Back to Journals » Clinical Epidemiology » Volume 15
Temporal Trends in the Disease Burden of Colorectal Cancer with Its Risk Factors at the Global and National Level from 1990 to 2019, and Projections Until 2044
Authors Liu Y, Zhang C , Wang Q, Wu K, Sun Z, Tang Z, Zhang B
Received 15 September 2022
Accepted for publication 5 January 2023
Published 12 January 2023 Volume 2023:15 Pages 55—71
DOI https://doi.org/10.2147/CLEP.S388323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Yang Liu,1 Chao Zhang,2 Qianwen Wang,1 Kangze Wu,3 Zhouyi Sun,1 Zhe Tang,1 Bo Zhang3
1The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, Zhejiang, 322000, People’s Republic of China; 2Center for Evidence-Based Medicine and Clinical Research, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, 442000, People’s Republic of China; 3The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310000, People’s Republic of China
Correspondence: Bo Zhang, The Second Affiliated Hospital, Zhejiang University School of Medicine, 88 Jiefang Road, Hangzhou, Zhejiang, 310000, People’s Republic of China, Tel/Fax +86-0571-87783563, Email [email protected]
Background: This study aimed to evaluate the global colorectal cancer(CRC) trend and the relevant risk factors from 1990 to 2019 and for better policymaking and resource allocation.
Methods: Data on CRC, including incidence, mortality and disability adjusted life year (DALY) rates, were extracted from the 2019 Global Burden of Disease (GBD) study. The estimated annual percentage changes (EAPCs) were calculated to assess the temporal trend of incidence, mortality and DALYs. The Bayesian age-period-cohort model(BAPC) was used to predict the future burden of CRC.
Results: In 2019, a total of 2.17 million CRC cases were reported worldwide, a 157% increase from 1990. In high-social demographic index (SDI) regions, the trend of age-standardized incidence rate(ASIR) tended to decrease, while the proportion of people under 50 years of age tended to increase. Although the number of deaths and DALYs increased, the age-standardized death rate (ASDR) and age-standardized DALY rate decreased. The CRC burden was growing fastest in middle-SDI regions, especially in East Asia, followed by low SDI regions. In addition, the milk intake, High-BMI and high fasting plasma glucose play a more important role in on CRC. The predicted cases and deaths in global continued to increase to 2044. And there is an upward trend in ASIR for both men and women.
Conclusion: In developed regions, the CRC burden continues to decrease, while the CRC burden become more and more severe in developing regions. Overall, the burden of CRC will rising in the near future. Therefore, reasonable resource allocation and prevention policies should be implemented. Developing countries needs more attention.
Keywords: global burden of disease, colorectal cancer, disability-adjusted life year, estimated annual percentage change, age-standardized incidence rate, Bayesian age-period-cohort
Introduction
Colorectal cancer (CRC) is a major component of the global cancer burden, accounting for approximately one-tenth of all cancer cases and deaths.1 Among all types of cancer, the incidence of CRC ranks third, while the mortality rate ranks second.2 Surgical resection and adjuvant chemotherapy are the basic treatment, with prevention being the primary strategy.3,4 In recent years, new, less invasive, and efficient inspection methods have been developed, which have reduced the CRC morbidity and mortality rates to a certain extent.5 Globally, the incidence and mortality of CRC are increasing annually and are expected to exceed 2.2 million new cases and 1.1 million deaths by 2030.6,7 The incidence and mortality of CRC vary widely by age, sex, region, and socioeconomic development.8 Typically, 90% of patients with CRC are aged >50 years, but the number of patients aged <50 years has rapidly increased over the past 20 years.9,10 This may be due to genetic and lifestyle factors. The lifestyle factors that affect the development of CRC include poor eating habits, lack of exercise and sedentary habits, smoking, passive smoking, and excess body weight. Although these factors have increased the number of cases and deaths, they are potentially modifiable and can even have a protective effect.11 By adjusting the risk factors to make population-level changes toward a healthier lifestyle, in some high-risk countries, the incidence of CRC has either decreased or remained stable.12 However, the morbidity and mortality rates are rapidly increasing in several countries with low and medium levels of economic development.7
The Global Burden of Disease (GBD) study estimated 369 diseases and injuries in 204 countries and regions; it provided the data on the incident cases, deaths, and disability-adjusted life-years (DALYs) of CRC from 1990 to 2019, thus allowing a full evaluation of the global burden and trends of CRC. Our research reveals the effects of the socio-demographic index (SDI), geographic location, gender, and age on the incidence rate, death rate, and DALYs of CRC, which updates, supplements, and expands previous research8 to provide new insights for the development of CRC prevention plans in different countries.
Methods
Study Data
This study used data on the incidence rate and death rate of CRC and DALY loss attributable to CRC and the relevant risk factors from 1990 to 2019 obtained from the Global Health Data Exchange(GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool), based on gender, age, region, and country. The 204 countries and territories were divided into five levels by SDI, high, high middle, middle, low middle, and low SDI was calculated using several social factors, including the fertility rate of the population aged <25 years, the education level of the population aged >15 years, and per capita income.13 According to geographical contiguity, the world was divided geographically into 21 different GBD regions, such as Andean Latin America, Western Europe, and Central Asia. The data released from the (GHDx) Query Tool did not require informed patient consent and was publicly available. In addition, the GBD study data followed the guidelines for Accurate and Transparent Health Estimation Reporting for Population Health Research(GATHER).
Statistical Analysis
The annual age-standardized incidence rate (ASIR), age-standardized death rate (ASDR), age-standardized DALY rate, and the corresponding estimated annual percentage changes (EAPCs) were used to assess the trends in the incidence and mortality of CRC. DALY is calculated by adding the years of life with disabilities(YLDs) and years of life lost(YLLs).14 YLD are estimated by combining prevalence estimates with disability weight(DW) associated with various phases of cancer survival, including diagnosis/treatment, remission, metastatic/disseminated and terminal. DW indicates the magnitude of the health loss associated with the outcome, usually in the range 0–1, with 1 indicating a state to death and 0 indicating a state to full health. And the Global Burden Of Disease captured community-representative data through some large population surveys and open-access internet surveys to estimate DW. Age-standardized rate (ASR) is an important indicator that can effectively exclude the influence of age factors to compare the morbidity, mortality, and DALYs between regions or countries with different age structures. Moreover, the ASR trend was used as a basis for determining the changes in human disease patterns and risk factors. The following formula was used to calculate the ASRs (age-standardized incidence/death/DALY rate) (per 100,000 population),15 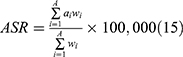 , where ai represents the specific age ratio of the i−th age group, wi represents the number (or weight) of the corresponding age group in the chosen reference standard population, and A represents the number of age groups. Assuming that the natural logarithm of ASR changes linearly with time, EAPC was introduced, a widely accepted method to describe the trend of ASR in a specific time interval.16 The following regression model was used to calculate the natural logarithm of the rates, y = α + βx + ε, where y denotes ln (ASR), x denotes the calendar year, and ε denotes the error term (EAPC = 100 × (exp(β) − 1)); moreover, its 95% confidence interval (CI) can also be obtained using the abovementioned method. The relationship between EAPC and ASR trends can be explained using this method. The trend of ASR is deemed to1 increase when the EAPC and the lower limit of its CI are >0,2 decrease when the EAPC and the upper limit of its CI are <0, and3 stabilize under other conditions. In order to quantify the risk factors, the GBD study incorporated the comparative risk assessment framework that offer a synthesizing evidence on risks and risk–outcome associations.17 In this study, we select deaths and DALYs to model the attributable burden of colorectal cancer to risk.
, where ai represents the specific age ratio of the i−th age group, wi represents the number (or weight) of the corresponding age group in the chosen reference standard population, and A represents the number of age groups. Assuming that the natural logarithm of ASR changes linearly with time, EAPC was introduced, a widely accepted method to describe the trend of ASR in a specific time interval.16 The following regression model was used to calculate the natural logarithm of the rates, y = α + βx + ε, where y denotes ln (ASR), x denotes the calendar year, and ε denotes the error term (EAPC = 100 × (exp(β) − 1)); moreover, its 95% confidence interval (CI) can also be obtained using the abovementioned method. The relationship between EAPC and ASR trends can be explained using this method. The trend of ASR is deemed to1 increase when the EAPC and the lower limit of its CI are >0,2 decrease when the EAPC and the upper limit of its CI are <0, and3 stabilize under other conditions. In order to quantify the risk factors, the GBD study incorporated the comparative risk assessment framework that offer a synthesizing evidence on risks and risk–outcome associations.17 In this study, we select deaths and DALYs to model the attributable burden of colorectal cancer to risk.
For cancer burden prediction, several models have been proposed, such as exponential regression model,18 generalized linear models,19 Nordpred model, Joinpoint model and Bayesian age-period-cohort (BAPC) model.20 Among these models, the BAPC model was shown to have a relatively lower error rate, especially for short-term projections, thus we chose it to predict the trend of CRC through 2044.21–23 In a word, the age-period-cohort model, regarded as a log-linear Poisson model, assumes a multiplying effects of age, period and cohort: 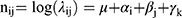 In the formula, i (1 ≤ i ≤ I) stand for the age group at time nodes j (1 ≤ j ≤J); μ, αij,βij and γk are the intercept, age, period, and cohort effect; k = M (I–i) + j, represents cohort index, which influence by multiple parameters, such as age, period index and period interval et al. M represents how many times the age group intervals are the periodic intervals. For instance, M is 5 in our study, since 5-year age groups and yearly data were used. And based on the data on 2019, the predicted results are compared. We used the CRC incidence in 2019 as the baseline, with an increase of 1% per year as a negative reference and a decrease of 1% per year as an optimistic reference. R package BAPC and INLA was used to implement a BAPC model integrated nested Laplace approximations. To verify the stability of the prediction model, nordpred age-period-cohort analysis was run by the Nordpred package in R. All statistical analyses were performed using R (version 4.4.1).
In the formula, i (1 ≤ i ≤ I) stand for the age group at time nodes j (1 ≤ j ≤J); μ, αij,βij and γk are the intercept, age, period, and cohort effect; k = M (I–i) + j, represents cohort index, which influence by multiple parameters, such as age, period index and period interval et al. M represents how many times the age group intervals are the periodic intervals. For instance, M is 5 in our study, since 5-year age groups and yearly data were used. And based on the data on 2019, the predicted results are compared. We used the CRC incidence in 2019 as the baseline, with an increase of 1% per year as a negative reference and a decrease of 1% per year as an optimistic reference. R package BAPC and INLA was used to implement a BAPC model integrated nested Laplace approximations. To verify the stability of the prediction model, nordpred age-period-cohort analysis was run by the Nordpred package in R. All statistical analyses were performed using R (version 4.4.1).
Results
Incidence Burden of Colorectal Cancer
The number of CRCs worldwide has been increasing annually, and 2.166×106 (95% uncertainty interval (UI), 1.996–2.342) CRC cases have been reported in 2019, a 157% increase compared with the 842.1 (95%UI, 810.41–868.57) cases reported in 1990. Moreover, ASIR showed an increasing trend from 22.25/100,000 persons (95% UI, 21.29–22.97) in 1990 to 26.71/100,000 persons (95% UI, 24.58–28.89) in 2019, with an average annual rate of 0.58% (Table 1). In the past 30 years, the annual incidence rate of men (0.95%) has increased much higher than that of women (0.11%). In all age groups, the incidence rate was higher in men than in women (Figure 1A). The number of CRC cases in men was also higher than that in women, and the incidence peak at the age of 65–69 years and 70–74 years, respectively. However, in the >85-year age groups, the number of cases was much higher in women than in men. In all SDI and geographic regions, ASIR was higher in men than in women in 2019 (Figure 1B). More detail about Age distribution of incidence (per 100,000) for CRC in countries are shown in Table S1. Based on the results of the SDI level analysis, as presented in Table 1, only the ASIR trend of the high SDI region decreased, and a sharp increase was observed in the middle-SDI region. At the global level, the incidence of CRC in younger individuals was much higher than that in older patients, especially in those aged 30–34 years; this can also be observed in the high and high-middle SDI regions (Figure 1C).
 |
Table 1 The Incident Cases and ASIR in 1990 and 2019 and Its Temporal Trends |
For geographic regions, except for high-income Asia Pacific, other regions with the highest ASIR, including Australasia and high-income North America, the ASIR for CRC declined (Figure 1D). Sub-Saharan Africa and South Asia had the lowest ASIR for CRC (Table S2). In terms of CRC incidence in both men and women, East Asia showed the largest increase in ASIR for CRC. With the increase in the SDI, the ASIR of each region from 1990 to 2019 increased (Figure 2A). However, the four regions with the highest SDI, including Western Europe, high-income Asia Pacific, high-income North America, and Australasia, had a downward trend in ASIR. The largest increase was observed in Equatorial Guinea, followed by Vietnam and China. The largest decrease was observed in Austria, followed by Kyrgyzstan and Luxembourg (Figure 3A; Tables S3 and S4 and Figure S1).
Death Burden of Colorectal Cancer
Worldwide, the number of CRC-related deaths was 9.264×106 ((95% UI, 0.831–1.011)) in 2019 and increased to 109.56% from 1990 to 2019, but the ASDR of CRC declined (Table 2). In 1990–2019, the ASDR of men was higher than that of women, and the gap widened. As shown in Figure 4A, the mortality rates in both sexes increased with age. At the SDI level, the ASDR trend in the high SDI region decreased, stabilized in the high-middle SDI region, and increased in the middle, low-middle, and low SDI regions. The ASDR of the high SDI region decreased in every age group; in the other SDI regions, the ASDR of younger individuals decreased, while the ASDR of the elderly increased (Figure 4C). Table S5 shows age distribution of death rate for CRC in different countries in 2019.
 |
Table 2 The Death Cases and ASDR in 1990 and 2019 and Its Temporal Trends |
On observation from the geographic regions level, except for Andean Latin America, men in other regions had higher ASDR than women (Figure 4B). Meanwhile, the ASDR of each region from 1990 to 2019 had a nonlinear relationship with SDI; until the value of SDI reached about 0.75, a downward trend was observed (Figure 2B). In Figure 4D, the ASDR of women was much higher than that of men in the low SDI region, which can also be found in Figure 1D and Figure S2. In Eritrea, Marshall Islands, and Uganda, the abovementioned phenomena were more obvious (Figures S3-S5). In 2019, the ASDR of CRC differed substantially between countries and territories, with Greenland, Brunei Darussalam, and Greenland showing the highest ASDRs. By contrast, Bangladesh, Somalia, and Nepal showed the lowest ASDRs (Figure S6). Equatorial Guinea, Cabo Verde, and Vietnam showed the largest ASDR increase; meanwhile, Austria, Singapore, and Luxembourg showed the largest ASDR decline (Figure 3B), details are shown in Tables S3 and S6.
DALYs Burden of Colorectal Cancer
At the global level, 24.284.09×106 (95% UI, 22.614–25.723) DALYs were reported in 2019, doubling the number of DALYs in 1990, but the age-standardized DALY rate slightly decreased with an EAPC of −0.21 (Table 3). In 2019, the burden of CRC DALY rate showed a pattern similar to that of the death burden, regardless of age, sex, or region (Figures S2 and S7-S9; Table S7). Based on the SDI level, the high-middle SDI region had the highest age-standardized DALY rate, followed by the high SDI region. These two regions showed a decreasing trend in age-standardized DALY rates.
 |
Table 3 The DALY and Age-Standardized DALY Rate in 1990 and 2019 and Its Temporal Trends |
Central Asia is the only region that showed an increase in ASDR, but the trend of the age-standardized DALY rate declined. The increase in the age-standardized DALY rate was the largest in Equatorial Guinea, followed by Lesotho and Vietnam. The decrease in the age-standardized DALY rate was largest in Austria, followed by Singapore and Luxembourg (Figure 4C); details are shown in Tables S2, S3 and S8, and Figures S10 and S11.
Projection of Colorectal Cancer Trends
With the cancer prediction model and CRC data derived from the GBD website, the morbidity and mortality of CRC in the next 25 years were projected. From 2020 to 2044, the global trend of CRC will not be optimistic, especially among males. For both male and female, the ASIR for CRC will continue to increase, due to the population growth and aging (Figure S12). But the ASDR were predicted to rise slowly for males and to fall slowly for females after 2035 years, largely due to advances in treatment (Figure 5A). And the predicted results from the Nordpred age-period-cohort analysis show a similar trend (Figure S13).
During 2020 to 2044, the CRC case number will reach 6.098×106, about 3 times that of 2019. In Figure 5B, the predicted incidence of males is much higher than the negative reference (1% increased rate annually), while females are close to the negative reference. That means that existing prevention policies and their scope are insufficient to limit the growth of the global burden of CRC, therefore the importance of prevention and treatment of CRC should be further emphasized. In addition, although the increase in overall deaths is lower than the negative reference, the number of male deaths will be higher than the negative reference after 2037. Meanwhile, the number of deaths among female will remain stable before 2040.
Risk Factors Attributable to Colorectal Cancer Burden
In 2019, the three highest attributable proportions of risk factors for CRC were diet low in whole grains (15.8% [6.1–20.7%]), diet low in milk (15.3% [9.9–20.7%]), and smoking (12.9% [8.6–17.5%]), which was different from those reported in a previous study, which analyzed the data from 2017 (the top three risk factors, diet low in calcium, alcohol use, and diet low in milk). Compared with 1990, the risk caused by diets low in milk has exceeded the risk caused by smoking. The attributable risk proportions for CRC caused by smoking, alcohol use, and diet high in processed meat decreased, while proportions caused by high fasting plasma glucose level and high body mass index (BMI) increased (Figure 6A). The proportion of risk factors contributing to CRC deaths differed by country and age (Tables S9 and S10). In 2019, along with age, the attributable proportion of diet low in milk and calcium for CRC decreased, but the proportion of high fasting plasma glucose and low physical activity levels increased. Moreover, the attributable proportion associated with smoking for CRC deaths initially increased with age, but eventually decreased, peaking at ages 60–64 years and 65–69 years (Figure S14).
At the regional level, Central Europe and Australasia had highest attributable proportion of smoking and alcohol use for CRC deaths, respectively, in 2019. The highest attributable proportion of diet in low milk for CRC deaths was observed in Central Sub-Saharan Africa in 1990–2019 and far surpassed other countries. The proportion of deaths attributable to diet low in whole grain and calcium was highest in Central Asia (21%) and Southeast Asia (25%), respectively (Figure S15; Tables S11 and S12).
The proportions of risk factors for CRC DALY that can be attributed had a similar pattern to CRC deaths (Figure 6B; Figures S16 and S17). The attributable proportion of risk factors for both CRC death and DALY differed by gender. Except for low physical activity, the risk contributed by other factors for CRC was higher in men compared with that in women (Figure 6C and D). In women, high BMI and high fasting plasma glucose level were the 8th and 4th leading risk of CRC-related death, respectively, in the past 30 years, which was not observed in men (Figures S18 and S19). Compared with that reported in 2017, diets low in milk and high BMI accounted for a greater proportion of women’s CRC risk in 2019 (Figures S20 and S21).
Discussion
This study, based on GBD2019, evaluated the up-to-date global, regional, and national patterns in incidence, mortality, and DALYs associated with CRC and leading risk factors for CRC deaths and DALYs. The number of cases, deaths, and DALYs of CRC have increased tremendously over the last 30 years globally. And with the exception of ASIR, ASDR and age-standardized DALY rates have declined from 1990 to 2019. At present, the major burden of CRC is shifting toward the developing regions from developed regions. Looking at the next 25 years, the global colorectal burden will continue to grow. The following reasons which we speculate might be partly explained this increase:1) the population growth and population aging;24 2) low-SDI regions which have strong potential to increase burden without well-established prevention and treatment systems;25 3) advances in screening methods lead to higher detection rates;26 4) increased dietary risks (such as lack of milk, whole grain, and calcium, etc.) due to resource shortages since the outbreak of COVID-19;27 5) the westernization of life pattern of low-development region with the development of economy. Given this changing trend in burden, CRC will increasingly be the primary public health burden in some developing countries with lower incidence rates currently.
Decades ago, more than 90% of patients with CRC were aged >50 years.10 The incidence of CRC in the younger individuals worldwide has increased rapidly, mainly reported in the high SDI and high-middle SDI regions, which is consistent with the results of previous research.28,29 However, this mechanism remains unclear. A possible hypothesis is that early exposure to potential risk factors may lead to genetic changes in colorectal epithelial cells, gut microbiota, and host immunity.30 In addition, changes in lifestyle habits during adulthood and adolescence, such as increased intake of sugary drinks, also increase the risk of CRC.31 The reason for the decreased incidence of CRC in older individuals is that precancerous polyps are removed during early screening.32 The change in the age of onset means that the target population for screening also needs to be changed. At present, CRC screening in most countries is performed after the age of 50 years, and the American Cancer Society recommends the initiation of screening at the age of 45 years.26,33 Moreover, each country should appropriately relax the age of the population targeted for CRC screening in consideration of the economic benefits.
From 1990 to 2019, gender differences increased, and the burden of CRC in men was much higher than that in women. Two abnormal situations require attention. In the Andean Latin American region, the burden of CRC was higher in women than in men, which has also been reported in previous studies.29 Another phenomenon was observed in the low-SDI region, the burden of CRC in women has high rate of increase compared with that in men, which may be due to lifestyle and genetics. For example, in Latin American countries, residents tend to have excessive sugar intake, especially women, but high sugar intake increases the risk of survival in patients with CRC.34,35 At present, this phenomenon has not been reported in other studies, and further research is required to determine its possible causes. Meanwhile, countries in Andean Latin America and low SDI regions should consider the importance of abnormal gender differences when formulating prevention and treatment policies.
Currently, the region with the highest rate of growing burden of CRC is East Asia, mainly in China, primarily due to industrialization and economic development, leading to westernization of lifestyle.36,37 CRC screening projects are still in the early stages, and the coverage and participation rate of the population are relatively low.38,39 Nearly 50% of the increase in CRC burden in China may be attributed to seven major risk factors.40 Therefore, China must take measures to improve its shortcomings, and should further optimize the screening policy, adopt noninvasive methods, increase the acceptance of citizens, and expand the scope of screening.38
Africa has always been regarded as a region with low CRC burden,41 however, some countries in Africa, Equatorial Guinea, and Lesotho have the fastest rate of increase of CRC burden. Another concern is the high mortality rate of CRC in Africa and Oceania, despite its low incidence. In a report of CRC survival in sub-Saharan Africa, the 5-year survival rate of CRC patients was very low, and the mortality rate was approximately 3 times that of developed countries.42 For low-income and middle-income countries, CRC screening programs should be cost-effective and culturally acceptable, healthcare infrastructure needs to be improved, and policies that can promote healthy lifestyles and reduce the involvement of high-risk factors should be developed.43,44 In addition, subsequent treatment is the key to reducing mortality. Fortunately, sub-Saharan Africa has made great achievements in universal health coverage since 2010.45 What needs to be done further is how to allocate resources reasonably, improve the quality of medical care, reduce medical costs, and attain equitable healthcare. Without the help of other countries is hard to accomplish, thus we hope that countries with spare capacity can provide medical assistance to low-income countries and jointly improve the global CRC burden.
In 2019, diet in low milk, which increases the risk of CRC, was more prevalent in women than before.46 In general, dietary risks were reduced compared with that reported in 1990 but remained the number 1 risk factor of CRC. This means that although multiple dietary interventions have been formulated, it is difficult to achieve an optimal global diet.47 However, we believe that the attributable proportion of dietary risks for CRC burden will increase during the COVID-19 pandemic. On the one hand, the global disruptions in the supply of food, such as vegetables, meat products and milk. On the other hand, the economic depression has caused low-income households to cut back on non-essential spending.48 Ultimately reduces dietary diversity, increasing CRC risk. In addition, the effects of high BMI, high fasting plasma glucose level and low physical activity level, which have become more important over the past 30 years, cannot be ignored. These factors are directly related to obesity, which currently affects more than 2 billion people worldwide in the world.49 In the era of the new crown epidemic, working from home has become the new normal for work in various countries, and the risk of obesity is also increasing due to easy access and excessive intake.50 Over the past 30 years, more than 40 countries have implemented drinking guidelines, which have effectively controlled alcohol intake, but this is still far from enough; alcohol use was still the primary risk factor for CRC in the Australasia region.51 The risk of smoking has decreased significantly, but it remains the number 1 risk factor of CRC in men. In this dilemma modification caused by COVID-19, each country should recognize the inadequacy of its own dietary diversity and formulate the appropriate dietary policy and adopt stronger policies for smoking and alcohol use. However, efforts should not only include the proposal of relevant policies but also the implementation of these policies at the individual level.52,53
At present, there is growing evidence that inflammatory bowel disease, antibiotic misuse, and diabetes are risk factors for early-onset colorectal cancer. However, no investigation for these variables in the GBD database. A meta-analysis demonstrates that long-term inflammatory bowel disease increases the risk of developing CRC by approximately 2–3 times, and that the risk accumulates over time.54 A case-control study of 4029 patients with the use of antibiotics determined that with increased antibiotic use comes increased risk of CRC.55 And in a large cohort study, diabetes was significantly associated with colon cancer.56 Thus, efforts should be undertaken to define the role of those risk factors in increasing of CRC burden.
Our study has some limitations. The quality and quantity of the GBD data determine the accuracy of our results.57 If real data of disease burden were unavailable, GBD data were estimated using modeling methods, and hence uncertainty existed.58 Besides, the data collection performed in the GBD study aimed at obtaining country-specific estimates, and the data collection and coding of each country were different, which may lead to data processing bias. Third, the short-term trend of colorectal cancer burden may be masked due to assessment of the long-term trend from 1990 to 2019. Fourth, our predicted results may underestimate the change in colorectal cancer burden. Finally, due to the limited information available in the GBD database, we were unable to further analyze trends in colorectal cancer burden by histological subtype. Despite these limitations, based on large samples of cancer data and advanced modeling methods, our results demonstrate past, present, and future trends in CRC burden.
Conclusion
Globally, ASIR of CRC increased annually, while ASDR and age-standardized DALY rates of CRC decreased. The CRC was predicted to impose a heavier burden on global health care system in the future due to growing population and increasing exposure risk. Compared with decreasing CRC burden in the developed areas, the CRC burden in developing countries is increasing rapidly. The incidence of CRC increased in the younger individuals in the high SDI region, signifying the need to strengthen CRC screen at the age< 50 years. The risk factors associated with obesity, including high BMI, high fasting plasma glucose level, and low physical activity level, should be paid more attention to. Consequently, based on the abovementioned studies, reasonable resource allocation, prevention policies and new effective treatment strategies should be implemented to prevent the burden of CRC from increasing, particularly in developing countries.
Abbreviations
ASDR, Age-standardized death rate; ASIR, Age-standardized incidence rate; ASR, Age-standardized rate; DALY, Disability-adjusted life year; EAPC, Estimated annual percentage change; GBD, Global burden of disease; SDI, Social-demographic index; UI, Uncertainty interval; BMI, Body−mass index; CI, confidence interval; BAPC, Bayesian age-period-cohort.
Data Sharing Statement
The datasets for this article are available from the Global Health Data Exchange query tool (http://ghdx.healthdata.org/gbd-results-tool).
Ethics Approval and Consent to Participate
This study was approved by The Fourth Affiliated Hospital, College of Medicine, Zhejiang University. The data obtained from the Global Health Data Exchange Query Tool did not require informed patient consent.
Acknowledgments
We would like to thank the Global Burden of Disease for providing open access to the database.
Funding
This work was supported by grants from the National Natural Science Foundation of China, No. 81570698 (Dr. Bo Zhang) and the key project of Natural Science Foundation of Zhejiang Province (LZ20H160002), key research and development project of Zhejiang province (2021C03048).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi:10.1016/S0140-6736(13)61649-9
4. Roncucci L, Mariani F. Prevention of colorectal cancer, How many tools do we have in our basket? Eur J Intern Med. 2015;26(10):752–756. doi:10.1016/j.ejim.2015.08.019
5. Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. doi:10.1038/521S1a
6. Barbirou M, Woldu HG, Sghaier I, et al. Western influenced lifestyle and Kv2.1 association as predicted biomarkers for Tunisian colorectal cancer. BMC Cancer. 2020;20(1):1086. doi:10.1186/s12885-020-07605-7
7. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi:10.1136/gutjnl-2015-310912
8. Safiri S, Sepanlou SG, Ikuta KS. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017, a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):913–933. doi:10.1016/S2468-1253(19)30345-0
9. Lancet Oncology T. Colorectal cancer, a disease of the young? Lancet Oncol. 2017;18(4):413. doi:10.1016/S1470-2045(17)30202-4
10. Patel SG, Ahnen DJ. Colorectal cancer in the young. Curr Gastroenterol Rep. 2018;20(4):15. doi:10.1007/s11894-018-0618-9
11. Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. doi:10.3322/caac.21440
12. Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–49.e15. doi:10.1053/j.gastro.2020.02.068
13. Yang X, Zhang T, Zhang H, Sang S, Chen H, Zuo X. Temporal trend of gastric cancer burden along with its risk factors in China from 1990 to 2019, and projections until 2030, comparison with Japan, South Korea, and Mongolia. Biomark Res. 2021;9(1):84. doi:10.1186/s40364-021-00340-6
14. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010, a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2197–2223. doi:10.1016/S0140-6736(12)61689-4
15. Liu Z, Jiang Y, Yuan H, et al. The trends in incidence of primary liver cancer caused by specific etiologies, results from the global burden of disease study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–683. doi:10.1016/j.jhep.2018.12.001
16. Hankey BF, Ries LA, Kosary CL, et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control. 2000;11(1):31–35. doi:10.1023/A:1008953201688
17. Murray CJ, Lopez AD. On the comparable quantification of health risks, lessons from the global burden of disease study. Epidemiology. 1999;10(5):594–605. doi:10.1097/00001648-199909000-00029
18. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020, global burden of disease study. Lancet. 1997;349(9064):1498–1504. doi:10.1016/S0140-6736(96)07492-2
19. Stock C, Mons U, Brenner H. Projection of cancer incidence rates and case numbers until 2030, A probabilistic approach applied to German cancer registry data (1999-2013). Cancer Epidemiol. 2018;57:110–119. doi:10.1016/j.canep.2018.10.011
20. Riebler A, Held L. Projecting the future burden of cancer, Bayesian age-period-cohort analysis with integrated nested Laplace approximations. Biom J. 2017;59(3):531–549. doi:10.1002/bimj.201500263
21. Yu J, Yang X, He W, Ye W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int J Cancer. 2021;149(5):993–1001. doi:10.1002/ijc.33617
22. Du Z, Chen W, Xia Q, Shi O, Chen Q. Trends and projections of kidney cancer incidence at the global and national levels, 1990-2030, a Bayesian age-period-cohort modeling study. Biomark Res. 2020;8:16. doi:10.1186/s40364-020-00195-3
23. Knoll M, Furkel J, Debus J, Abdollahi A, Karch A, An SC. R package for an integrated evaluation of statistical approaches to cancer incidence projection. BMC Med Res Methodol. 2020;20(1):257. doi:10.1186/s12874-020-01133-5
24. Gu D, Andreev K, Dupre ME. Major trends in population growth around the world. China CDC Weekly. 2021;3(28):604–613. doi:10.46234/ccdcw2021.160
25. Dieleman J, Campbell M, Chapin A. Evolution and patterns of global health financing 1995-2014, development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet. 2017;389(10083):1981–2004. doi:10.1016/S0140-6736(17)30874-7
26. Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults, 2018 guideline update from the American cancer society. CA Cancer J Clin. 2018;68(4):250–281. doi:10.3322/caac.21457
27. de Paulo Farias D, Dos Santos Gomes MG. COVID-19 outbreak, What should be done to avoid food shortages? Trends Food Sci Technol. 2020;102:291–292. doi:10.1016/j.tifs.2020.06.007
28. Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–2185. doi:10.1136/gutjnl-2019-319511
29. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. 2020;158(2):341–353. doi:10.1053/j.gastro.2019.07.055
30. Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18(4):230–243. doi:10.1038/s41571-020-00445-1
31. Hur J, Otegbeye E, Joh HK, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut. 2021;70(12):2330–2336. doi:10.1136/gutjnl-2020-323450
32. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008, a joint guideline from the American cancer society, the US multi-society task force on colorectal cancer, and the American college of radiology. CA Cancer J Clin. 2008;58(3):130–160. doi:10.3322/CA.2007.0018
33. Zavoral M, Suchanek S, Majek O, et al. Colorectal cancer screening, 20 years of development and recent progress. World J Gastroenterol. 2014;20(14):3825–3834. doi:10.3748/wjg.v20.i14.3825
34. Zoltick ES, Smith-Warner SA, Yuan C, et al. Sugar-sweetened beverage, artificially sweetened beverage and sugar intake and colorectal cancer survival. Br J Cancer. 2021;125(7):1016–1024. doi:10.1038/s41416-021-01487-7
35. Fisberg M, Kovalskys I, Gómez G, et al. Total and added sugar intake, assessment in eight Latin American countries. Nutrients. 2018;10:4. doi:10.3390/nu10040389
36. Keum N, Giovannucci E. Global burden of colorectal cancer, emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–732. doi:10.1038/s41575-019-0189-8
37. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China, good or bad news from the 2018 global cancer statistics? Cancer Commun. 2019;39(1):22. doi:10.1186/s40880-019-0368-6
38. Chen H, Li N, Ren J, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. 2019;68(8):1450–1457. doi:10.1136/gutjnl-2018-317124
39. Callinan S, Mojica-Perez Y, Wright CJC, et al. Purchasing, consumption, demographic and socioeconomic variables associated with shifts in alcohol consumption during the COVID-19 pandemic. Drug Alcohol Rev. 2021;40(2):183–191. doi:10.1111/dar.13200
40. Gu MJ, Huang QC, Bao CZ, et al. Attributable causes of colorectal cancer in China. BMC Cancer. 2018;18(1):38. doi:10.1186/s12885-017-3968-z
41. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–378. doi:10.3322/caac.20038
42. Gullickson C, Goodman M, Joko-Fru YW, et al. Colorectal cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index, A population-based registry study. Int J Cancer. 2021;149(8):1553–1563. doi:10.1002/ijc.33715
43. Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138(4):345–355. doi:10.1161/CIRCULATIONAHA.117.032047
44. Schliemann D, Ramanathan K, Matovu N, et al. The implementation of colorectal cancer screening interventions in low-and middle-income countries, a scoping review. BMC Cancer. 2021;21(1):1125. doi:10.1186/s12885-021-08809-1
45. Malagón-Rojas JN. Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990-2019, a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1250–1284. doi:10.1016/S0140-6736(20)30750-9
46. Kawada T. Milk intake and risk of colorectal cancer. J Nutr. 2019;122(1):120. doi:10.1017/S000711451900059X
47. Afshin A, Penalvo J, Del Gobbo L, et al. CVD prevention through policy, a review of mass media, food/menu labeling, taxation/ subsidies, built environment, school procurement, worksite wellness, and marketing standards to improve diet. Curr Cardiol Rep. 2015;17(11):98. doi:10.1007/s11886-015-0658-9
48. Laborde D, Martin W, Swinnen J, Vos R. COVID-19 risks to global food security. Science. 2020;369(6503):500–502. doi:10.1126/science.abc4765
49. Caballero B. Humans against obesity, who will win? Adv Nutr. 2019;10(suppl_1):S4–s9. doi:10.1093/advances/nmy055
50. Birimoglu Okuyan C, Begen MA. Working from home during the COVID-19 pandemic, its effects on health, and recommendations, The pandemic and beyond. Perspect Psychiatr Care. 2022;58(1):173–179. doi:10.1111/ppc.12847
51. LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and cancer, A statement of the American society of clinical oncology. Am J Clin Oncol. 2018;36(1):83–93. doi:10.1200/JCO.2017.76.1155
52. Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60.e16. doi:10.1053/j.gastro.2014.12.035
53. Levy D, Huang A, Havumaki J, Meza RJ. The role of public policies in reducing smoking prevalence, results from the Michigan SimSmoke tobacco policy simulation model. Cancer Causes Control. 2016;27(5):615–625. doi:10.1007/s10552-016-0735-4
54. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis, a meta-analysis. Gut. 2001;48(4):526–535. doi:10.1136/gut.48.4.526
55. Dik VK, van Oijen MG, Smeets HM, Siersema PD. Frequent use of antibiotics is associated with colorectal cancer risk, results of a nested case-control study. Dig Dis Sci. 2016;61(1):255–264. doi:10.1007/s10620-015-3828-0
56. Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–1167. doi:10.1093/aje/kwh161
57. Liu Z, Jiang Y, Yuan H, et al. The trends in incidence of primary liver cancer caused by specific etiologies, results from the global burden of disease study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–683.
58. Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017, results from the global burden of disease study 2017. J Hematol Oncol. 2019;12(1):140. doi:10.1186/s13045-019-0828-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






