Back to Journals » International Medical Case Reports Journal » Volume 13
Temporal Profile and Treatment of Purpureocillium lilacinum Keratitis Secondary to Herpes Zoster Reactivation Following Influenza Vaccination
Authors Nguyen LN, Parikh SU, Batliwala SY, Davis AS, Riaz KM
Received 4 June 2020
Accepted for publication 18 August 2020
Published 22 September 2020 Volume 2020:13 Pages 455—459
DOI https://doi.org/10.2147/IMCRJ.S265724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lindsey N Nguyen,1 Suparshva U Parikh,1 Shehzad Y Batliwala,2 Alexander S Davis,3 Kamran M Riaz2
1College of Medicine, University of Oklahoma, Oklahoma City, OK, USA; 2Dean McGee Eye Institute, Department of Ophthalmology, University of Oklahoma, Oklahoma City, OK, USA; 3Division of Ophthalmology, University of New Mexico, Albuquerque, NM, USA
Correspondence: Kamran M Riaz
Dean McGee Eye Institute, Department of Ophthalmology, University of Oklahoma, 608 Stanton L Young Blvd, Oklahoma City, OK 73104, USA
Tel +1 405 271-1095
Fax +1 405 271-3680
Email [email protected]
Purpose: To report a temporal profile of Purpureocillium lilacinum keratitis (PLK) secondary to immune dysfunction induced by the combination of reactivation of herpes zoster dermatitis and recent influenza vaccination that suggests a possible association, including successful medical management.
Methods: A 64-year-old contact lens wearer presented with left eye keratitis days after receiving an influenza vaccination and subsequent development of herpes zoster lesions on the flank. Patient was initially treated for bacterial keratitis with fortified antibiotics and oral valacyclovir for her concurrent zoster. Pharmacotherapy was changed to topical voriconazole after cultures were positive for Purpureocillium lilacinum. Topography and anterior segment OCT demonstrated scarring at multiple levels within the cornea with irregular astigmatism. A literature review was conducted to identify mechanisms that demonstrate a temporal link between influenza vaccination, herpes zoster reactivation, and fungal keratitis.
Results: After the conclusion of topical therapy, the central corneal infiltrate regressed and a partial light-blocking anterior stromal scar remained. Best corrected visual acuity improved from 20/400 to 20/25.
Conclusion: Transient systemic immune dysregulation, secondary to influenza vaccination and reactivation of systemic herpetic disease, compounded by contact lens wear, may create a favorable environment for opportunistic fungal keratitis. This case highlights the importance of adequately assessing and treating for existing comorbidities in the successful treatment of mycotic keratitis.
Keywords: fungal keratitis, Purpureocillium lilacinum, herpes zoster, influenza vaccination
Introduction
Fungal keratitis remains a challenging disease entity, often due to delay in diagnosis and institution of appropriate pharmacotherapy.1 A rare, but extremely visually impairing cause of mycotic keratitis is caused by Purpureocillium lilacinum, which is a saprophytic fungus ubiquitous in air, soil, and decomposing vegetation.2 Previous reports have discussed this mycotic infection to have a predilection for immunocompromised patients with considerable resistance to commonly deployed antifungal agents.1 This report describes a case of Purpureocillium lilacinum keratitis (PLK) in an otherwise immunocompetent contact lens wearing patient with potential immune dysfunction occurring after influenza vaccination followed by clinically significant zoster dermatitis reactivation. A transient immunodeficient state after vaccine administration may have created an environment for opportunistic keratomycosis.
Case Report
A 64-year-old female patient was referred to our institution for management of a corneal ulcer of the left eye unresponsive to topical fluoroquinolone antibiotics. At the time of presentation, the patient had significant blurry vision, pain, and photophobia of the affected eye. She denied trauma or exposure to vegetable matter, but endorsed a history of soft contact lens misuse, including periodic extended and overnight contact lens wear (monthly wear Bausch & Lomb SofLens 38® cleaned with Biotrue®, non-hydrogen peroxide cleaning solution). Prior to development of ocular symptoms, the patient had a two-week history of reactivation of herpes zoster dermatitis along her left flank, which developed approximately four days after receiving influenza vaccination (inactivated quadrivalent vaccine, Fluzone®; Sanofi Pasteur Inc., Lyon, France). Of note, the patient reported a long-standing history of episodic reactivation of herpes zoster dermatitis after her yearly influenza vaccine without ocular symptoms previously. She had no other significant ocular history, including any history of previous ocular infections.
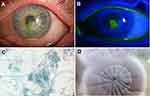
On examination, the patient’s best corrected visual acuity was 20/20 in the right eye and 20/400 in the left eye. Examination of the right eye was unremarkable. Slit-lamp examination of the left eye revealed a central corneal infiltrate with feathery borders measuring 2.4 mm x 2.0 mm with an overlying epithelial defect with associated satellite lesions, 2+ stromal edema, and presence of keratic precipitates with hypopyon inferiorly (Figure 1A and B).
Routine corneal scrapings were performed using blood, chocolate, Sabourad’s and non-nutrient agars, as well as thioglycolate broth, as previously described in the literature.2 Empirical treatment with hourly fortified vancomycin and tobramycin was initiated, along with oral valacyclovir 1 gram three times daily.
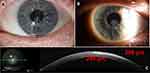
Three days later, the preliminary corneal cultures on blood and Saboraud’s agars revealed fungal elements (Figure 1C). Fortified antibiotics were decreased and the patient was started on hourly topical voriconazole. Final culture and speciation results indicated Purpureocillium lilacinum as the causative agent (Figure 1D). After approximately 3 months of voriconazole monotherapy, the infiltrate regressed, leaving behind a partially light-blocking anterior stromal scar (Figure 2A–C). The patient’s best corrected visual acuity improved to 20/25 in the left eye.
Discussion
Purpureocilium lilacinum keratitis (formerly Paecilomyces lilacinus) (PLK) has been previously described as a rare, opportunistic keratomycosis in immunocompromised patients.1,3 PLK may be difficult to treat because of the organism’s resiliency and ability to survive at a wide range of temperatures and pH levels.4 Corneal infection may be traced to two different qualities of this organism. First, P. lilacinum is able to sporulate in a pattern significantly different from filamentous fungi, which often leads to misidentification and incorrect treatment.4 Second, the organism produces hydrolytic enzymes and toxins that disrupt intact cell membranes, including corneal epithelium. Along with other factors such as tropical climates, vegetable matter exposure, and contact lens misuse, this may explain the increased ocular susceptibility in certain patient populations.
PLK in an immunocompetent patient may occur due to disruption of corneal epithelium integrity secondary to trauma, surgery, and contact lens misuse, which creates a local state of immune dysfunction and predisposes the eye to infection.3 P. lilacinum is an especially challenging mycotic infection as there is usually a delay in diagnosis, variable history in concurrent topical corticosteroid use, and a well-known resistance to traditional antifungal agents such as amphotericin B, fluconazole, griseofulvin, and echinocandins. Newer second-generation triazoles such as voriconazole, posaconazole, albaconazole, isavuconazole, and ravuconazole have relatively greater success as they have lower minimum inhibitory concentration.4 Although our patient responded well to voriconazole, treatment with antifungal drugs often still fails. The disease frequently requires alternative treatment such as therapeutic and/or emergency surgical intervention, as the prognosis of PLK remains poor due to the recalcitrant nature of this infection to medical therapy.1,4-6
While PLK has been previously described in the literature, we postulate our case is instructive given the sequence of events that occurred prior to the development of corneal infection. First, the patient had a long-standing history of contact lens misuse without previous ocular infection. Second, the patient received an influenza vaccine that led to a reactivation of herpes zoster dermatitis (HZD). Subsequent to the appearance of HZD, the patient developed PLK.
Reactivation of dormant herpes zoster virus leading to HZD typically arises during immunocompromised or weakened immunity states, such as cancer, HIV, immunosenescence, and long-term corticosteroid use.7,8 Together, the influenza vaccine and HZD may have caused a synergistic immune dysfunction that predisposed the patient to opportunistic ocular infections, especially in context of the patient’s contact lens misuse. As cell-mediated immunity is the primary defense mechanism against fungal infections, compromised or dysfunctional cell-mediated immunity may have created an ideal environment for opportunistic PLK.9
Reports in the non-ophthalmic literature have indicated a relationship between the influenza vaccine and immune dysfunction.10,11 One proposed mechanism is that the virus present in inactivated influenza vaccines inhibits STAT3 activation, leading to the dysregulation of cell-mediated immune response.7 STAT3 plays a significant role in T-lymphocyte function through mediation of IL-6 dependent processes such as the prevention of apoptosis of T-cells; regulation of CD4+ and CD8+ T cells; and upregulation of CD8+ T cell-mediated responses to microbes like P. lilacinum.12,13
Another proposed mechanism involves alterations of T-regulatory cell frequency.14 T-regulatory cells principally maintain immunologic homeostasis through negative control of excessive inflammation and autoimmunity, especially to protect healthy tissues during response to infections.14 A full discussion regarding the role of T-regulatory cells is beyond the scope of this report. However, what is clear is that a careful balance is needed between functional T-regulatory cells and effector T-cells to uphold a viable cell-mediated immune system. Vaccination, including the influenza vaccine, can decrease the frequency and function of T-regulatory cells. Depletion of these cells may enable a state of immune dysfunction, rather than true immunocompromise, that allows for opportunistic infections.14
A third mechanism involves exacerbation of autoimmune neurologic disease, with onset associated with influenza virus vaccination10,11 This proposed process involves molecular mimicry, wherein an antigen in the influenza vaccine interacts with a naturally occurring antigen found in systemic neural cells.13 As stated previously, our patient had a yearly history of herpes zoster reactivation following influenza vaccine without any ocular symptoms previously. Given that the herpes zoster virus resides in neural cells, it is possible that an autoimmune process involving molecular mimicry occurred after vaccine administration leading to the reactivation of the latent herpes zoster virus.13,15 Reactivation of herpes zoster can augment the immune dysfunction caused by the influenza vaccine.15 These events, in addition to the local trauma caused by contact lens misuse, may have created a favorable environment for the development of PLK.1
It is difficult to point to a single factor as the sole causative etiology of infection in our patient. Indeed, it is possible that contact lens misuse alone led to PLK; hence, we have described this as a keratomycosis with temporal association rather than causation. We believe the current case report is important for several reasons. First, the influenza vaccine likely contributed to a transient immune dysfunctional state which may have created a favorable environment for PLK. Second, because it is possible for PLK to occur simply due to contact lens misuse, clinicians may use this case to counsel patients about judicious contact lens use, especially when receiving systemic vaccination. Third, patients with recurring episodes of reactivated viral dermatitis may be appropriate candidates for systemic viral prophylaxis, especially if a vaccination is administered. The potential use of antivirals or vaccination may help these patients avoid recurrent herpes zoster infection in the future, and thus, protect them from ophthalmic opportunistic infections such as PLK in the context of zoster eye disease. Currently, many clinicians use prolonged oral antivirals in this patient population; studies, such as the Zoster Eye Disease Study (ZEDS), are currently being undertaken to determine optimal practice.16 Finally, a comprehensive review of a patient’s complete history of systemic pathologies and initiation of appropriate topical antimycotic therapy are of paramount importance to help decrease the need for surgical intervention and optimize the potential for visual rehabilitation in this challenging patient population. In our patient’s situation, despite advanced PLK at the time of presentation, she responded favorably well to topical voriconazole and did not require any surgical intervention.
Abbreviations
PLK, Purpureocillium lilacinum keratitis; HZD, herpes zoster dermatitis.
Consent for Publication
The authors certify that they have obtained all the appropriate consent forms from the patient in the study. Patient has provided written informed consent for both the case details and accompanying images to be published. The patient has given consent for all images and other clinical information to be reported to the journal without identifying information and has been reassured that all due efforts will be made to conceal identity of the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conceptions, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in draft, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors would like to acknowledge the Dean McGee Eye Institute and the Eaton Balyeat Grant Committee for their financial support with this manuscript.
Disclosure
KMR reports Bausch and Lomb and Beaver-Visitec, Inc (Speaker, Consultant) and no other potential conflicts of interest for this work. The remaining authors declare that they have no competing interests for this work.
References
1. Malecha MA, Tarigopula S, Malecha MJ. Successful treatment of paecilomyces lilacinus keratitis in a patient with a history of herpes simplex virus keratitis. Cornea. 2006;25(10):1240–1242. doi:10.1097/01.ico.0000230497.99648.8d
2. Leck A. Taking a corneal scrape and making a diagnosis. Community Eye Health. 2009;22(71):42–43.
3. Wu PC, Lai CH, Tan HY, Ma DH, Hsiao CH. The successful medical treatment of a case of paecilomyces lilacinus keratitis. Cornea. 2010;29(3):357–358. doi:10.1097/ICO.0b013e3181af7626
4. Chew R, Dorman A, Woods ML. Purpureocillium lilacinum keratitis: a case series and review of the literature. Can J Ophthalmol. 2016;51(5):382–385. doi:10.1016/j.jcjo.2016.05.013
5. Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep. 2013;7(3):209–218. doi:10.1007/s12281-013-0150-1
6. Sanitato JJ, Kelley CG, Kaufman HE. Surgical management of peripheral fungal keratitis (keratomycosis). Arch Ophthalmol. 1984;102(10):1506–1509. doi:10.1001/archopht.1984.01040031226023
7. Matsubara H, Konishi T, Saito K, et al. Herpes zoster duplex in a patient with influenza A and bacterial superinfection. J Dermatol. 2020;47(1):e32–e33. doi:10.1111/1346-8138.15099
8. Randolph SA. Shingles. Workplace Health Saf. 2015;63(11):528. doi:10.1177/2165079915607499
9. Blanco JL, Garcia ME. Immune response to fungal infections. Vet Immunol Immunopathol. 2008;125(1–2):47–70. doi:10.1016/j.vetimm.2008.04.020
10. Principi N, Esposito S. Vaccine-preventable diseases, vaccines and Guillain-Barre’ syndrome. Vaccine. 2019;37(37):5544–5550. doi:10.1016/j.vaccine.2018.05.119
11. Sasaki T, Suzuki Y, Ishida K. Autoimmune hepatitis following influenza virus vaccination. Medicine. 2018;97(30):e11621. doi:10.1097/MD.0000000000011621
12. Kuchipudi SV. The complex role of STAT3 in viral infections. J Immunol Res. 2015;2015:272359. doi:10.1155/2015/272359
13. Mahony R, Gargan S, Roberts KL, et al. A novel anti-viral role for STAT3 in IFN-α signalling responses. Cell Mol Life Sci. 2017;74(9):1755–1764. doi:10.1007/s00018-016-2435-3
14. de Wolf ACMT, van Aalst S, Ludwig IS, et al. Regulatory T cell frequencies and phenotypes following anti-viral vaccination. PLoS One. 2017;12(6):e0179942. doi:10.1371/journal.pone.0179942
15. Akmatov MK, Riese P, Trittel S, et al. Self-reported diabetes and herpes zoster associated with a weak humoral response to the seasonal influenza A H1N1 vaccine antigen among the elderly. BMC Infect Dis. 2019;19(1). doi:10.1186/s12879-019-4214-x.
16. Lo DM, Jeng BH, Gillespie C, Wu M, Cohen EJ. Current practice patterns and opinions on the management of recent-onset or chronic herpes zoster ophthalmicus of Zoster Eye Disease Study investigators. Cornea. 2019;38(1):13–17. doi:10.1097/ICO.0000000000001732
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.