Back to Journals » Journal of Hepatocellular Carcinoma » Volume 1
Telaprevir-containing regimen for treatment of hepatitis C virus infection in patients with hepatocellular carcinoma awaiting liver transplantation: a case series
Authors Torres H, Kaseb A, Mahale P, Miller E, Frenette C
Received 11 February 2014
Accepted for publication 7 April 2014
Published 16 July 2014 Volume 2014:1 Pages 109—114
DOI https://doi.org/10.2147/JHC.S60867
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Harrys A Torres,1 Ahmed Kaseb,2 Parag Mahale,1 Ethan Miller,3 Catherine Frenette4
1Department of Infectious Diseases, Infection Control and Employee Health, 2Department of Gastrointestinal Medical Oncology, 3Department of Gastroenterology, Hepatology and Nutrition, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 4Department of Liver Transplantation, Weill Cornell Medical College, The Methodist Hospital, Houston, TX, USA
Abstract: In patients who undergo liver transplantation (LT), allograft failure secondary to hepatitis C virus (HCV) recurrence after LT accounts for two-thirds of graft failures and deaths. Achievement of sustained virologic response before LT eliminates the risk of HCV recurrence. Only a limited number of studies have evaluated the role of antiviral treatment before LT. No published data are available regarding the use of HCV protease inhibitors before LT. We report our experience using the combination of telaprevir, pegylated interferon alfa-2a (PegIFN alfa-2a), and ribavirin in three patients with HCV-associated hepatocellular carcinoma (HCC) awaiting LT. Two patients had not received, and one had had a partial response to HCV therapy (PegIFN alfa-2a plus ribavirin). All three patients had genotype 1b and were started on telaprevir and full doses of PegIFN alfa-2a and ribavirin. Treatment was planned to be continued until the day of LT or 48 weeks total, whichever came first. One patient still had detectable HCV RNA after 24 weeks of antivirals and was, therefore, excluded from further analysis. The other two patients had undetectable HCV RNA at the end of antiviral therapy. In one of these patients, HCV RNA remained undetectable after LT; the other patient experienced viral relapse. HCV therapy was tolerated by all patients; no patient required permanent discontinuation of therapy because of toxic effects. All three patients experienced hematologic toxic effects. Only one patient required treatment discontinuation, due to progression of HCC. The use of telaprevir-containing regimens appears to be safe in selected patients with HCV-associated HCC awaiting LT, but more studies are warranted to evaluate the safety and efficacy of this treatment combination to prevent post-LT viral recurrence.
Keywords: telaprevir, hepatitis C virus, hepatocellular carcinoma, liver transplantation
Introduction
Hepatitis C virus (HCV)-associated cirrhosis is the leading indication for adult liver transplant (LT) throughout the world.1–5 In patients undergoing LT, allograft failure secondary to HCV recurrence after LT accounts for two-thirds of graft failures and deaths.6 Achievement of a sustained virologic response before LT eliminates the risk of such recurrence.7,8 Only a limited number of studies have evaluated the role of antiviral treatment before LT,4 most of them including patients who underwent LT for HCV-induced end-stage liver disease. In these studies, approximately 20% of patients remained HCV-free after LT.9–11 Unfortunately, patients included in these studies have been heterogeneous with respect to reasons for LT, infecting genotypes, and duration of pre-LT therapy.10–12 In a randomized controlled trial of pre-LT antiviral therapy to prevent post-LT recurrence of HCV (patients treated with standard-of-care therapy with peginterferon alfa-2b plus ribavirin), 22% of 23 treated patients with genotypes 1, 4, or 6 achieved a post-LT virologic response.13 This study included a heterogeneous group of patients with chronic HCV with and without hepatocellular carcinoma (HCC).13
To our knowledge, no published data are available regarding the use of direct-acting antiviral agents such as first generation protease inhibitors in HCV-infected patients with compensated cirrhosis and HCC. Herein, we report the impact of the combination of telaprevir, a US Food and Drug Administration–approved direct-acting antiviral, along with standard-of-care therapy on prevention of post-LT HCV recurrence in three patients with genotype 1 infection and HCV-associated HCC.
Patients and methods
In this study, we described the characteristics and outcomes of three patients with genotype 1 HCV-associated HCC treated for HCV infection with a combination of telaprevir, pegylated interferon alpha-2a (PegIFN alfa-2a), and ribavirin while awaiting LT. These patients were treated at The University of Texas MD Anderson Cancer Center from September 1, 2011, to October 31, 2012. Patient demographics, information on HCC management, and laboratory data were obtained from the patients’ medical records. This retrospective study was approved by the MD Anderson Institutional Review Board.
HCV RNA quantification, sequencing, and genotyping
HCV RNA levels were measured at baseline (before treatment initiation); after 2, 4, 12, 24, 36, and 48 weeks of treatment; and 4 and 8 weeks after LT.
HCV RNA in serum was quantified using a commercially available polymerase chain reaction method (COBAS® TaqMan® HCV Test; Roche Molecular Systems Inc., Pleasanton, CA, USA) with a quantification range of 43 IU/mL to 69,000,000 IU/mL (1.63–7.84 log10 IU/mL). HCV genotype was determined using the Trugene® 5′NC HCV genotyping kit (Siemens Healthcare Diagnostics Inc., Erlangen, Germany), a commercial genotyping method using direct sequence analysis of the 5′ noncoding region.
Antiviral treatment
The planned treatment was the combination of telaprevir, PegIFN alfa-2a, and ribavirin for 12 weeks followed by the combination of PegIFN alfa-2a and ribavirin for an additional 36 weeks, for a total of 48 weeks of therapy (which is the standard-of-care treatment for cirrhotic patients14), or until the day of LT, whichever came first.
The starting doses were as follows: PegIFN alfa-2a, 180 μg subcutaneously once weekly; ribavirin, 1,000 mg orally daily for patients weighing less than 75 kg and 1,200 mg orally daily for patients weighing 75 kg or more; and telaprevir, 750 mg orally three times a day.
Results
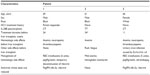
Patient demographics and treatment characteristics are summarized in Table 1. The mean age of the patients was 65 years. Two patients were African American men, and one patient was a white woman. All patients had genotype 1b infection; regarding interleukin 28B polymorphism, two patients had the CT genotype, and one patient had the TT genotype. Two patients had not received, and one had had a partial response to, prior HCV therapy with PegIFN alfa-2a and ribavirin.
Pre-LT HCV RNA levels
All patients were started at full doses of each antiviral drug. In all three patients, HCV RNA levels were markedly lower (HCV RNA level <1.63 log10 IU/mL, the lower limit of the quantification range of the test used) at 4 weeks after treatment initiation compared to baseline (Table 2 and Figure 1). In patient 1, antiviral therapy was discontinued after 24 weeks because the patient underwent LT at that time. In patient 2, HCV RNA levels remained detectable after 24 weeks of antiviral therapy, and, therefore, the patient was excluded from further analysis for this study due to virologic failure pre-LT. In patient 3, antiviral therapy was discontinued after 11 weeks because of progression of HCC. Patients 1 and 3 had undetectable HCV RNA levels at the end of therapy prior to LT (Table 2 and Figure 1).
  | Figure 1 HCV RNA levels during treatment and after liver transplant (LT). |
Post-LT HCV RNA levels
As noted above, patients 1 and 3 had undetectable HCV RNA levels prior to LT. In patient 1, HCV viral load was detectable at a very low level (HCV RNA <1.63 log10 IU/mL) at 4 weeks after LT and was higher (6.38 log10 IU/mL) at 8 weeks after LT (Table 2 and Figure 1). In patient 3, the viral load remained undetectable until 11 weeks after LT, when the patient developed severe antibody-mediated graft rejection and died.
Adverse events related to HCV therapy
HCV therapy was tolerated by all patients with no permanent discontinuation of therapy due to toxic effects. Hematologic toxic effects were observed in all patients (Table 1). In patients 1 and 3, management of hematologic toxic effects required reduction of PegIFN alfa-2a and ribavirin doses and administration of growth factors, along with red blood cell transfusions.
In patient 1, the dose of PegIFN alfa-2a was reduced twice because of thrombocytopenia, the lowest dose being 90 μg per week. The dose of ribavirin was reduced to 600 mg per day because of anemia, and the dose was never increased back to the starting dose. Ribavirin was also temporarily discontinued once because of severe anemia, but was reinitiated after correction of anemia with red blood cell transfusion. In patient 3, the dose of PegIFN alfa-2a was reduced twice because of thrombocytopenia to 90 μg per week, and the dose of ribavirin was reduced because of anemia to 600 mg per day.
There were no adverse events related to administration of growth factors except for the development in patient 3 of a bland thrombus in the left and right portal veins that could be attributed to the use of eltrombopag. Progression of HCC as the cause of such thrombosis could not be excluded.
Concomitant transcatheter arterial chemoembolization
Two patients underwent transcatheter arterial chemoembolization with drug-eluting beads of doxorubicin while undergoing treatment for HCV and awaiting LT. No clinically relevant interactions between transcatheter arterial chemoembolization and HCV therapy were observed after these oncologic procedures.
Discussion
HCC is a major complication of chronic liver disease and is the third most common cause of cancer-related deaths worldwide.15 Currently, orthotopic LT remains the best treatment option for patients with HCC within the Milan criteria,16 with cure rates up to 95% in some series.16 Patients with HCC and compensated cirrhosis are one of the ideal target groups for pre-LT antiviral therapy.9 In these patients, unlike other HCV-infected patients, the indication for LT is not liver decompensation but rather the cancer. The increased incidence of HCC in the US has resulted in a dramatic increase in the number of patients awaiting LT17 and concomitant increased wait times for all patients. Given these prolonged wait times, for patients with adequate liver function who are awaiting LT for HCC-related reasons, treatment of HCV can be considered as achievement of a sustained virologic response before LT could eliminate the risk of post-LT HCV recurrence.9,10
The International Liver Transplantation Society proposed that patients with a Child-Pugh-Turcotte score no greater than 7 and Model for End-Stage Liver Disease score no greater than 18 should be strongly considered for interferon-based treatment.5 No published data are available regarding the use of first generation protease inhibitors, such as telaprevir, in patients with HCV-induced HCC. Our case series reported here sheds light on these issues.
In terms of efficacy, two of the three patients analyzed had no detectable HCV RNA by the end of treatment. In one of these two patients, HCV viral load remained undetectable at the last follow-up, almost 3 months after LT. In the remaining patient, viral relapse occurred. Unfortunately, our findings are too limited to draw any meaningful conclusion on the efficacy of telaprevir-containing regimens to prevent post-LT viral recurrence.
Before the introduction of direct-acting antiviral agents, there was a general consensus that a low, accelerating dose regimen of interferon and ribavirin was the preferred treatment for patients with HCV-associated HCC, awaiting LT.5 As achievement of low viral load at week 4 of treatment is crucial in determining whether to continue telaprevir-based therapy,14 full doses of both PegIFN alfa-2a and ribavirin were administered initially to all patients in our series, as previously done in cirrhotic patients awaiting LT.12 None of the three patients in our series required permanent discontinuation of this triple combination therapy due to toxic effects.
The use of telaprevir-containing regimens in patients on the waiting list for LT appears to be safe. Although all patients developed hematologic toxic effects as the major side effect, these were managed with the standard approach of reduction in the dose of PegIFN alfa-2a and/or ribavirin, blood cell transfusion, or use of hematopoietic growth factors. Only one patient required treatment discontinuation, and this was due to progression of HCC, not the toxic effects of antiviral therapy.
Late in 2013, sofosbuvir – a nucleotide analog NS5B polymerase inhibitor – was approved by the US Food and Drug Administration in combination with ribavirin for treatment of chronic HCV in patients with HCC awaiting LT, given its good tolerability and a post-LT virologic response of 64%.18 Telaprevir, a cytochrome P450 3A and P-glycoprotein substrate and inhibitor, and an immunosuppressive agent, is expected to interact with a number of other drugs.19 Unlike protease inhibitors, sofosbuvir is not metabolized by cytochrome P450 enzymes and may, therefore, not be associated with clinically relevant drug interactions.20
In conclusion, the use of telaprevir-containing regimens appears to be safe in selected patients with HCV-associated HCC awaiting LT, but more studies are warranted to evaluate the safety and efficacy of this treatment combination to prevent post-LT viral recurrence. However, with the approval of newer and better tolerated direct-acting antivirals (eg, sofosbuvir) for this patient population, the use of first generation protease inhibitors such as telaprevir has fallen out of favor in some recently published guidelines.21
Acknowledgment
We thank Stephanie Deming for editorial assistance.
Disclosure
Dr Torres is a consultant for Gilead Sciences, Merck and Co, Inc., Novartis, Astellas, Pfizer, Theravance, Inc., Genentech, and Vertex Pharmaceuticals, and has received research grants from Merck and Co, Inc. and Vertex Pharmaceuticals. The other authors declare that they have nothing to disclose regarding funding or conflict of interest with respect to this manuscript. This paper was presented in part at The Liver Meeting, November 9–13, 2012, Boston, Massachusetts, USA.
References
Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. | |
Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997; 26(3 suppl 1):62S–65S. | |
Murphy EL, Bryzman S, Williams AE, Co-Chien H, Schreiber GB, Ownby HE, et al. Demographic determinants of hepatitis C virus seroprevalence among blood donors. JAMA. 1996;275(13):995–1000. | |
Li KK, Neuberger J. The management of patients awaiting liver transplantation. Nat Rev Gastroenterol Hepatol. 2009;6(11):648–659. | |
Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9(11):S1–S9. | |
Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122(4):889–896. | |
Ghobrial RM, Steadman R, Gornbein J, Lassman C, Holt CD, Chen P, et al. A 10-year experience of liver transplantation for hepatitis C: analysis of factors determining outcome in over 500 patients. Ann Surg. 2001;234(3):384–93; discussion 393–394. | |
Neumann UP, Berg T, Bahra M, Puhl G, Guckelberger O, Langrehr JM, et al. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation. 2004;77(2):226–231. | |
Terrault NA. Hepatitis C therapy before and after liver transplantation. Liver Transpl. 2008;14(Suppl 2):S58–S66. | |
Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42(2):255–262. | |
Forns X, Garcia-Retortillo M, Serrano T, Feliu A, Suarez F, de la Mata M, et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39(3):389–396. | |
Carrión JA, Martinez-Bauer E, Crespo G, Ramirez S, Perez-del-Pulgar S, Garcia-Valdecasas JC, et al. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: A retrospective study. J Hepatol. 2009;50(4):719–728. | |
Everson GT, Terrault NA, Lok AS, Rodrigo DR, Brown RS Jr, Saab S, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752–1762. | |
Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(4):1433–1444. | |
Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma – epidemiological trends and risk factors. Dig Dis. 2009;27(2):80–92. | |
Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. | |
Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11(9):1773–1784. | |
Sofosbuvir [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2013. | |
Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011;54(1):20–27. | |
Asselah T. Sofosbuvir for the treatment of hepatitis C virus. Expert Opin Pharmacother. 2014;15(1):121–130. | |
American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) [homepage on the Internet]. Recommendations for Testing, Managing, and Treating Hepatitis C; 2014. Available from: http://hcvguidelines.org. Accessed February 10, 2014. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.