaSure procedure ranged from 30.0% to 75.0%. Patients who reported being satisfied with the NovaSure procedure ranged from 85.0% to 94.0%. In randomized controlled trials with other global endometrial ablation modalities, amenorrhea rates at 12 months with the NovaSure procedure ranged from 43.0% to 56.0%, while other modalities ranged from 8% to 24%. In addition, this manuscript reviews the following: the NovaSure technology; use of the NovaSure procedure in the office setting; intraoperative and postoperative pain; effects on premenstrual syndrome (PMS); dysmenorrhea; special circumstances, including presence of uterine disease, history of cesarean delivery, coagulopathy, or use of anticoagulant medication; post-procedure uterine cavity assessment and cancer risk; contraception and pregnancy; and safety.
Keywords: abnormal uterine bleeding, menorrhagia, endometrial ablation, NovaSure®
Introduction
Abnormal uterine bleeding (AUB) is defined as an irregularity in the timing, amount, or duration of menstrual bleeding. Estimates suggest that 10% to 30% of reproductive-aged women suffer from AUB, depending on whether it is defined objectively by measuring the amount of blood loss or subjectively by using patient-reported information.1 However it is defined, AUB contributes considerably to medical care costs, as well as significantly affects the quality of life and productivity of women who suffer from it.1
Hysterectomy is the leading treatment for AUB in women for whom medical therapy has failed or is contraindicated; however, complication rates are high and can include infection; injury to the bowel, bladder or ureter; nerve damage; and postoperative thromboembolism.2 While global endometrial ablation (GEA) offers a less invasive alternative to hysterectomy, the first-generation ablation devices require high operative skill and are associated with a greater risk of uterine perforation and intraoperative fluid overload.3 Second-generation devices simplify the ablation procedure by not using resectoscopes and appear to require less training and experience than resectoscopic endometrial ablation.4 In addition, second-generation devices can be used under local anesthesia in an office-based setting.5,6
US Food and Drug Administration (FDA)-approved second-generation devices use a variety of technologies to ablate the endometrium, including thermal balloon ablation (ThermaChoice® Uterine Balloon Therapy; Johnson & Johnson, New Brunswick, NJ, USA [FDA approval obtained in 1997]), cryoablation (Her Option™; Cooper Surgical, Trumbull, CT, USA [FDA approval obtained in 2001]), heated free fluid (Hydro ThermAblator [HTA™] System; Boston Scientific, Natick, MA, USA [FDA approval obtained in 2001]), bipolar radiofrequency ablation (NovaSure® endometrial ablation; Hologic, Inc, Bedford, MA, USA [FDA approval obtained in 2001]), and microwave ablation (MEA® System, previously produced by Microsulis Medical Limited, Denmead, UK [FDA approval obtained in 2003]).7–11 In addition to the FDA-approved devices, one thermal balloon device (Cavaterm™; Pnn Medical SA, Kvistgaard, Denmark [CE mark obtained in 1995]) is available outside the US.
This review examines the peer-reviewed literature describing prospective studies that report amenorrhea rates, patient satisfaction, and surgical reintervention rates following the NovaSure endometrial ablation procedure. A search of the English-language literature published from 2000 to 2011 was conducted using PubMed. Key medical subject headings and search terms were “NovaSure”, “bipolar radiofrequency ablation”, “endometrial ablation”, and “premenopausal.” Review articles, case reports, retrospective studies, and studies that did not report menstrual bleeding outcomes or did not follow patients for at least 6 months were excluded. Ten prospective studies, six single-arm NovaSure trials, and four randomized controlled trials (RCTs) comparing NovaSure ablation with other GEA modalities met the inclusion criteria and were reviewed. The follow-up periods ranged from 6 to 60 months. Amenorrhea rates for the NovaSure procedure ranged from 30.0% to 75.0%. Patients who reported being satisfied with the NovaSure procedure ranged from 85.0% to 94.0%. In RCTs with other GEA modalities, amenorrhea rates at 12 months with the NovaSure procedure ranged from 43.0% to 56.0%, while other modalities ranged from 8% to 24%.
In addition, this manuscript reviews the following: the NovaSure technology; use of the NovaSure procedure in the office setting; intraoperative and postoperative pain; effects on premenstrual syndrome (PMS); dysmenorrhea; special circumstances, including presence of uterine disease, history of cesarean delivery, coagulopathy or use of anticoagulant medication; post-procedure uterine cavity assessment and cancer risk; contraception and pregnancy; and safety.
NovaSure impedance-controlled endometrial ablation system
The NovaSure system comprises a disposable ablation device, a radiofrequency (RF) generator (RF controller), a suction line desiccant, and a carbon dioxide canister.12 The ablation device is a conformable, bipolar electrode array housed within a protective sheath and mounted on an expandable frame. It also contains an intrauterine measuring system, which determines the uterine cavity width (cornua-to-cornua distance). When the ablation device is deployed within the uterine cavity, the sheath withdraws into the endocervical canal to protect the endocervix from thermal injury. The RF controller is a constant power output generator with a maximal power delivery of 180 watts. Using the uterine cavity width and length dimensions, the RF controller calculates the appropriate power output to ensure complete ablation of the endometrium. During ablation, tissue impedance is continuously monitored, and the procedure terminates once a level of 50 ohms is achieved. Actual treatment time may vary up to 120 seconds12 to accommodate different endometrial thicknesses. The RF controller also has a cavity integrity assessment system, which determines whether a defect or perforation exists in the uterine wall before RF energy is delivered to the uterus. Carbon dioxide is delivered into the uterine cavity at a safe flow rate and pressure through the central lumen of the ablation device. When 50 mmHg is achieved for 4 seconds, uterine integrity is confirmed. A vacuum pump in the RF controller applies suction to bring the endometrial lining into contact with the electrode array and simultaneously removes by-products of the ablation, steam, and blood from the uterine cavity.12
Compared to other GEA techniques, the NovaSure procedure has the shortest treatment time, requires no uterine pretreatment, and can be performed at any time during the menstrual cycle.12,13
Preoperative evaluation of women before GEA
Practitioners should complete a thorough medical history and evaluation of women with AUB before GEA.1 Additional diagnostic testing should include assessment of current medical therapy to eliminate iatrogenic causes of AUB and endometrial biopsy to eliminate hyperplasia and carcinoma. In a Practice Bulletin published by the American College of Obstetricians and Gynecologists (ACOG), the importance of excluding malignancy prior to ablation is emphasized.4 Assessment of a patient’s self-reported blood loss, quality of life, and fertility concerns will help further determine whether the patient is a candidate for GEA.
Efficacy of NovaSure GEA
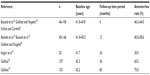
Six single-arm studies (eight publications) prospectively examined the use of NovaSure endometrial ablation in women and reported rates of amenorrhea, patient satisfaction, and surgical reintervention.5,14–20
Amenorrhea rates after GEA with NovaSure
The most comprehensive clinical trial data on the safety and long-term efficacy of the NovaSure procedure were reported by Gallinat18,19 and Gallinat and Nugent20 over a 60-month follow-up period. Safety and efficacy were first demonstrated for the NovaSure procedure in a controlled observational pilot study that enrolled 107 women and used the Pictorial Blood Loss Assessment Chart (PBLAC) to assess bleeding symptoms.20 Successful reduction in bleeding occurred in 98% of the patients by 12 months. At the 6-month follow-up, the amenorrhea rate was 46.2% and, at 12 months, it was 58.6%. Only four (3.9%) patients reported menorrhagia at 12 months. Of these patients, one had a PBLAC score of 800 and was considering additional ablation; one had a PBLAC score of 250 and then had a second ablation with resulting PBLAC score of 19; and two other patients (with PBLAC scores of 219 and 125) were satisfied with their outcomes and did not undergo further treatment. All patients who reported amenorrhea at 12 months’ follow-up also reported amenorrhea at 36 months.18 Seven additional patients reported amenorrhea without having menopausal symptoms, making the final 36-month amenorrhea rate 65%. By 60 months post-procedure, 75% of the patients reported amenorrhea and 2% reported menorrhagia.19 A McNemar test for significance of change identified considerable improvement in amenorrhea rates between 12 and 36 months (58% and 65%, respectively; P=0.0253) and between 36 and 60 months (65% and 75%, respectively; P=0.0047).
Similar rates of amenorrhea were reported in the other NovaSure single-arm trials (Table 1). One study reported an amenorrhea rate of 64% at the 6-month follow-up.5 Another study reported amenorrhea rates at 6 and 12 months of 50% and 58%, respectively.16 Between 12 and 48 months’ follow-up, a Canadian group reported amenorrhea rates from 43.1% to 58.0%, respectively.15 One study of 20 patients reported an amenorrhea rate of 30% at 24 months posttreatment.14 Fulop et al followed 75 patients with a median follow-up period of 7.8 years (range 6–8.6 years).17 At the 7-year follow-up point, 56 patients were evaluable for bleeding outcomes. As not all patients were available for follow-up, an amenorrhea rate based on the intent-to-treat population was not provided, although the authors calculated an actuarial amenorrhea rate of 88.9% (95% confidence interval [CI]: 79.5%–95.3%).17
  | Table 1 Single-arm prospective trials: amenorrhea rates after treatment with the NovaSure® endometrial ablation device (Hologic, Inc, Bedford, MA, USA) |
Patient satisfaction after NovaSure GEA
Three single-arm prospective studies have assessed patient satisfaction during follow-up periods ranging from 6 to 48 months.5,14,15 Patient satisfaction ranged from 85.0% to 94.0%.5,14 In one of the largest prospective studies of NovaSure endometrial ablation, Baskett et al reported on 200 patients with 1 to 4 years of follow-up; of the 146 women with greater than 1-year follow-up, 97.3% would recommend the NovaSure procedure.15
Rates of reintervention after NovaSure GEA
The rate of reintervention was low for the women who were prospectively recruited to single-arm clinical trials for treatment with the NovaSure procedure for GEA (Table 2). At 60 months’ follow-up, Gallinat reported that only three hysterectomy procedures were performed within their cohort of 107 (2.8%).19 Busund et al reported that only two (4.4%) women within their cohort of 45 had undergone hysterectomy due to continuous bleeding at 12 months.16 The highest reintervention rate (8.2%) for prospectively recruited women was reported in a study by Baskett et al: over 1 to 4 years of follow-up, ten women had a hysterectomy and two had second ablations, out of 146 women observed from an original cohort of 200 patients.15
  | Table 2 Single-arm prospective trials: surgical reinterventions after treatment with the NovaSure® endometrial ablation device (Hologic, Inc, Bedford, MA, USA) |
Randomized Clinical Trials
NovaSure versus rollerball ablation
The FDA pivotal trial performed by Cooper et al examined the safety and effectiveness of the NovaSure procedure compared to wire loop resection with rollerball ablation.12 To evaluate treatment effectiveness, the primary outcome measure was PBLAC scores at 12 months. Study success, defined as a PBLAC score of ≤75, was achieved for 88.3% of the NovaSure patients and 81.7% of the rollerball patients. The average PBLAC pretreatment score was 562 for both groups, and 3-month posttreatment scores were 48 for the NovaSure group and 63 for the rollerball group. By 6 months, the PBLAC scores were reduced to 28 and 42, respectively, and stabilized. By 12 months, amenorrhea rates were 40.9% and 35.4%, respectively, while patient satisfaction was not different between groups (92.8% for NovaSure and 93.9% for rollerball). Hysterectomy was performed for three women in the NovaSure arm and two in the rollerball arm. A total of six women had persistent menorrhagia and required either repeat ablation or medical therapy, of which four women were in the NovaSure arm and two were in the rollerball arm.
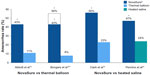
Subsequent RCTs compared the outcomes of patients treated with NovaSure endometrial ablation and other second-generation devices. These studies consistently showed that amenorrhea rates were significantly greater in NovaSure-treated patients than in patients treated with other ablation devices (Figure 1).
NovaSure versus thermal balloon ablation
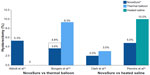
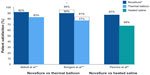
In the first report comparing the NovaSure and ThermaChoice procedures, amenorrhea at 3, 6, and 12 months was the primary outcome for a double-blind RCT.21 Enrollment was targeted to 82 patients using a 2:1 ratio; however, after 44 patients were enrolled and treated, a technical failure was discovered in the NovaSure controller. Therefore, enrollment was increased to 126 patients (82 in the NovaSure arm and 44 in the ThermaChoice arm), and the data were subsequently analyzed in two sets: one that included all of the data (Group A) and one that included only the data from women who were treated after the equipment failure was noted (Group B). At 3, 6, and 12 months, the amenorrhea rates were 40%, 43%, and 41% in the NovaSure arm and 12%, 10%, and 8% in the ThermaChoice arm, respectively (Figure 1). Exclusion of the data from before the NovaSure controller malfunction resulted in higher amenorrhea rates in the NovaSure arm: 52%, 55%, and 56% at 3, 6, and 12 months, respectively. The amenorrhea rates in the NovaSure arm were higher than the amenorrhea rates in the ThermaChoice arm at all times and before and after exclusion (P<0.001). A hysterectomy was performed in four patients in the NovaSure group and four patients in the ThermaChoice group (relative risk 0.47, 95% CI: 0.07 to −3.30) (Figure 2). Patient satisfaction was higher for NovaSure endometrial ablation than for ThermaChoice (Figure 3).
A follow-up questionnaire was given to this cohort at 60 months with ≥90% total response rate.22 Of the women surveyed, 48% in the NovaSure arm and 32% in the ThermaChoice arm reported amenorrhea. These rates increased when only the data from the women treated after the controller failure were analyzed (64% for the NovaSure arm and 37% for ThermaChoice arm). Regardless of the data exclusion for the controller malfunction, the amenorrhea rates were significantly higher for the NovaSure arm than for the ThermaChoice arm (P<0.001). At 60 months’ follow-up, hysterectomy was performed in 9.8% of women in the NovaSure arm and 12.9% in the ThermaChoice arm (hazard ratio 1.2, 95% CI: 0.35–4.00). One woman in each arm was dissatisfied and had reablation with the NovaSure procedure. Clark et al performed a single-blind, randomized clinical trial (Comparison of Office Endometrial Ablation Techniques [COAT]) to examine the NovaSure and ThermaChoice III procedures in an office-based setting.23 Procedure time was significantly shorter in the NovaSure group by 6.2 minutes on average (P≤0.001). Treatment was completed in all women randomized to the NovaSure arm, whereas it was terminated in 5% of women randomized to the ThermaChoice III arm due to patient discomfort. Postoperative hysteroscopy recorded complete destruction of the endometrium in 88% of women in the NovaSure arm and 58% of women in the ThermaChoice III arm (P=0.002). No serious intraoperative complications were reported in either arm. Of the patients treated with ThermaChoice III, 23% reported that the procedure was unacceptable, compared to 6% of NovaSure patients (P=0.08), and 50% of ThermaChoice III patients would have the same treatment again compared to 69% of NovaSure patients (P=0.2). At 12 months, amenorrhea rates were significantly higher in NovaSure-treated women (56%) than in ThermaChoice III–treated women (23%) (P=0.02). At 12 months, one patient in the NovaSure arm and three patients in the ThermaChoice III arm had reintervention.
One study directly compared the amenorrhea rates after the use of the Cavaterm or NovaSure ablation systems.24 At 12 months, the rate of amenorrhea in the Cavaterm treatment arm was significantly less than that in the NovaSure arm (12% versus 43%; P=0.04), but patient satisfaction at 12 months was not different between the two groups (83% Cavaterm versus 92% NovaSure). No difference in the reintervention rates between the two treatment groups was found.
NovaSure versus heated saline
One double-blind RCT compared the efficacy of the NovaSure and HTA procedures.25 Patients were enrolled in a 1:1 ratio for treatment with either NovaSure (n=82) or HTA (n=78). Procedure time with NovaSure endometrial ablation was 11.8 minutes on average, compared to 27.8 minutes for HTA (P<0.001). At 12 months, significantly more women were reporting amenorrhea in the NovaSure arm than in the HTA arm (47% NovaSure versus 24% HTA) (Figure 1). At 12 months, 87% of patients treated with NovaSure endometrial ablation were completely satisfied, compared with 68% of patients treated with HTA (Figure 3). At 12 months, five (6%) patients in the NovaSure arm required surgical reintervention, compared with 17 (24%) in the HTA arm. At 60 months’ follow-up, amenorrhea rates were 55.4% and 35.3% in the NovaSure and HTA groups, respectively.26 The number of surgical reinterventions was more than double in the HTA group when compared to the NovaSure group: 23 versus eleven, respectively.
Network meta-analysis: GEA
Over the last 10 years, the methodology for statistical comparisons has evolved from conventional pairwise meta-analysis to include network meta-analysis, which allows for indirect comparison across trials based on a common comparator. A 2012 systematic review of the literature on second-generation endometrial ablation devices yielded over 700 articles and abstracts.27 After critical evaluation, 19 RCTs involving over 3,200 women were deemed appropriate for a meta-analysis; five studies were comparisons between second-generation GEA technologies, and 14 were comparisons between second-generation and first-generation devices such as rollerball and/or transcervical resection of the endometrium. Outcome measures used to evaluate the treatments were rate of amenorrhea, rate of heavy bleeding, and dissatisfaction at 12 months, or at 2 years if 12-month data were not available. Treatment with the NovaSure procedure resulted in higher rates of amenorrhea than thermal balloon ablation (odds ratio [OR] 2.51, 95% CI: 1.53–4.12, P<0.001). There was a significantly increased risk of persistent heavy bleeding after free-fluid ablation compared to NovaSure (OR 2.19, 95% CI: 1.07–4.50, P=0.03), reduced rates of amenorrhea (OR 0.36, 95% CI: 0.19–0.67, P=0.004), and increased rates of dissatisfaction (OR 4.79, 95% CI: 1.07–21.5, P=0.04). The authors concluded that “bipolar radio frequency and microwave ablative devices are more effective than thermal balloon and free-fluid ablation in the treatment of heavy menstrual bleeding with second-generation endometrial ablation devices.”27
GEA in an office-based setting
Safety, feasibility, and efficacy of the NovaSure procedure in an office-based setting using local anesthesia were evaluated in two single-arm studies5,6 and one randomized clinical trial.23 Penninx et al used a visual analog scale (VAS) to measure pain during cervical dilation and at 4 hours and 24 hours after the procedure.6 No intraoperative or postoperative complications were reported, and no premature termination of the procedure occurred in any of the 33 women. Four women developed a vasovagal reaction during the procedure. Median pain scores were 3.0 (range, 1.0–7.0) during dilation and 5.1 (range, 0.0–10.0) for the entire procedure. At 24 hours post-ablation, 23 women had a pain score of 0. All women were satisfied with their outcomes and only two patients reported that they would not undergo the procedure again.
In another study, Kalkat and Cartmill assessed the feasibility and efficacy of using the NovaSure procedure with local anesthesia in 50 women.5 All of the procedures were completed and 94% of women were discharged on the same day as their procedure; three women required overnight admission for pain relief. Preference for general anesthesia for future treatment was 14%. Patient satisfaction was 86% and 94% at 4 and 6 months, respectively.
Intraoperative and postoperative pain
One prospective multicenter clinical trial examined intraoperative and postoperative pain associated with the NovaSure and ThermaChoice procedures; however, treatment assignment was not randomized but based on patient choice.28 Pain was assessed by a VAS, a numeric rating scale, and a brief pain inventory form. No uterine pretreatment was performed in women who underwent treatment with the NovaSure procedure, while women who chose ThermaChoice received a 3-minute suction dilation and curettage before ablation. Intraoperative anesthesia consisted of a combination of paracervical block and intravenous sedation. No serious intraoperative adverse events occurred in either group. Both VAS and numeric rating scale scores were significantly better with NovaSure compared to ThermaChoice (P<0.0001). Postoperative pain indices varied over time, but were significantly lower for the NovaSure procedure (P<0.0001). Fewer NovaSure-treated patients than ThermaChoice-treated patients experienced postoperative nausea and vomiting: 5.4% versus 33% (P<0.0001).
Two other studies reported on pain during GEA.23,24 In one study, no statistical differences were reported for intraoperative pain scores for women treated with either the NovaSure or ThermaChoice procedures; however, all NovaSure procedures were completed and two (5%) balloon procedures were not completed because of patient discomfort.23 In the other study, NovaSure ablation was found to be significantly less painful than ablation performed with Cavaterm (P=0.01).24
Effect of GEA on PMS
One single-arm cohort study examined the effects of NovaSure on symptoms of PMS.29 Participants were surveyed before and 4 to 6 months after GEA. Survey instruments included the Daily Symptom Report and Daily Record of Severity of Symptoms. Before NovaSure ablation, the self-reported VAS scores for PMS symptoms and for menstrual pain were 7.4 (symptoms not specified) and 7.3, respectively. The VAS scores for both PMS symptoms and menstrual pain significantly decreased to 2.6 (P<0.05) and 2.2 (P<0.05), respectively, after NovaSure ablation. In addition, 97% of women reported improvement in their PMS symptoms after ablation, and the results of both the Daily Symptom Report (P<0.05) and the Daily Record of Severity of Symptoms (P<0.05) improved after ablation. All of the women reported improvement in heavy menstrual bleeding after ablation and 44% reported amenorrhea at 6 months.
While not examined as either primary or secondary outcomes, two studies did characterize the effect of NovaSure ablation on PMS symptoms.12,23 Comparing the NovaSure and ThermaChoice III procedures in an office-based setting, Clark et al found no difference between treatment arms at 6 months for reported improvements in PMS symptoms among women enrolled in the COATS trial.23 Emotional symptoms improved by 61% with NovaSure versus 50% with ThermaChoice III, and physical symptoms improved by 71% with NovaSure versus 67% with ThermaChoice III. In the NovaSure FDA pivotal trial, Cooper et al reported a significant reduction in the number of women reporting PMS symptoms post-ablation.12 Approximately two-thirds of the patients had symptoms prior to endometrial ablation, compared with a little over one-third at 6 and 12 months posttreatment.
Effect of GEA on dysmenorrhea
Five studies, including one retrospective30 and four prospective12,21–23,25 RCTs, evaluated the impact of GEA on dysmenorrhea.
Cooper et al reported that 56% of patients in both NovaSure and rollerball groups experienced dysmenorrhea before ablation.12 At 12 months posttreatment, 21% of women in the NovaSure arm and 34% of women in the rollerball arm reported dysmenorrhea.
When comparing NovaSure and ThermaChoice, severe dysmenorrhea was present at baseline in more than 30% of all women and was significantly reduced in both treatment groups (P=0.001).21 When the analysis was limited to patients randomized after discovery of a controller malfunction, the decrease in severe dysmenorrhea at 12 months was significantly greater in the NovaSure group than in the ThermaChoice group (P=0.001). By 60 months after treatment, both groups had a significant reduction in dysmenorrhea (P=0.001).22 No significant difference in the dysmenorrhea rate was found between the arms: 14% of NovaSure-treated and 25% of ThermaChoice-treated women had dysmenorrhea. Limiting the analysis to the patients treated after discovery of the controller malfunction, however, resulted in a significant difference in the reduction of dysmenorrhea rates between treatments (dysmenorrhea rate decreased from 35% to 13% for NovaSure and 31% to 25% for ThermaChoice; P=0.001). Clark et al compared the improvements in dysmenorrhea between NovaSure- and ThermaChoice III-treated women at 3, 6, and 12 months.23 At 12 months, 78% of NovaSure-treated women had improvement in dysmenorrhea versus 57% of ThermaChoice III-treated women (P=0.1).
In a study by Penninx et al, 37% of the NovaSure-treated women and 40% of the HTA-treated women had moderate or severe dysmenorrhea at baseline.25 At 12 months post-ablation, 21% and 14% had dysmenorrhea, respectively, which was not significantly different.
In the single-arm retrospective review of patient charts by Elmardi et al, dysmenorrhea was present in 49.5% of women before NovaSure ablation and decreased to 21.9% at 18 months posttreatment.30
Special circumstances in the use of NovaSure
Uterine disease or pathology
No study included women with diagnoses of either hyperplasia or carcinoma of the uterus before ablation. Most studies used inclusion criteria that ensured no uterine pathology was present before ablation.6,14,16,20,21,24 Two studies specified exclusion criteria of fibroids and polyps >2 cm.12,25 Sabbah and Desaulniers specified inclusion criteria of submucous fibroids up to 3 cm and found that the amenorrhea rate was 69% at 1 year.31 Another study included women with intracavitary lesions not greater than 3 cm; submucosal fibroids were identified in five women and endometrial polyps in six women treated with either the NovaSure or ThermaChoice III procedures; however, no specific outcome data were reported for these women.23
History of cesarean delivery
Only one study has reported the safety and efficacy of the NovaSure procedure in women who have a history of cesarean delivery.32 Of the 704 women enrolled, 162 had one or more cesarean deliveries, and 542 were either nulliparous or had vaginal deliveries; only patients with a history of low-transverse sections were included. GEA was performed using either NovaSure or thermal balloon ablation; however, no analysis was made between the type of GEA and delivery mode. Five years after their GEA procedure, 19.8% of women in the cesarean group and 20.8% of women in the without-cesarean group had amenorrhea (P=0.76). Adjusting for confounding factors did not affect likelihood of post-procedure amenorrhea. The 5-year cumulative failure rate was 11.3% and likelihood of treatment failure was similar for both groups. The only intraoperative complication was uterine perforation: two occurred in women in the cesarean group (1.2%) and two occurred in women in the without-cesarean group (0.4%). All perforations that occurred were fundal in location and remote from the lower-segment uterine scar. The authors concluded that GEA has similar efficacy and safety in women who have had at least one or more previous low-transverse cesarean deliveries compared to women with no history of cesarean delivery.32 One case of vesicouterine fistula post-ablation reported in the literature was incorrectly attributed to NovaSure ablation, and the report was subsequently retracted.33,34
Known coagulopathy or use of anticoagulant medication
Women with excessive vaginal bleeding associated with coagulopathy or anticoagulation therapy present a unique challenge for GEA treatment. One study retrospectively compared GEA outcomes for women who were being treated for a coagulopathy to a reference cohort.35 The coagulopathy and reference arms comprised patients treated with the ThermaChoice or NovaSure procedures. Two women in the coagulopathy arm on warfarin at the time of the procedure had treatment failures; however, the authors did not report the GEA device used for these patients. Treatment failures occurred in 5% of women with NovaSure ablation and 8% in women with ThermaChoice ablation; no difference in treatment failures was found between NovaSure-treated and ThermaChoice-treated women. Complications included intraoperative perforation (n=1), cervical laceration (n=1), hematometra (n=1), and pregnancy (n=1) in the coagulopathy group, and volume overload (n=1) and post-procedural pelvic pain (n=5) in the reference group. The authors did not distinguish between the GEA device type and the occurrence of adverse events, but did mention that both devices were found to be equally effective and concluded that GEA appears to be an effective option for treatment of women with coagulopathy.35
These same patients received a follow-up SF-12 Short Form Health Survey (SF-12®) questionnaire.36 Median time between treatment and the survey mailing was 33 months (range, 14.6–57.9 months) in the coagulopathy arm and 30.7 months (range, 10.2–68.5 months) in the reference arm (P=0.49). Patients in both arms reported significant improvements in menorrhagia-specific, health-related quality-of-life measures. The SF-12 scores were significantly lower in the coagulopathy group. Patient satisfaction was not different between the arms (95% versus 84%; P=0.6), but the amenorrhea rate was higher in the coagulopathy arm than in the reference arm (57% versus 46%, P=0.02). Reinterventions at the second follow-up included hysterectomy (n=1 in the coagulopathy arm and n=5 in the reference arm; P>0.99). The authors concluded that GEA is an effective treatment choice for women with coagulopathy presenting with AUB.
Evaluating the uterine cavity and cancer risk after GEA
As more women choose endometrial ablation as an alternative to hysterectomy, questions arise regarding the long-term incidence of endometrial cancer and feasibility of diagnosis in these women. The topic of a potential delay in cancer diagnosis after endometrial ablation was discussed in ACOG Practice Bulletin 81:
An early concern about endometrial ablation was the potential for delaying the diagnosis of a subsequent endometrial carcinoma. However, it appears in most instances, an intrauterine cavity remains, allowing egress of bleeding from retained endometrium.4
One study identified 509 women who had an endometrial ablation between 1978 and 1994.37 The women were contacted by mail or phone to determine if they had been diagnosed or treated for endometrial cancer; 5,063 total women-years of follow-up were calculated, with two cases of endometrial cancer identified. There was no significant difference between this observed number of endometrial cancers and the expected number based on US Surveillance, Epidemiology, and End Results (SEER) data. A similar study from Denmark identified 367 patients treated by endometrial ablation between 1990 and 1996.38 In addition to a questionnaire regarding the incidence of endometrial cancer, the subjects were registered through the national cancer registry and were verified by checking of medical records. The authors observed three women with incidental endometrial cancer at follow-up, as compared to the expected number of 6.8 cases, concluding that there was no increase in the incidence of endometrial cancer after endometrial ablation.
Most patients diagnosed with endometrial cancer after ablation develop AUB and have risk factors. In an early review of the literature on eight reported cases of endometrial cancer after ablation by resection or coagulation, the majority of patients had significant risk factors or were poor candidates for endometrial ablation.39 Six patients had postmenopausal bleeding unresponsive to hormonal therapy and five had endometrial complex hyperplasia on pre-ablation curettings.
A more recent systematic review of the literature, including a case report of endometrial cancer diagnosed 5 years after NovaSure ablation, confirmed that most women with post-ablation endometrial cancer present with AUB and pain.40 Over 75% of the women with post-ablation endometrial cancer were diagnosed in stage I, consistent with the typical presenting stage among women without a history of ablation. In addition to the case report of endometrial cancer after NovaSure ablation, most of the women with post-ablation endometrial cancer could be evaluated by endometrial sampling or hysteroscopy when they presented with symptoms. In two cases (11.8%), pre-hysterectomy evaluation was not possible due to scarring and intrauterine adhesions secondary to the ablation. The authors concluded that most patients with endometrial cancer after GEA present with symptoms such as bleeding and pelvic pain.40 Preoperative diagnosis can be obtained in most cases, contrary to concerns that the diagnosis of endometrial cancer is delayed and may be difficult to achieve.
Contraception and pregnancy after GEA
As discussed in ACOG Practice Bulletin 81, women should be counseled to use contraception after ablation.4 The incidence of pregnancy after endometrial ablation is estimated at 0.7%. More than half of such pregnancies were found not to be carried to term because of spontaneous miscarriage or personal choice to terminate.41 Pregnancy after GEA poses significant risk of major complications. All of the clinical trials reviewed herein selected women based on the desire for no future fertility; however, post-procedural pregnancies have been reported after NovaSure ablation. A small report evaluating pregnancy after NovaSure ablation demonstrated that pregnancies that continued beyond the first trimester were associated with poor obstetrical outcomes.42
Safety of NovaSure endometrial ablation
Few complications associated endometrial ablation have been reported in the medical literature.43 Minor complications associated with the NovaSure procedure may include bleeding, infection, uterine perforation, and device failure.44 More serious complications may include bowel injury, cardiac arrest, urinary tract injury, carbon dioxide embolus, sepsis, and death.45 It is recognized that inappropriate use and physician error are contributors to device-related complications.44,46 In the most recent review of the Manufacturer and User Facility Device Experience (MAUDE) database, Brown and Blank noted the possibility of false tracking with the NovaSure device leading to transmural thermal injury, thus highlighting the importance of on-label use of the device and proper technique in deployment and seating procedure.46
In the NovaSure pivotal trial of 175 patients, one intraoperative adverse event related to bradycardia was reported.12 Within 24 hours of the procedure, six patients reported pelvic pain/cramping and three experienced nausea and/or vomiting. Postoperative adverse events occurring within 2 weeks included hematometra (0.6%), urinary tract infection (0.6%), vaginal infection (0.6%), pelvic pain/cramping (0.6%), and nausea and/or vomiting (0.6%). Subsequent adverse events reported were hysterectomy (1.7%), hematometra (0.6%), urinary tract infection (1.1%), vaginal infection (2.9%), endometritis (1.1%), pelvic inflammatory disease (1.1%), hemorrhage (0.6%), and pelvic pain/cramping (2.9%). There was no significant difference in the incidence of adverse events between the NovaSure cohort and subjects treated with loop resection plus rollerball.
Post-ablation infection or endometritis after the NovaSure procedure is uncommon, but it has been reported: the incidence of post-ablation endometritis in clinical studies ranges from 0.6%12 to 5%.24 ACOG, recognizing the relatively small risk of infection, does not recommend routine administration of prophylactic antibiotics to the general patient population undergoing endometrial ablation;47 however, special consideration for prophylactic antibiotics should be considered in patients with a history of pelvic inflammatory disease.47
Long-term complications of endometrial ablation include the risk of hematometra or post-ablation tubal sterilization syndrome. One study described an incidence as high as 10% in women after rollerball ablation.48 The authors posited that women with previous tubal occlusion may have retrograde bleeding from persistent or regenerated endometrium into the proximal fallopian tube with no egress. In women without a tubal ligation, contracture or synechiae at the cornua area post-ablation could lead to a cornual hematometra. If the upper endocervical canal is ablated and consequently occluded, the patient could develop a central hematometra. The NovaSure pivotal study reported an incidence of <1% of post-ablation tubal sterilization syndrome and/or hematometra.7 Nonetheless, patients undergoing NovaSure endometrial ablation who have previously had a tubal ligation are at risk of developing post-ablation tubal sterilization syndrome, which can occur as late as 10 years post-procedure.45
With more serious and rare complications, it is necessary to identify large patient populations. Studies47 that reviewed the MAUDE database provide no information on the absolute rate of adverse events because the number of cases performed annually is not known. As the authors noted:
Vast under-reporting of adverse events also likely exists, resulting in unknown numerator data. This, in combination with lack of denominator data, makes the Manufacturer and User Facility Device Experience data unsuitable for determining adverse event rates.47
National studies in Scotland and England reported on the complications associated with first-generation endometrial ablation devices. One patient in the Scottish Audit Study was ultimately determined to have had a visceral injury.49 In the Minimally Invasive Surgical Techniques – Laser, EndoThermal or Endorescetion (MISTLETOE) study of 10,686 subjects, the incidence of thermal injury to the bowel was one in 1,700.50
Hologic, Inc, maintains post-market quality assurance tracking of all reportable complications through its representatives and by direct communication with health care providers. By applying the number of devices shipped, Hologic estimates the rate of bowel injury after NovaSure endometrial ablation is less than one event in 10,000 cases (Hologic, Inc, data on file, 2013).
Conclusion
In the 10 years since the NovaSure procedure was FDA-approved for use in GEA, significant data have been generated that provide a favorable safety profile for the use of the procedure in premenopausal women for the treatment of AUB. Rates of reintervention are low and patient satisfaction is high. Rates of amenorrhea and reduction in heavy menstrual bleeding are consistently higher for women treated with NovaSure endometrial ablation than for women treated with other second-generation ablation devices and, importantly, these rates appear to be stable over time.
Acknowledgment
Dr Gimpelson would like to thank Hologic, Inc, for providing editorial and indirect financial support for this publication.
Disclosure
Dr Gimpelson is a consultant to Hologic, Inc, the manufacturer of the NovaSure endometrial ablation device, and Boston Scientific, the manufacturer of the Genesys HTA device. Dr Gimpelson was an investigator in the Phase III trial for the NovaSure device and an investigator in the Phase III trial for the Genesys HTA device. Dr Gimpelson has received royalties from Cooper Surgical, the manufacturer of the Her Option device, and a research grant from Ethicon, a subsidiary of Johnson and Johnson Ethicon (Gyneclamp), the manufacturer of the ThermaChoice Uterine Balloon system. Dr Gimpelson did not receive any compensation for this project.
References
Committee on Practice Bulletins – Gynecology. Practice bulletin 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120(1):197–206. | |
Davies A, Hart R, Magos A, Hadad E, Morris R. Hysterectomy: surgical route and complications. Eur J Obstet Gynecol Reprod Biol. 2002;104(2):148–151. | |
Morgan H, Advincula AP. Global endometrial ablation: a modern day solution to an age-old problem. Int J Gynaecol Obstet. 2006;94(2):156–166. | |
Committee on Practice Bulletins—Gynecology. Practice Bulletin 81. Endometrial ablation. Obstet Gynecol. 2007;109(5):1233–1248. | |
Kalkat RK, Cartmill RS. NovaSure endometrial ablation under local anaesthesia in an outpatient setting: an observational study. J Obstet Gynaecol. 2011;31(2):152–155. | |
Penninx JP, Mol BW, Bongers MY. Endometrial ablation with paracervical block. J Reprod Med. 2009;54(10):617–620. | |
US Food and Drug Administration, Center for Devices and Radiological Health. P970021 ThermaChoice® Uterine Balloon Therapy System Approval, December 12, 1997. Available from: http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=1645. Accessed on January 6, 2014. | |
US Food and Drug Administration, Center for Devices and Radiological Health. P000032 Her Option™ Uterine Cryoblation Therapy™ System Approval Letter, April 20, 2001. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/P000032A.pdf. Accessed on January 6, 2014. | |
US Food and Drug Administration, Center for Devices and Radiological Health. P000040 Hydro ThermAblator® Endometrial Ablation System Approval Letter, April 20, 2001. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/P000040a.pdf. Accessed on January 6, 2014. | |
US Food and Drug Administration, Center for Devices and Radiological Health. P010013 NovaSure™ Impedance Controlled Endometrial Ablation System Approval Letter, September 28, 2001. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/P010013a.pdf. Accessed on January 6, 2014. | |
US Food and Drug Administration, Center for Devices and Radiological Health. P020031 Microsulis Microwave Endometrial Ablation (MEA) System Approval Letter, September 23, 2003. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf2/P020031a.pdf. Accessed on January 6, 2014. | |
Cooper J, Gimpelson R, Laberge P, et al. A randomized, multicenter trial of safety and efficacy of the NovaSure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9(4):418–428. | |
Zarek S, Sharp HT. Global endometrial ablation devices. Clin Obstet Gynecol. 2008;51(1):167–175. | |
Asgari Z, Moini A, Samiee H, Tehranian A, Mozafar-Jalali S, Sabet S. Endometrial ablation with the NovaSure system in Iran. Int J Gynaecol Obstet. 2011;114(1):73–75. | |
Baskett TF, Clough H, Scott TA. NovaSure bipolar radiofrequency endometrial ablation: report of 200 cases. J Obstet Gynaecol Can. 2005;27(5):473–476. | |
Busund B, Erno LE, Grønmark A, Istre O. Endometrial ablation with NovaSure GEA, a pilot study. Acta Obstet Gynecol Scand. 2003;82(1):65–68. | |
Fulop T, Rákóczi I, Barna I. NovaSure impedance-controlled endometrial ablation: long-term follow-up results. J Minim Invasive Gynecol. 2007;14(1):85–90. | |
Gallinat A. NovaSure impedance-controlled system for endometrial ablation: three-year follow-up on 107 patients. Am J Obstet Gynecol. 2004;191(5):1585–1589. | |
Gallinat A. An impedance-controlled system for endometrial ablation: five-year follow-up of 107 patients. J Reprod Med. 2007;52(6):467–472. | |
Gallinat A, Nugent W. NovaSure impedance-controlled system for endometrial ablation. J Am Assoc Gynecol Laparosc. 2002;9(3):283–289. | |
Bongers MY, Bourdrez P, Mol BW, Heintz AP, Brölmann HA. Randomised controlled trial of bipolar radio-frequency endometrial ablation and balloon endometrial ablation. BJOG. 2004;111(10):1095–1102. | |
Kleijn JH, Engels R, Bourdrez P, Mol BW, Bongers MY. Five-year follow-up of a randomised controlled trial comparing NovaSure and ThermaChoice endometrial ablation. BJOG. 2008;115(2):193–198. | |
Clark TJ, Samuels N, Malick S, Middleton L, Daniels J, Gupta J. Bipolar radiofrequency compared with thermal balloon endometrial ablation in the office: a randomized controlled trial. Obstet Gynecol. 2011;117(5):1228. | |
Abbott J, Hawe J, Hunter D, Garry R. A double-blind randomized trial comparing the Cavaterm and the NovaSure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003;80(1):203–208. | |
Penninx JP, Mol BW, Engels R, et al. Bipolar radiofrequency endometrial ablation compared with hydrothermablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116(4):819–826. | |
Penninx JP, Herman MC, Mol BW, Bongers MY. Five-year follow-up after comparing bipolar endometrial ablation with hydrothermablation for menorrhagia. Obstet Gynecol. 2011;118(6):1287–1292. | |
Daniels JP, Middleton LJ, Champaneria R, et al; International Heavy Menstrual Bleeding IPD Meta-analysis Collaborative Group. Second generation endometrial ablation techniques for heavy menstrual bleeding: network meta-analysis. BMJ. 2012;344:e2564. | |
Laberge PY, Sabbah R, Fortin C, Gallinat A. Assessment and comparison of intraoperative and postoperative pain associated with NovaSure and ThermaChoice endometrial ablation systems. J Am Assoc Gynecol Laparosc. 2003;10(2):223–232. | |
Lukes AS, McBride RJ, Herring AH, Fried M, Sherwani A, Dell D. Improved premenstrual syndrome symptoms after NovaSure endometrial ablation. J Minim Invasive Gynecol. 2011;18(5):607–611. | |
Elmardi A, Furara S, Khan F, Hamza M. NovaSure impedance controlled system for endometrial ablation: the experience of the first UK reference centre. J Obstet Gynaecol. 2009;29(5):419–422. | |
Sabbah R, Desaulniers G. Use of the NovaSure Impedance-Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13(5):467–471. | |
Khan Z, El-Nashar SA, Hopkins MR, Famuyide AO. Efficacy and safety of global endometrial ablation after cesarean delivery: a cohort study. Am J Obstet Gynecol. 2011;205(5):450. e1–e4. | |
Rooney KE, Cholhan HJ. Vesico-uterine fistula after endometrial ablation in a woman with prior cesarean deliveries. Obstet Gynecol. 2010;115(2 Pt 2):450–451. | |
Evantash E. Vesico-uterine fistula after endometrial ablation in a woman with prior cesarean deliveries. Obstet Gynecol. 2010;115(4):869. Available from: http://journals.lww.com/greenjournal/Fulltext/2010/04000/Corrections.38.aspx. Accessed January 17, 2014. | |
El-Nashar SA, Hopkins MR, Feitoza SS, et al. Global endometrial ablation for menorrhagia in women with bleeding disorders. Obstet Gynecol. 2007;109(6):1381–1387. | |
El-Nashar SA, Hopkins MR, Barnes SA, et al. Health-related quality of life and patient satisfaction after global endometrial ablation for menorrhagia in women with bleeding disorders: a follow-up survey and systematic review. Am J Obstet Gynecol. 2010;202(4):348. e1–e7. | |
Neuwirth RS, Loffer FD, Trenhaile T, Levin B. The incidence of endometrial cancer after endometrial ablation in a low-risk population. J Am Assoc Gynecol Laparosc. 2004;11(4):492–494. | |
Krogh RA, Lauszus FF, Guttorm E, Rasmussen K. Surgery and cancer after endometrial resection. Long-term follow-up on menstrual bleeding and hormone treatment by questionnaire and registry. Arch Gynecol Obstet. 2009;280(6):911–916. | |
Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;179(3 Pt 1):569–572. | |
AlHilli MM, Hopkins MR, Famuyide AO. Endometrial cancer after endometrial ablation: systematic review of medical literature. J Minim Invasive Gynecol. 2011;18(3):393–400. | |
Lo JS, Pickersgill A. Pregnancy after endometrial ablation: English literature review and case report. J Minim Invasive Gynecol. 2006;13(2):88–91. | |
Smith SE, Bacher-Lind L. Pregnancy outcomes following a NovaSure® endometrial ablation procedure. J Minim Invasive Gynecol. 2012;19(6):S21. | |
Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102(6):1278–1282. | |
Della Badia C, Nyirjesy P, Atogho A. Endometrial ablation devices: review of a manufacturer and user facility device experience database. J Minim Invasive Gynecol. 2007;14(4):436–441. | |
NovaSure Impedance-controlled Endometrial Ablation System: Instructions for Use and Controller’s Manual. Marlborough, MA: Hologic, Inc; 2010. | |
Brown J, Blank K. Minimally invasive endometrial ablation device complications and use outside of the manufacturers’ instructions. Obstet Gynecol. 2012;120(4):865–870. | |
ACOG Committee on Practice Bulletins – Gynecology. ACOG practice bulletin No 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113(5):1180–1189. | |
McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment and prevention. J Minim Invasive Gynecol. 2007;14(4):399–406. | |
[No authors listed]. A Scottish audit of hysteroscopic surgery for menorrhagia: complications and follow up. Scottish Hysteroscopy Audit Group. Br J Obstet Gynaecol. 1995;102(3):249–254. | |
Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the MISTLETOE study. Minimally Invasive Surgical Techniques – Laser, EndoThermal or Endorescetion. Br J Obstet Gynaecol. 1997;104(12):1351–1359. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.