Back to Journals » Journal of Pain Research » Volume 15
Temporal Summation and Aftersensations of Second Pain in Women with Myofascial Temporomandibular Disorder Differ by Presence of Temporomandibular Joint Pain
Authors Santiago V, Janal MN, Cook DB, Raphael KG
Received 8 July 2022
Accepted for publication 8 October 2022
Published 19 October 2022 Volume 2022:15 Pages 3275—3286
DOI https://doi.org/10.2147/JPR.S381640
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Keith
Vivian Santiago,1 Malvin N Janal,2 Dane B Cook,3,4 Karen G Raphael1
1Department of Oral & Maxillofacial Pathology, Radiology & Medicine, New York University College of Dentistry, New York, NY, USA; 2Department of Epidemiology and Health Promotion, New York University College of Dentistry, New York, NY, USA; 3Research Service, William S. Middleton Memorial Veterans Hospital, Madison, WI, USA; 4Department of Kinesiology, University of Wisconsin-Madison School of Education, Madison, WI, USA
Correspondence: Vivian Santiago, Department of Oral & Maxillofacial Pathology, Radiology & Medicine, New York University College of Dentistry, 137th East 25th Street, Rm 731, New York, NY, 10010, USA, Tel +1 212 998-9419, Email [email protected]
Purpose: Mechanisms underlying myofascial temporomandibular disorder (mTMD) are poorly understood. One theory is dysfunction in the central mediation of pain, specifically in enhanced facilitatory pain modulation. Because mechanisms leading to central sensitization may differ for joint and muscle pain, this study of mTMD addressed phenotypic heterogeneity by temporomandibular (TM) joint pain in the examination of quantitative sensory testing (QST).
Patients and Methods: The stimulus dependent increase in second pain (temporal summation (TS)) and associated aftersensations (AS) were examined across groups of women with mTMD with TM joint pain and without, and a demographically matched control group.
Results: TS was slightly more evident in mTMD without joint pain vs with (p = 0.035), but AS were most robustly persistent in the group with joint pain vs without (p < 0.002).
Conclusion: While both subgroups demonstrated evidence of central sensitization relative to controls on one of two measures, differences in QST results, if replicated, may point to possible differences in the mechanisms that yield central sensitization. Alternatively, it may represent methodological artifacts that need to be addressed. Therefore, greater consideration should be given to symptom-based phenotypes in studies examining TS and AS.
Keywords: endogenous pain modulation, facilitatory pain modulation, central sensitization, muscle pain, wind-up
Introduction
The causes of painful myofascial Temporomandibular Disorder (mTMD), an orofacial pain condition reportedly affecting more than 10% of women in the United States,1 continue to elude researchers. Although many treatment options are available (eg, self-management, medications, physical therapy, intraoral appliances, etc.),2 as described by the National Academy of Sciences’ recent report on TMDs, “[e]vidence about the safety and efficacy of these treatments is sparse.” Moreover, innovative research approaches to study biopsychosocial mechanisms of TMD pain are essential to develop safe and effective treatments.2
The neurobiological theories of mTMD have converged on potential central nervous system modulatory pain mechanisms.3–5 One such mechanism is enhanced excitatory pain modulation leading to, or as a marker of central sensitization.5–8 Central sensitization is a phenomenon of pain hypersensitivity due to “a prolonged but reversible increase in excitability and synaptic efficacy of neurons in central nociceptive pathways”, that can be triggered by nociceptive inputs.9 Wind-up is one mechanism associated with central sensitization. It is “a frequency-dependent increase in the excitability of spinal cord neurones, evoked by electrical stimulation of afferent C-fibers”.10 In humans, temporal summation (TS) of second pain is a perceptual correlate of wind-up.10 Second pain11 to heat stimuli is a C-fiber mediated slow burning pain that usually comes about 1 second after a heat pulse and follows the A-delta fiber mediated first pain. In healthy humans, TS of second pain has been used to infer central nervous system excitability to nociceptive stimuli.10,12 The study of aftersensations has evolved as an extension of TS procedures where pain is examined for several minutes after stimulation ceases and interpreted as a measure of central sensitization in the post stimulation period.13,14
Despite increasing research on endogenous pain modulation in mTMD, consistent evidence of enhanced TS in mTMD has not emerged.6–8 Evidence of enhanced TS in mTMD for mechanical pain was reported by all15–19 but one20 of six studies while results for TS of heat pain were consistently null.6,21–23 One seldom explored factor in TS studies is whether phenotypic heterogeneity poses a challenge to causal inference. For instance, 8 of the 17 identified published studies on TS include a mix of TMD diagnoses,24–28 while the remaining studies were more specific in their diagnostic sampling of myofascial TMD.15–23 This heterogeneity of case definitions may contribute to inconsistent results. To our knowledge, no studies have compared results across TMD diagnostic categories (ie, articular vs myogenic) to examine this possibility, and studies of TS specifically in mTMD patients show mixed results.
Even studies specifically on mTMD are phenotypically heterogenous because most individuals meeting mTMD criteria also meet criteria for at least one other articular TMD subtype.29–31 This heterogeneity by joint pain may reflect a mix of neurobiological mechanisms driving pain. For example, the mechanism for joint pain may be largely peripheral in nature (ie, inflammation of the joint)32 while no reliable peripheral cause for the muscle-based pain in mTMD has been isolated.4 Our previous research found background masseter muscle activity during sleep was lower among mTMD with joint pain potentially reflecting inhibition of muscle activity due to inflammatory joint pain.33 If joint pain reflects varying degrees of peripheral and central pain mechanisms, accounting for it may reduce the variability seen in psychophysical assessments. Without a similar alternative peripheral explanation for central sensitization when no joint pain is present, perhaps mTMD with muscle-only pain may serve as clearer test of dysfunction in central mediation of pain. This may, in turn, help isolate dysfunction in endogenous pain modulation to better study its etiology, improve research on mechanisms of myofascial TMD, and help accelerate therapeutic research of clinical importance.
The present study employs the novel approach of taking TM joint pain into account in the examination of psychophysical data on excitatory pain modulation in myofascial TMD. The aims are to examine (1) temporal summation (TS) and (2) aftersensations (AS) of heat pain stimuli among women with research diagnosed myofascial TMD34 with and without TM joint pain on palpation, and controls. The expectation is that isolating muscle-only pain will provide a clearer picture of central modulatory mechanisms driving myofascial TMD pain. As a first step, we hypothesize that results of quantitative sensory testing will differ by presence or absence of TM joint pain.
Materials and Methods
Sample
Data were drawn from the Sleep Bruxism and Central Sensitization in Myofascial Pain Study (R01DE018569), a case-control study of adult women positive for mTMD and a group of demographically matched controls without mTMD.21,35 All subjects were recruited via advertisements or during treatment at a university dental clinic. The exclusion criteria were history of trauma to the face and dental treatment in the 48 hours prior to the research clinical examination.21,35 The original study and the present study complied with the Declaration of Helsinki and with ethical oversight by the NYU Medical School Institutional Review Board (IRB#07‐303 and IRB-FY2019-3403). All subjects underwent informed consent procedures before enrollment. For inclusion in the present analyses, completed psychophysics procedures were required (ie, at least 1 completed trial during the testing phase including four ratings of pain over repeated stimuli followed by ratings of aftersensations).
Measures
Clinical Research Diagnosis of Myofascial TMD and Fibromyalgia
Myofascial TMD clinical research diagnosis was determined via clinical research examination using the Research Diagnostic Criteria for TMD (RDC-TMD).21,34,35 Participants were classified as mTMD positive per the RDC-TMD34 if they self-reported pain in the orofacial region, reported three or more tender points with palpation out of the 20 muscle sites palpated, and reported elicited and spontaneous pain on the same side of the face. Comorbid fibromyalgia was assessed via clinical research examination in accordance with American College of Rheumatology 1990 criteria.36
Presence or Absence of TM Joint Pain Among mTMD Cases
The presence or absence of TM joint pain was based on the data obtained during the clinical research examination. For analysis, women who met criteria for mTMD were designated as having muscle and joint pain (MJ-pain) if they reported any pain on palpation of TM joint sites (on either side of the face) and as muscle only-pain (M-pain) if they did not report pain on palpation at these sites.33
Psychophysical Procedures
All subjects were trained on quantitative sensory testing (QST) procedures, with practice on how to focus on second pain sensations occurring shortly after first pain, prior to the formal testing phase during the same day.21 The data included here represent the results of the testing phase. TS and AS pain ratings were based on a 100-point numerical rating scale (NRS) with anchors ranging from warm at 10 to intolerable pain at 100. Heat pain stimuli were delivered by the Pathway Thermal Sensory analyzer (Medoc Ltd., Ramat Yishai, Israel) through a 27 mm diameter CHEPS thermode applied on the thenar eminence of the non-dominant hand.
The full set of TS-AS data included a total of 12-time intervals (T) per trial, each with recorded NRS ratings, with the possibility of multiple trials per person (Table 1) if the test stimulus failed to evoke a 10-point increase in the 1st to 15th stimulus (full details can be found in previous publication).21 The heat stimulus was presented, held for 0.7 seconds and then repeated after 2 seconds for a total of 15 stimuli. Participants were asked to rate their second pain after the 1st, 5th, 10th and 15th application of the heat stimulus, ie, at T1 through T4. After the last pain rating (ie, 15th stimulus) in each TS trial, the heat was preprogrammed to cease. Presence and intensity of any AS were measured at 15 second intervals during a 2-minute period after the heat ceased or until participants reported no AS on two consecutive intervals, at which point an NRS of 0 was imputed for any remaining time points. The values of AS are the remaining 8 ratings (and associated time intervals). As such, the TS train included ratings across four timepoints (T1 through T4) and the AS train reflects ratings across 8 time points (T5 through T12).
 |
Table 1 Subject Demographic, Clinical and Psychophysical Characteristics |
Categorical Patterns of Change in Pain Based on 10-Points
No specific standard exists for what degree of change is required to demonstrate summation. As described below, to examine TS, the present study models all changes in pain ratings across the 15 heat stimuli. To explore trajectories of change, a previous publication used a 10-point difference between the pain rating at T1 and T4 (1st and 15th stimulus) to define three patterns: a 10-point summation pattern if the NRS increase by at least 10 points, a decreasing pattern if the NRS decreased by at least 10 points, and the steady pattern defined as NRS ratings remaining within 10 points.21 Since, as described below, we wanted a dichotomous measure of summation to aid in the interpretation of AS results, we explored the frequency of these 10-point-based patterns by clinical group and stratified the models of TS and AS results by whether summation occurred based on ≥10-points.
Demographics and Clinical Characteristics
Demographic characteristics were obtained via interview and included age, duration of facial pain, and various pain measures. Characteristic pain intensity was defined as the average of current, average, and worst pain in the past 6 months, each on a 0 to 100 scale (0 = no pain to 100 = bad as it could be). Bodily pain across all groups was measured via the SF36 bodily pain severity question which assesses the severity of pain in the last four weeks rated on a scale from none=1 to very severe=6. Use of non-steroidal anti-inflammatory drugs (NSAIDs) was assessed via participant self-report.
Since previous research identified differences in masticatory muscle activity between mTMD subgroups operationalized as background electromyography (EMG) recordings of the masseter muscle activity during sleep,33 sleep background EMG was examined. Background activity was computed as the EMG activity remaining after that attributable to sleep bruxism and movement artefacts was removed.35 These data were obtained during the second of two nights of laboratory sleep data with polysomnography (PSG) and EMG to measure sleep masticatory muscle activity.35 For parsimony and consistent with previous analyses,33 high sleep background EMG was defined as 4th quartile sleep background EMG vs the 1st through 3rd quartiles.
Analytic Strategy
Given the nested nature of the data resulting from the TS protocol, ie, repeated pain ratings per trial and repeated trials per person, a 3-level linear mixed model with random intercepts was specified to account for the random effects of participants and trials. Models estimated fixed effects of group [control, muscle only (M-pain), and muscle and joint (MJ-pain)] and time (T1 through T4 time intervals representing the 1st, 5th, 10th and 15th stimulus) and their interaction, adjusted for each trial’s stimulus temperature. A variance component (VC) structure was used. The improved fit of different models was ascertained by examining the Corrected Quasi-likelihood under Independence Model Criterion (QICC).
In addition to the nested nature of the data, the analysis of AS required consideration of censoring as individuals’ pain resolved before 2 minutes had elapsed after the last stimulation. Therefore, generalized estimating equations (GEE) with a survival link and autoregressive-1 (AR-1) covariance structure were used to model persistence or decay of aftersensations. The AS outcome was the dichotomized pain rating of NRS ≥5 versus NRS <5. GEE models estimated odds of NRS ≥5 by case groups and time interval and their interaction adjusting for stimulus temperature and pain rating following the last stimulus presentation (T4).
A few additional adjustments to both the TS and AS models were explored to address alternative peripheral explanations that may influence the QST results. To ensure that TS and AS results reflect mTMD-specific phenomena, analyses were adjusted for bodily pain and comorbid fibromyalgia. To address possible peripheral nociceptive input due to muscle activity, models were adjusted for high sleep background EMG, which we previously showed differed by presence or absence of joint pain.33 Use of NSAIDs was also explored as a potential confounder. Because a previous publication identified a variety of pain trajectories in that may contribute to a significant interaction term (ie, decreasing trend and steady within 10-point fluctuations in pain), the final TS and AS models were stratified by the categorical a-priori 10-point change in pain pattern of each trial to ensure the pattern of results held among those trials where there was no doubt of summation.
Statistical modeling was conducted using IBM SPSS v. 27,37 with some descriptive analyses conducted in StataSE 17.38
Results
Participant Characteristics
Table 1 describes the various demographic and clinical characteristics observed across the mTMD subgroups (MJ-pain and M-pain) and the controls. Mean characteristic pain intensity was generally higher among the MJ-pain versus the M-pain subgroup, but bodily pain, fibromyalgia research diagnosis, and duration of facial pain did not differ between case subgroups. As reported previously,33 high sleep background masticatory muscle EMG was more frequent in the M-pain subgroup. Use of opioids was rare (<5%) and not statistically different in cases than controls (data not shown). Use of NSAIDs was more common among cases but not different between mTMD subgroups (Table 1) and was not associated with stimulus-dependent pain ratings (p-values: T1 = 0.16; T2 = 0.35; T3 = 0.35; T4 = 0.65).
Characteristics Across QST Trials
A total of 621 trials were included with up to 12 ratings of pain. The mean stimulus temperature of each trial did not differ between diagnostic groups but did differ by type of 10-point change in pain pattern with increasing stimulus temperatures occurring across decreasing, steady and ≥10-point summation trials, respectively (Mean stimulus temperature (SD): 46.4°C (1.2), 47.1°C (1.5), 47.8°C (1.8); p < 0.00).
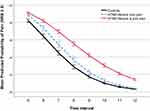
Figure 1 illustrates the mean pain ratings at each of the 12-time intervals, not yet accounting for nesting within trials and individuals. The descriptive pattern that is observed shows a slope of increasing pain ratings for the mTMD subgroups, particularly steep for the M-pain subgroup, over the first four time points (reflecting the 15 heat stimuli). After the last stimulus at T4, the pain ratings drop precipitously for all groups. The mTMD groups, especially the MJ-pain subgroup, show slower decreases in pain ratings over the remaining eight time points (total of 2 minutes in 15-second intervals).
Temporal Summation
The linear mixed model of mean pain ratings across the four time points (corresponding to the 15 heat stimuli) adjusted for the trials’ stimulus temperature revealed a small but statistically significant interaction between the three groups and time interval (p = 0.004; partial eta2=0.01; Figure 2) with steepest slope in the M-pain subgroup (M-pain vs MJ-pain: p = 0.035; partial eta2=0.01). In uncontrolled descriptive analyses, the proportion of a-priori ≥10-point summation trials was higher among cases than controls, but did not differ between mTMD subgroups (Table 1). When the analysis of change in pain ratings was limited to trials with this pre-determined ≥10-point summation pattern (N = 170, 27% of trials) the difference in slope was slightly steeper for the M-pain subgroup relative to MJ-pain but not significant (p = 0.06; partial eta2=0.02; Figure 3) potentially reflecting decrease in power, but similar pattern of results. In separate models comparing each subgroup to controls among trials with ≥10-point summation pattern, only the M-pain subgroup reported steeper increase in pain over time, although not significant (p = 0.06; partial eta2=0.03). These results did not change appreciably after adjustments for bodily pain, comorbid fibromyalgia, high sleep background masticatory muscle EMG or use of NSAIDs (data not shown). Together, these data suggest a small difference in temporal summation between subgroups, with a steeper slope in the M-pain subgroup reflecting greater increases in second pain with repeated heat stimuli.
Aftersensations
GEE model-based estimated probabilities of AS (NRS ≥5 vs less) are illustrated in Figures 4 and 5. The shallowest slope, indicating the longest persistence of AS, was seen in the mTMD subgroup with joint pain. The odds of continuing pain reports in this group were 16% higher relative to controls and 17% higher relative to the M-pain subgroup (OR = 1.16, 95% CI = 1.06, 1.27 p < 0.001 and OR = 1.17, 95% CI = 1.06, 1.27 p = 0.002, respectively; Figure 4). AS persistence in the MJ-pain subgroup was particularly evident among trials with at least a 10-point increase in pain, where the odds of continuing AS were 42% higher relative to controls and 16% higher relative to the M-pain subgroup (OR = 1.42, 95% CI = 1.51, 1.75, p = 0.001 and OR = 1.160, 95% CI = 0.99, 1.36, p = 0.059, respectively; Figure 5). However, these greater odds of AS persistence among the MJ-pain subgroup vs controls were also observed among trials with the decreasing 10-point pattern of changes in pain over the 15 heat stimuli (OR = 1.30, 95% CI = 1.04, 1.62, p = 0.022), with no statistically significant difference in the steady pattern (steady pattern: OR = 1.06, 95% CI = 0.94, 1.20, p = 0.369).
In contrast to the persistent AS in MJ-pain subgroup, AS in the M-pain subgroup had a non-significant elevated persistence only among trials with summation of at least 10-points, where the odds of continuing pain reports were 23% higher relative to controls (OR = 1.23, 95% CI = 0.98, 1.56; p = 0.081; Figure 5). These patterns of results did not change appreciably after adjustments for bodily pain, comorbid fibromyalgia, high sleep background masticatory muscle EMG or use of NSAIDs (data not shown).
Discussion
As hypothesized, the presence or absence of TM joint pain yielded different patterns of TS and AS among women with mTMD. The TS results suggest enhanced central sensitization in mTMD in the absence of joint pain, although the small magnitude of the enhanced TS effect must be interpreted with caution until replicated. In contrast, AS were more likely to persist in mTMD with joint pain.
Aftersensations are often conceptualized as reflecting a lingering excitation of the central nervous system specifically triggered by temporal summation.14,39 Therefore, to interpret AS in this manner, we would expect that AS would be more pronounced in trials with clear evidence of summation and in subgroups with greater TS – ie, in the mTMD subgroup with muscle only pain. This was not the case. While persistence of AS among those with joint pain was more likely following trials that produced at least a 10-point summation of second pain, it was also present after trials showing a decreasing response pattern during stimulation. This makes interpretation of AS results difficult.
The TS results are generally consistent with the mixed findings in the literature with one positive40 and four negative studies22–24,41 reporting enhanced TS to thermal stimuli in groups of TMD patients inclusive of myofascial pain. The two studies specifically on myofascial TMD are among the null studies.22,23 One was primarily on myofascial pain22 while the second was on myofascial pain comorbid with tension-type headache.23 No studies, to our knowledge, examine TS in mTMD by presence or absence of joint pain. Studies have focused on arthralgia or osteoarthritis of the TM joint without excluding myofascial pain, but have not explored myofascial pain in the analyses.25,27 In those studies,25,27 enhanced mechanical wind-up was reported, but heat pain summation was not explored. Whether results differ by presence or absence of muscle pain was not examined. Moreover, it is unclear whether differences in results by pain stimulus modalities reflect differences in underlying neurobiology of heat versus mechanical pain (ie, relative contributions of A-delta and C-fiber input) and/or, differences in the reliability of the QST procedures.
More intense AS or slower decay of AS in mTMDs of heat21–23,41 and mechanical18,41 pain have been reported in other studies,18,22,23,41 including the parent study.21 However, this appears to be the first study to examine AS by coexisting joint pain among mTMD patients.
To interpret and integrate our QST results, two key points must be considered. First, “central sensitization” is a normal process that may be induced by nociceptive input at the periphery (eg, via peripheral sensitization). This is supported by studies that, with the use of capsaicin injections, induce similar TS and AS in healthy controls as that observed in neuropathic pain patients without such injections.14 Therefore, evidence of central sensitization using these measures does not by definition reflect dysfunctional central processes (ie, central dysfunction in the initiation or exaggeration of central sensitization). Second, wind-up is one potential mechanism that has been associated with central sensitization, but others are theorized.10 The QST results of the present study support that some central sensitization is present across mTMD subgroups and, if replicated, may suggest multiple mechanisms of central sensitization.
When both joint and muscle pain are present, as is often the case in mTMD, our QST results show no increased wind-up relative to controls but significantly more persistent AS than any group. This may suggest that joint pain is primary and muscle pain secondary, potentially reflecting an interrelated mix of peripheral and central sensitization. Under this scenario, muscle pain may be referred (ie, larger receptive fields and/or recruitment of non-nociceptive Aβ fibers) thus reflective of peripherally induced central sensitization.42 Alternatively, results may reflect pre-existing deficits in central inhibition in the MJ-pain subgroup which these analyses do not directly address.
In the absence of joint pain, the small enhanced TS effect may be evidence of central sensitization via wind-up. In the absence of an alternative hypothesis of peripherally induced central sensitization for the muscle pain, this may be more indicative of centrally initiated central sensitization. If replicated, these results may provide a clue for how to differentiate the origin of central sensitization in efforts to isolate or identify a primary dysfunction in the central mediation of myofascial pain. This in turn may improve the ability to identify therapeutic targets to best treat the specific presentations of myofascial TMD.
Strengths and Potential Limitations
Particular strengths of this study were the relatively large sample size, participant training to differentiate first and second pain sensations, and multiple trials which allowed variations in stimulus temperature where needed to maximize the likelihood of summation. Additionally, various factors were considered that may have differed across groups potentially biasing results (ie, NSAID use, fibromyalgia research diagnosis, bodily pain ratings, high background EMG).
Nevertheless, this study has potential limitations. First, mTMD was defined using RDC/TMD,34 not the updated DC/TMD criteria.43 For example, in addition to pain on palpation, DC/TMD asks whether the pain is familiar or similar to what the patient has been experiencing.43 Although this does not reduce the validity of results, it does limit their generalizability to the updated criteria. Second, TM joint pain was based only on palpation during research clinical examination. Future work should include additional examination of joint pain including more extensive joint and muscle diagnostics, as well a QST evaluation of peripheral sensitization. Third, not all trials yielded summation of pain. This is not uncommon as TS involves “complex testing procedures, which are challenging to standardize and perform”,13 and have “often been associated with ceiling or floor effects and low response rates.”13 This common failure to yield summation may largely reflect that QST procedures for TS may not adequately translate the wind-up process seen in animals. It is unclear whether repeated failure to summate may also be informative of differences in central processes, so we included all trials, modeled all changes in pain, and explored the more extreme ≥10-point summation dichotomy. Fourth, we cannot rule out a ceiling effect for trials with higher ratings at T1, which are more common in the joint pain group. However, nearly 90% of trials in each group reported pain at or under 50 points at T1. Restricting the TS linear mixed model to this group yielded similar results, ruling out that the TS findings are due to a ceiling effect. Nonetheless, we recommend replication in a study with the above noted improvements.
Conclusion
This study found that the presence or absence of TM joint pain may contribute to response heterogeneity in QST of heat pain among women with myofascial TMD. Therefore, given the potential difference in the interplay of peripheral and central sensitization in joint vs muscle pain, to understand the mechanisms of muscular pain in myofascial TMD, it may be important to consider the presence and role of articular pain.
Acknowledgments
This work was supported by NIDCR (5K01DE028292 and 3K01DE028292-03S1) and uses data from a completed study funded by NIDCR (R01DE018569).
Disclosure
Dr Vivian Santiago reports grants from NIH, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Janal MN, Raphael KG, Nayak S, Klausner J. Prevalence of myofascial temporomandibular disorder in us community women. J Oral Rehabil. 2008;35(11):801–809. doi:10.1111/j.1365-2842.2008.01854.x
2. National Academies of Sciences, Engineering and Medicine. Temporomandibular Disorders: Priorities for Research and Care. Washington (DC): Press NA; 2020. Available from: https://www.nap.edu/catalog/25652/temporomandibular-disorders-priorities-for-research-and-care.
3. Fernandez-de-las-Penas C, Svensson P. Myofascial temporomandibular disorder. Curr Rheumatol Rev. 2016;12(1):40–54. doi:10.2174/1573397112666151231110947
4. Cairns BE. Pathophysiology of TMD pain--basic mechanisms and their implications for pharmacotherapy. J Oral Rehabil. 2010;37(6):391–410. doi:10.1111/j.1365-2842.2010.02074.x
5. Jessri M, Sultan AS, Tavares T, Schug S. Central mechanisms of pain in orofacial pain patients: implications for management. J Oral Pathol Med. 2020;49(6):476–483. doi:10.1111/jop.13062
6. La Touche R, Paris-Alemany A, Hidalgo-Perez A, Lopez-de-Uralde-Villanueva I, Angulo-Diaz-Parreno S, Munoz-Garcia D. Evidence for central sensitization in patients with temporomandibular disorders: a systematic review and meta-analysis of observational studies. Pain Pract. 2018;18(3):388–409. doi:10.1111/papr.12604
7. Moana-Filho EJ, Herrero Babiloni A, Theis-Mahon NR. Endogenous pain modulation in chronic orofacial pain: a systematic review and meta-analysis. Pain. 2018;159(8):1441–1455. doi:10.1097/j.pain.0000000000001263
8. Meng H, Dai J, Li Y. Quantitative sensory testing in patients with the muscle pain subtype of temporomandibular disorder: a systemic review and meta-analysis. Clin Oral Investig. 2021;25(12):6547–6559. doi:10.1007/s00784-021-04171-5
9. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(Suppl 3):S2–S15. doi:10.1016/j.pain.2010.09.030
10. Herrero JF, Laird JM, López-García JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61(2):169–203. doi:10.1016/S0301-0082(99)00051-9
11. Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol. 1977;69(1):167–171. doi:10.1111/1523-1747.ep12497942
12. Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66(2–3):105–108. doi:10.1097/00006396-199608000-00001
13. Staud R, Godfrey MM, Mejia M, Ramanlal R, Riley JL, Robinson ME. Usefulness of ramp & hold procedures for testing of pain facilitation in human participants: comparisons with temporal summation of second pain. J Pain. 2020;21(3):390–398. doi:10.1016/j.jpain.2019.08.004
14. Gottrup H, Kristensen AD, Bach FW, Jensen TS. Aftersensations in experimental and clinical hypersensitivity. Pain. 2003;103(1–2):57–64. doi:10.1016/S0304-3959(02)00415-3
15. Araújo Oliveira Ferreira DM, Costa YM, de Quevedo HM, Bonjardim LR, Rodrigues Conti PC. Experimental psychological stress on quantitative sensory testing response in patients with temporomandibular disorders. J Oral Facial Pain Headache. 2018;32(4):428–435. doi:10.11607/ofph.2046
16. Garrett PH, Sarlani E, Grace EG, Greenspan JD. Chronic temporomandibular disorders are not necessarily associated with a compromised endogenous analgesic system. J Orofac Pain. 2013;27(2):142–150. doi:10.11607/jop.943
17. Sarlani E, Garrett PH, Grace EG, Greenspan JD. Temporal summation of pain characterizes women but not men with temporomandibular disorders. J Orofac Pain. 2007;21(4):309–317.
18. Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Evidence for up-regulated central nociceptive processing in patients with masticatory myofascial pain. J Orofac Pain. 2004;18(1):41–55.
19. Hilgenberg-Sydney PB, Kowacs PA, Conti PC. Somatosensory evaluation in dysfunctional syndrome patients. J Oral Rehabil. 2016;43(2):89–95. doi:10.1111/joor.12344
20. Welte-Jzyk C, Pfau DB, Hartmann A, Daubländer M. Somatosensory profiles of patients with chronic myogenic temporomandibular disorders in relation to their painDETECT score. BMC Oral Health. 2018;18(1):138. doi:10.1186/s12903-018-0601-8
21. Janal MN, Raphael KG, Cook DB, Sirois DA, Nemelivsky L, Staud R. Thermal temporal summation and decay of after-sensations in temporomandibular myofascial pain patients with and without comorbid fibromyalgia. J Pain Res. 2016;9:641–652. doi:10.2147/JPR.S109038
22. Raphael KG, Janal MN, Anathan S, Cook DB, Staud R. Temporal summation of heat pain in temporomandibular disorder patients. J Orofac Pain. 2009;23(1):54–64.
23. Sato H, Saisu H, Muraoka W, Nakagawa T, Svensson P, Wajima K. Lack of temporal summation but distinct aftersensations to thermal stimulation in patients with combined tension-type headache and myofascial temporomandibular disorder. J Orofac Pain. 2012;26(4):288–295.
24. Ribeiro-Dasilva MC, Goodin BR, Fillingim RB. Differences in suprathreshold heat pain responses and self-reported sleep quality between patients with temporomandibular joint disorder and healthy controls. Eur J Pain. 2012;16(7):983–993. doi:10.1002/j.1532-2149.2011.00108.x
25. Kothari SF, Baad-Hansen L, Hansen LB, et al. Pain profiling of patients with temporomandibular joint arthralgia and osteoarthritis diagnosed with different imaging techniques. J Headache Pain. 2016;17(1):61. doi:10.1186/s10194-016-0653-6
26. Gil-Martínez A, Grande-Alonso M, La Touche R, Lara-Lara M, López-López A, Fernández-Carnero J. Psychosocial and somatosensory factors in women with chronic migraine and painful temporomandibular disorders. Pain Res Manag. 2016;2016:3945673. doi:10.1155/2016/3945673
27. Kothari SF, Baad-Hansen L, Oono Y, Svensson P. Somatosensory assessment and conditioned pain modulation in temporomandibular disorders pain patients. Pain. 2015;156(12):2545–2555. doi:10.1097/j.pain.0000000000000325
28. Yang G, Baad-Hansen L, Wang K, Fu K, Xie QF, Svensson P. Somatosensory abnormalities in Chinese patients with painful temporomandibular disorders. J Headache Pain. 2016;17:31. doi:10.1186/s10194-016-0632-y
29. Rammelsberg P, LeResche L, Dworkin S, Mancl L. Longitudinal outcome of temporomandibular disorders: a 5-year epidemiologic study of muscle disorders defined by research diagnostic criteria for temporomandibular disorders. J Orofac Pain. 2003;17(1):9–20.
30. Schiffman EL, Truelove EL, Ohrbach R, et al. The research diagnostic criteria for temporomandibular disorders. I: overview and methodology for assessment of validity. J Orofac Pain. 2010;24(1):7–24.
31. Machado LP, Nery Cde G, Leles CR, Nery MB, Okeson JP. The prevalence of clinical diagnostic groups in patients with temporomandibular disorders. Cranio. 2009;27(3):194–199. doi:10.1179/crn.2009.029
32. Jennings EA, Williams MC, Staikopoulos V, Ivanusic JJ. Neurobiology of temporomandibular joint pain: therapeutic implications. Semin Orthod. 2012;18(1):63–72. doi:10.1053/j.sodo.2011.10.003
33. Santiago V, Raphael K. Absence of joint pain identifies high levels of sleep masticatory muscle activity in myofascial temporomandibular disorder. J Oral Rehabil. 2019;46(12):1161–1169. doi:10.1111/joor.12853
34. Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6(4):301–355.
35. Raphael KG, Janal MN, Sirois DA, et al. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 2013;40(12):883–891. doi:10.1111/joor.12112
36. Wolfe F, Clauw DJ, Fitzcharles M-A, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi:10.1002/acr.20140
37. IBM Corp. IBM SPSS Statistics for Windows, Version 27.0. Released 2020. Armonk, NY: IBM Corpx.; 2020.
38. StataCorp. Stata Statistical Software: Release 17. College Station, Tx: StataCorp LLC; 2019.
39. Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1–2):165–175. doi:10.1016/S0304-3959(00)00432-2
40. Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76(1–2):71–81. doi:10.1016/S0304-3959(98)00028-1
41. Greenspan JD, Slade GD, Bair E, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12(11 Suppl):T61–T74. doi:10.1016/j.jpain.2011.08.006
42. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi:10.1016/j.jpain.2009.06.012
43. Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and orofacial pain special interest. J Oral Facial Pain Headache. 2014;28(1):6–27. doi:10.11607/jop.1151
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.