Back to Journals » Clinical Ophthalmology » Volume 17
Temperature Change of Ophthalmic Viscosurgical Devices in a Bi-Chamber Set-Up at a Flow of 0 and 20mL/min
Authors Jensen NR , Ungricht EL, Harris JT, Zaugg B , Barlow WR , Murri MS, Olson RJ, Pettey JH
Received 8 September 2022
Accepted for publication 10 January 2023
Published 10 February 2023 Volume 2023:17 Pages 555—560
DOI https://doi.org/10.2147/OPTH.S389136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Nathan R Jensen,1,2 Emilie L Ungricht,1,2 Jacob T Harris,1,2 Brian Zaugg,1 William R Barlow,1 Michael S Murri,1 Randall J Olson,1 Jeff H Pettey1
1Department of Ophthalmology and Visual Sciences, John A. Moran Eye Center, University of Utah, Salt Lake City, UT, 84132, USA; 2University of Utah School of Medicine, Salt Lake City, UT, 84132, USA
Correspondence: Jeff H Pettey, John A. Moran Eye Center, University of Utah, 65 Mario Capecchi Drive, Salt Lake City, UT, 84132, USA, Tel +1 801-581-2352, Fax +1 801-581-3357, Email [email protected]
Purpose: To understand the role of ophthalmic viscosurgical devices (OVDs) in corneal incision contracture (CIC). Specifically, the aim was to evaluate with the tip of the phacoemulsification needle free of OVD, how various OVDs near the tip and sleeve may transmit thermal energy to the incision site.
Methods: A small chamber was filled with balanced saline solution (BSS), and a thin membrane was placed on the surface. OVD was placed atop the membrane. A temperature probe was placed in the OVD, while the handpiece pierced the membrane. The experiment was run both with and without flow and vacuum. Temperature measurements were gathered for each of the OVDs at four separate time points at 0 and 20mL/min flow.
Results: As expected, there was a more pronounced temperature increase in all test groups with no fluid flow. While the temperature increase was not significantly different from BSS for any of the OVDs tested at either 0 or 20mL/min, Viscoat showed the most variable results at both flow settings.
Conclusion: As long as the phaco tip is not in OVD, residual OVD near the incision is not exothermic and so not an additional risk for CIC.
Keywords: cornea, corneal incision contracture, phacoemulsification, flow, membrane, surface
Plain Language Summary
Standard cataract removal procedure involves phacoemulsification (phaco), using ultrasound energy for cataract emulsification. While this approach is generally safe, complications can occur, one being corneal incision contracture. This complication is due to a temperature rise, generally above 60°C, at the incision site. Factors like ophthalmic viscosurgical devices (OVDs), which have an exothermic reaction to ultrasound energy, can contribute to this complication when occlusion occurs.
This study aimed to assess if the phaco needle tip is free from OVD at 20mL/min and no flow rates, if residual OVD near the incision can still be exothermic.
We measured temperature on the membrane surface with the probe immersed in OVD, while the tip was positioned below the membrane submerged in BSS. We recorded temperature changes that occurred at 0, 10, 20 and 30 seconds of continual phacoemulsification. This was repeated using each OVD type with flow set at 0 and 20 mL/min.
We found that as long as there is some, even a low amount, of flow through the tip, any heat transmitted by OVD was not statistically different from BSS. Our findings demonstrated the importance of irrigation and aspiration of OVD to allow for free flow of BSS before ultrasound use.
Introduction
Phacoemulsification (phaco) is accompanied by various uncommon complications such as endophthalmitis, suprachoroidal hemorrhage, retinal detachment, and corneal incision contracture (CIC).1–4 The presence of ophthalmic viscosurgical devices (OVDs) has helped reduce many intraoperative complications of cataract surgery including endothelial damage.5 However, OVDs contribute to CIC, due to their variable ultrasound-induced exothermicity, especially during outflow occlusion.6 Previous studies have demonstrated that different OVDs have different protective effects upon the cornea.7 Additionally, different OVDs have varying levels of CIC risk during phacoemulsification.8 This present study aims to better understand how OVDs cause CIC. In CIC, heat from the phaco needle is transferred to the incision site and once this reaches 60°C, a CIC will happen within seconds. Any aspiration through the phaco needle rapidly cools any thermal build up at or near the phaco tip. The purpose of this study was to investigate conditions when the phaco tip is free of OVD but viscoelastic was still present near the corneal incision site, a typical clinical scenario. Our hypothesis was that the significant thermal energy may still transmit to the incision site.
Materials and Methods
Experimental Conditions
The test chamber used was plastic, with an approximate radius of 7.5 mm, height of 20 mm and volume of 3500 mm3. It was filled with balanced saline solution (BSS) and then a saran wrap membrane was placed on top of the BSS (Figures 1 and 2 [Rhino Software, Seattle, WA, USA]). While this volume did not represent any specific anatomical volume, the purpose of the chamber was to simulate the anterior chamber and the cornea in that they were two volumes that were separated by a thin layer. The four OVDs tested were Viscoat (Alcon, a dispersive OVD), DisCoVisc (Alcon, a medium viscosity dispersive [MVD] OVD), Healon5 (Johnson & Johnson Vision, a viscoadaptive OVD), and ProVisc (Alcon, a cohesive OVD). Henceforth, these OVDs will be identified by the following designations: Viscoat as OVD-1, DisCoVisc as OVD-2, Healon5 as OVD-3, and ProVisc as OVD-4. BSS (Alcon) was used as a control and for irrigation. We used a balanced 0.9 mm, 30-degree non-flared tip (Alcon) that features an aspiration bypass system (ABS). The handpiece was placed into the chamber and pierced minimally through the membrane so as to limit the amount of communication between the BSS in the chamber and the OVD on the surface of the membrane’s surface. The temperature probe was attached to an Omega Temperature Gauge (OM-EL-USB-TC-LCD; Omega Engineering, Norwalk, Connecticut) and then placed directly into the OVD, resting on the surface of the membrane, approximating where the wound would be, and maintaining a distance of at least 1 mm between the probe and the tip of the handpiece needle. Once a constant temperature was maintained, and/or 5 minutes had elapsed, the pedal of the Centurion Vision System phaco machine (Alcon Surgical, Fort Worth, Texas) was fully engaged. The continuous ultrasound ran for 30 seconds with an intraocular pressure (IOP) of 50 mm Hg, a vacuum of 0 mm Hg, and an aspiration of 12 cc/min on a continuous torsional setting at 60% power with linear torsional setting with 50% max power allowed with no longitudinal beyond intelligent phaco. Previous work has shown that present phaco technology requires active vacuum at the tip to overcome system resistance engineered to dampen post-occlusion surge in a peristaltic system. So, a setting of no vacuum will functionally result in no to very little fluid flow through the system.9 The gauge recorded temperature at 0, 10, 20 and 30 seconds. This procedure was repeated 10 times for each of the OVDs.
 |
Figure 1 Experimental setup. |
 |
Figure 2 Diagrammatic representation. |
The experiment was repeated with the phaco machine set to 700+ mm Hg vacuum (a specified setting on the machine) and 20 mL/min aspiration.
Statistical Analysis
The temperature change for each trial was calculated by subtracting the initial temperature from the final temperature. These values were averaged for each OVD to provide an average temperature increase at 0, 10, 20 and 30 seconds; and each OVD result was compared to BSS. These values were then evaluated with Prism software (GraphPad Software, San Diego, CA, USA) using ANOVA and Tukey’s multiple comparison test.
Results
At no or very little flow each OVD as well as BSS had an average increase of temperature from baseline to 30 seconds of at least 6.88°C with an average increase of 8.34°C. The differences from baseline at 30 seconds are: DisCoVisc (OVD-2) (6.88°C ± 1.22), Healon5 (OVD-3) (7.15°C ± 1.65), ProVisc (OVD-4) (7.31°C ± 1.46), BSS (8.30°C ± 5.32), Viscoat (OVD-1) (12.06°C ± 8.51). More than the other OVDs, Viscoat featured greater variation in temperature change; however, none of these increases in temperature were statistically significantly different from each other when adjusted for multiple comparisons. To make sure there was no experimental error, this was repeated. Viscoat continued to show a more varied temperature response; however, again, none of the differences were significant (Figure 3).
 |
Figure 3 OVD temperature change with low vacuum and low flow and presence of membrane. |
At 20mL/min flow, the average increase of temperature from baseline at 30 seconds was: Viscoat (0.57°C ± 0.90), DisCoVisc (0.66°C ± 0.68), ProVisc (0.79°C ± 0.73), Healon5 (1.22°C ± 0.32), and BSS (2.24°C ± 0.72) (Figure 4). None of these was significantly different from each other.
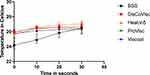 |
Figure 4 OVD temperature change in the presence of vacuum and flow and presence of membrane. |
Discussion
This study was designed to better understand the cause of CIC, in aiming to help mitigate this potential adverse effect of cataract removal through phacoemulsification.
The cornea is susceptible to changes in temperature that occur during phaco.10 Many studies have attempted to understand this rise of temperature through measurement of the temperature change that occurs at or around the tip of the handpiece.6,11–13 One primary purpose of this study was to increase understanding of how this temperature change may be occurring through a membrane as a surrogate of how temperature transmits from the tip in BSS with the sleeve surrounded by OVD. Previous studies have evaluated the thermic properties of different OVDs and how the choice of OVD may cause variable exothermicity secondary to ultrasound energy (after accounting for technique, flow, handpiece choice, and aspiration).6,14,15 This study is novel in that it aimed to understand the effect of choice of OVD that rests near the corneal incision as opposed to the OVD next to the handpiece tip, within the anterior chamber. Surgeons know that central OVD can easily be cleared by irrigation and aspiration; however, OVD near the incision could remain during ultrasound and theoretically still contribute to exothermic heat increase and a CIC. It is also important to note that each OVD used represents different OVD properties and as such it can be noted that dispersive OVD had the most variable change in temperature followed by medium viscosity dispersive, cohesive, and finally viscoadaptive.
Fortunately, this study concludes that whatever heat is transmitted by the OVD, it is clinically insignificant and not of any concern as long as the phaco tip is surrounded by BSS. It is well known that tip occlusion dramatically increases the temperature rise during ultrasound and is likely the greatest risk factor for a CIC; however, the potential for a moment of absolute occlusion to occur is decreased due to the ABS present in phacoemulsification tips which ensure a small amount of continuous flow into the anterior chamber. It is clear that CIC risk increases with OVD surrounding the tip with tip occlusion. The practical lesson is that a few seconds of irrigation and aspiration before using ultrasound is very protective of early OVD-induced CIC. Caution is advised if a lens particle occludes the tip in the surrounding OVD, and we advise surgeons to clear the central OVD or minimize phacoemulsification energy by using a lens chopper or other instruments to prevent CIC.16 Previous work has shown that using chopping techniques, higher flow and vacuum settings that are within the surgeon’s safety zone,5 and phaco settings that promote interval cooling can also be protective of CIC.16
So, why might just having the needle tip in BSS obviate residual OVD near the tip showing any exothermic reaction? One possibility is it is there, but we did not have the statistical power to detect it. However, even if there is a small amount of exothermic reaction, it is unlikely to be of any clinical significance because BSS alone often had more temperature increase than was the average for the OVDs tested. This suggests our result differences are random and not important. A more interesting and likely conclusion is the very causation of OVD exothermic reaction. In talking to an engineer who specializes in cavitation, he surmised that cavitational energy resulted in disulfide bond resonance and heat generation (unpublished communication). As cavitation is directed from the tip and rapidly falls off, OVD away from the tip does not get this effect; and so, the only thermal effect is friction between the tip and the sleeve which would be, as we found, equivalent for BSS and the OVDs tested. Causation of the variability of Viscoat is not clear and can also just be a statistical anomaly as the results were not significantly different. However, we did see this with both the no and 20mL/min flow experiments, and in both cases the mean temperatures were higher, although not significantly so. Viscoat has the lowest viscosity and is the most likely to leak through the membrane. Most likely what is happening is that some Viscoat did leak to the tip where the cavitation resulted in an exothermic reaction; hence, the small increase in temperature and the broad variability we found.
There are several limitations of this study. While the membrane puncture was as small as possible, thereby limiting the amount of transfer of BSS to the OVD to within the chamber, the size of the tear in the membrane may have varied. Therefore, possible transfer of fluids across the membrane was possible; however, the OVD loss was minimal as we observed a stable amount of OVD on the membrane surface during the experiment. Another limitation is that despite attempting to maintain an equal distance of the temperature probe to the tip of the handpiece, this distance may have varied slightly with each test. We recognize that the baseline temperature varied for one of the measurements; even though our focus was on the amount of change present, we recognize that more sophisticated equipment could aid in providing more accurate results. This study is limited in that all tips used feature an ABS. This system could have provided a protective aspect to the amount of temperature change. Further studies could evaluate the potential effect that may take place with a non-ABS tip. More robust data could be produced to evaluate each different OVD property by using multiple types of OVD that feature the same OVD properties. Finally, while we anticipate that heat transmission across a thin membrane such as saran wrap serves as an adequate model of how heat transmits to the corneal incision, we recognize that further studies could corroborate this assumption.
Conclusion
In summary, OVD exothermic heat transfer where the phaco needle tip is surrounded by BSS is clinically insignificant. Surgeons should irrigate and aspirate central OVD to ensure free flow of BSS before engaging ultrasound.
Abbreviations
ABS, aspiration bypass system; BSS, balanced saline solution; CIC, corneal incision contracture; IOP, intraocular pressure; MVD, medium viscosity dispersive; OVDs, ophthalmic viscosurgical devices; phaco, phacoemulsification.
Ethics Approval and Informed Consent
Since no human subjects were involved, approval from the University of Utah Institutional Review Board was not obtained.
Acknowledgments
Susan Schulman assisted with editing and manuscript preparation.
Funding
This study was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York, USA, to the Department of Ophthalmology and Visual Sciences, University of Utah, Salt Lake City, Utah, USA. Dr. Harris and Dr. Ungricht were awarded the NIH Ruth L. Kirschstein National Research Service Award (NRS) institutional training grant (T35EY026511). The biostatistical consultation for this publication was made possible with support from the Indiana Clinical and Translational Sciences Institute which is funded in part by Award Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The sponsors had no involvement in any of the stages from study design to submission of the manuscript for publication.
Disclosure
Dr. Randall J Olson is on the Board of Directors of Perceive Bio and the Scientific Advisory Board of Perfect Lens. Dr. Jeff H Pettey reports a consulting agreement for Lensar, outside the submitted work. Dr. Emilie L Ungricht reports grants from Alcon, outside the submitted work. The other authors report no conflicts of interest in this work.
References
1. Mansour HA, Mansour AM. Autologous tenon plug and patch in phacoburn. BMJ Case Rep. 2021;14(1):e238970. doi:10.1136/bcr-2020-238970
2. Khodabakhsh AJ, Zaidman G, Tabin G. Corneal surgery for severe phacoemulsification burns. Ophthalmology. 2004;111(2):332–334. doi:10.1016/j.ophtha.2003.06.004
3. Majid MA, Sharma MK, Harding SP. Corneoscleral burn during phacoemulsification surgery. J Cataract Refract Surg. 1998;24(10):1413–1415. doi:10.1016/S0886-3350(98)80239-3
4. Sugar A, Schertzer RM. Clinical course of phacoemulsification wound burns. J Cataract Refract Surg. 1999;25(5):688–692. doi:10.1016/S0886-3350(99)00021-8
5. Suzuki H, Igarashi T, Shiwa T, Takahashi H. Efficacy of ophthalmic viscosurgical devices in preventing temperature rise at the corneal endothelium during phacoemulsification. Curr Eye Res. 2016;41(12):1548–1552. doi:10.3109/02713683.2015.1136420
6. Floyd M, Valentine J, Coombs J, Olson RJ. Effect of incisional friction and ophthalmic viscosurgical devices on the heat generation of ultrasound during cataract surgery. J Cataract Refract Surg. 2006;32(7):1222–1226. doi:10.1016/j.jcrs.2006.01.107
7. Yildirim TM, Auffarth GU, Son HS, Khoramnia R, Munro DJ, Merz PR. Dispersive viscosurgical devices demonstrate greater efficacy in protecting corneal endothelium in vitro. BMJ Open Ophthalmol. 2019;4(1):e000227. doi:10.1136/bmjophth-2018-000227
8. Jurowski P, Gos R, Kusmierczyk J, Owczarek G, Gralewicz G. Quantitative thermographic analysis of viscoelastic substances in an experimental study in rabbits. J Cataract Refract Surg. 2006;32(1):137–140. doi:10.1016/j.jcrs.2005.11.025
9. Meyer JJ, Kuo A, Olson RJ. The risk of capsular breakage from phacoemulsification needle contact with the lens capsule: a laboratory study. Am J Ophthalmol. 2010;149(6):882–886.e1. doi:10.1016/j.ajo.2009.12.035
10. Buschschlüter S, Koch C, von Eicken J, Höh H. Computation of the temperature rise at the corneal endothelium during cataract surgery by modeling of heat generation inside the anterior chamber. Ultrasound Med Biol. 2014;40(10):2431–2444. doi:10.1016/j.ultrasmedbio.2014.05.017
11. Zacharias J. Laboratory assessment of thermal characteristics of three phacoemulsification tip designs operated using torsional ultrasound. Clin Ophthalmol. 2016;10:1095–1101. doi:10.2147/OPTH.S105065
12. Sippel KC, Pineda R. Phacoemulsification and thermal wound injury. Semin Ophthalmol. 2002;17(3–4):102–109. doi:10.1076/soph.17.3.102.14776
13. Nair S, Nair RU. Wound and surface temperatures in vivo in torsional and longitudinal modalities of ultrasound in coaxial microincisional cataract surgery. Clin Ophthalmol. 2017;11:249–255. doi:10.2147/OPTH.S123222
14. Modi SS, Davison JA, Walters T. Safety, efficacy, and intraoperative characteristics of DisCoVisc and Healon ophthalmic viscosurgical devices for cataract surgery. Clin Ophthalmol. 2011;5:1381–1389. doi:10.2147/OPTH.S22243
15. Bissen-Miyajima H. In vitro behavior of ophthalmic viscosurgical devices during phacoemulsification. J Cataract Refract Surg. 2006;32(6):1026–1031. doi:10.1016/j.jcrs.2006.02.039
16. Yamagami S, Yamagami H. Direct measurement of wound temperature during phacoemulsification. Ophthalmologica. 1998;212(1):50–52. doi:10.1159/000027260
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
