Back to Journals » Medical Devices: Evidence and Research » Volume 12
Technology update: dissolvable microneedle patches for vaccine delivery
Authors Rodgers AM , Cordeiro AS , Donnelly RF
Received 17 April 2019
Accepted for publication 8 August 2019
Published 19 September 2019 Volume 2019:12 Pages 379—398
DOI https://doi.org/10.2147/MDER.S198220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Aoife M Rodgers, Ana Sara Cordeiro, Ryan F Donnelly
School of Pharmacy, Queen’s University Belfast, Belfast, BT9 7BL, UK
Correspondence: Ryan F Donnelly
School of Pharmacy, Queen’s University Belfast, Medical Biology Centre, 97 Lisburn Road, Belfast BT9 7BL, UK
Tel +44 289 097 2251
Fax +44 289 024 7794
Email [email protected]
Abstract: Despite vaccination representing one of the greatest advances of modern preventative medicine, there remain significant challenges in vaccine distribution, delivery and compliance. Dissolvable microarray patches or dissolving microneedles (DMN) have been proposed as an innovative vaccine delivery platform that could potentially revolutionize vaccine delivery and circumvent many of the challenges faced with current vaccine strategies. DMN, due to their ease of use, lack of elicitation of pain response, self-disabling nature and ease of transport and distribution, offer an attractive delivery option for vaccines. Additionally, as DMN inherently targets the uppermost skin layers, they facilitate improved vaccine efficacy, due to direct targeting of skin antigen-presenting cells. A plethora of publications have demonstrated the efficacy of DMN vaccination for a range of vaccines, with influenza receiving particular attention. However, before the viable adoption of DMN for vaccination purposes in a clinical setting, a number of fundamental questions must be addressed. Accordingly, this review begins by introducing some of the key barriers faced by current vaccination approaches and how DMN can overcome these challenges. We introduce some of the recent advances in the field of DMN technology, highlighting the potential impact DMN could have, particularly in countries of the developing world. We conclude by reflecting on some of the key questions that remain unanswered and which warrant further investigation before DMNs can be utilized in clinical settings.
Keywords: microneedle patches, dissolvable, vaccine, cold chain, hazardous sharps waste, skin
Introduction
Vaccination is one of the greatest medical advances of modern preventative medicine. It is the most effective means of controlling the incidence of infectious disease, as evidenced from the elimination of smallpox and estimated avoidance of 2.5 million deaths per year from diphtheria, tetanus, whooping cough and measles.1 Despite this, current vaccination approaches face a number of challenges which impact upon vaccination compliance. Most vaccines are administered via intramuscular (IM) or subcutaneous (SC) injection, causing pain and discomfort and, in many cases, leading to poor compliance as a result of needle phobia.2,3 Hypodermic needles result in the creation of biohazardous sharps waste, requiring safe disposal to ensure needles are not reused, either intentionally or accidentally. This is notably problematic in the developing world, where the use of unsafe or inappropriate injection practices leads to the transmission of infectious diseases. Specifically, it is estimated that up to 33,800 HIV infections, 1.7 million hepatitis B infections and 315,000 hepatitis C infections arise every year as a result of unsafe injection practices.4 Additional challenges arise due to ineffective supply chains and vaccine wastage due to multi-dose vials or failures in cold chain systems.5,6
Oral vaccination is an alternative to that of parenteral, and has been approved for human use for a number of vaccines.7,8 Nonetheless, oral delivery of vaccines presents significant challenges, including decreased immunogenicity as a result of antigen digestion and degradation in the gastrointestinal tract, prior to the induction of appropriate immune responses. Thus, oral vaccination has been confined to a relatively small number of licensed vaccines including rotavirus, typhoid, cholera and some poliovirus vaccines.9 Transdermal vaccine delivery has also been investigated. The skin, however, by virtue of its protective function, serves as a barrier for delivering drugs or vaccines through the topical route. The skin’s stratum corneum acts as a barrier for topically applied drugs or vaccines, allowing only certain molecules such as those of low molecular weight (usually <350 Da) or lipophilic drugs to pass across it. Intradermal (ID) injection as an alternative delivery system into the viable skin layers is technically challenging, necessitating specialist training of health care providers. Considering the above-mentioned challenges with parenteral, oral and traditional ID delivery routes, recent research efforts have focused on addressing the urgent and unmet requirement for simplified, alternative methods for vaccine delivery.
Microneedle (MN) patches have been proposed as an innovative vaccine delivery platforms and a viable means of circumventing the challenges associated with conventional vaccine delivery.10 MNs are minimally invasive devices that consist of an array of microscopic needles attached to a base support or backing (Figure 1A and B) and are categorized into five main types, namely, hollow,11 solid,12 coated,13 swellable14 and dissolving MNs (DMN).10 DMNs show particular promise for vaccination purposes due to their self-disabling nature, prohibiting re-use. DMN are fabricated from fast dissolving materials such as polymers or sugars and the vaccine is incorporated within the matrix.15,16 These MNs have a “pin cushion” appearance and are applied to the skin in a manner similar to that of a plaster, bandage or conventional transdermal patch. Upon insertion of the needles in the skin, they come into contact with skin interstitial fluid and dissolve, simultaneously facilitating delivery of vaccines to the skin’s epidermis and dermis (Figure 1C and D).17,18 Therefore, DMN are the most promising MN for clinical use as they can overcome any safety issues caused by broken MNs from solid arrays which may remain in the skin post application.19 The needles of the array are typically 50–900 µm in length and as such, are long enough to penetrate the dermis, but are, in most cases, short and narrow enough to avoid stimulation of dermal nerves or puncture dermal blood vessels. Accordingly, DMNs facilitate a painless means of drug or vaccine delivery that is well accepted by patients.20
This review summarizes the barriers faced by current vaccination approaches. We introduce the field of DMN technology, providing an overview of the advantages this technology offers for ID vaccine delivery, in comparison to that of traditional SC, IM or ID injections. Specifically, the advantages of DMN from an immunological perspective are drawn upon. With this as a starting point, we subsequently provide an update on the recent advances which have been made using DMN for vaccination purposes, highlighting the potential and novelty of this technology. Extensive research efforts have been devoted to the development of innovative fabrication methods of DMN for vaccination purposes, with the aim of improving vaccine loading into needles and subsequent delivery.21–23 It is beyond the scope of this review to cover such aspects; however, we direct the reader to a number of recently published reviews which provide an insight into some of the challenges faced in the fabrication of DMN.24,25 Herein, we finally conclude by reflecting on some of the key questions which remain to be addressed for adoption of DMNs in a clinical setting.
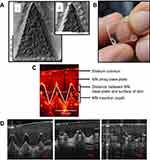 |
Figure 1 (A) Scanning electron microscope images of DMN, 500 µm in height and with 300 µm width at base. (B) A DMN, prior to application to the skin. (C, D) Representative optical coherence tomography (OCT) images showing DMN insertion in skin (C) and DMN dissolution in skin at time 0, 15 mins and 60 mins (D). Reproduced from Rodgers AM, McCrudden MT, Vincente-Perez EM, et al. Design and characterisation of a dissolving microneedle patch for intradermal vaccination with heat-inactivated bacteria: a proof of concept study. Int J Pharm. 2018;549(1–2):87–95. Creative commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.10 Abbreviation: DMN, dissolving microneedles. |
Circumventing vaccine delivery challenges: is the solution in the patch?
Despite the global impact vaccination has played in public health, there remain significant challenges with current vaccination approaches. As previously highlighted, the hypodermic needle and syringe are most commonly utilized for vaccine administration into the muscle tissue, despite the fact that the muscle is not a highly immunogenic organ.26 For this purpose, health care providers must acquire training for correct vaccine reconstitution and subsequent administration. This is particularly problematic in countries of the developing world where there are significant barriers to effective vaccination (as reviewed in4). In short, this is primarily due to logistical and economic challenges in relation to geographical accessibility and insufficient numbers of appropriately trained health care workers. The estimated cost of cold chain storage is $200–$300 million annually and failures in this system often result in vaccine shortages.10 As many vaccines require booster injections for induction of appropriate immune responses, this can also be difficult to implement in the developing world due to limited access to health care and difficulty in implementing vaccination programs. Accordingly, safer, cost-effective, innovative vaccine formulations are warranted, in order to improve coverage of current vaccines and help control the incidence of infectious disease.
Vaccine delivery to the skin via DMNs
The skin is an attractive site for vaccination. The physiology and anatomy of the skin has been well characterized and reviewed elsewhere.10,27 This organ serves as the first line of defense against pathogens, containing an abundant population of immune cells, including epidermal Langerhans cells (LC) and dermal dendritic cells (dDC), which can provide excellent targets for vaccine delivery.28 Since the work of Glenn and collaborators in the early 2000s, several clinical studies have shown the potential of transcutaneous (TC) administration route for vaccination.29–32 A Phase I trial by Combadière et al33 was the first study to demonstrate the superiority of this route, in comparison to that of IM. Specifically, it was shown that delivery of inactivated influenza vaccine via the TC route resulted in more efficient induction of influenza-specific CD8+ T cell responses, in comparison to that delivered via the IM route. Due to the substantial numbers of APCs present in the skin, vaccine delivery by this route may result in dose-sparing effects, thus permitting the induction of enhanced immune responses using lower doses of vaccine.34 As illustrated in Figure 2, DMNs inherently target these immune cells, providing a unique opportunity for enhanced vaccine immunogenicity and dose-sparing effects,35,36 concomitant to additional logistical advantages which will be discussed in further detail in the following subsections.
 |
Figure 2 A schematic representation of the skin structure illustrating the different routes of administration, namely intramuscular, subcutaneous and ID injections. DMN penetrate the skin’s stratum corneum barrier reaching the viable epidermis, whereas the hypodermic needle punctures the skin into the subcutaneous and muscle tissue. Reproduced from Leone M, Mönkäre J, Bouwstra JA, Kersten GF. Dissolving Microneedle Patches for Dermal Vaccination. Pharm Res. 2017;34(11):2223-2240. Creative commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.106 Abbreviations: DMN, dissolving microneedles; ID, intradermal. |
Opportunity for vaccine administration by untrained personnel
At present, the administration of vaccines necessitates delivery by a health care provider. This presents a challenge in situations whereby there is limited access to health care providers and facility-based care, particularly within the developing world countries. DMNs overcome the requirement for trained personnel for correct vaccine delivery because they are simply inserted by hand or utilizing an applicator device.37,38 This may be especially beneficial during, for example, mass vaccination campaigns, whereby vaccine administration by patients themselves, or lesser-trained health care providers would expand access to lifesaving vaccines. Concurrently, if vaccines were to be self-administrated by patients themselves, or by lesser-trained personnel, this could have significant cost savings.39
Considering this, authors have developed different studies to explore the opinions of health care professionals and the public on the use of MN.40,41 In a publication by Birchall and co-workers, all focus groups were in favor of MN, in comparison to that of hypodermic injections, although concerns were raised regarding the need for feedback mechanisms to reassure the user of successful MN insertion and delivery. More recently, Marshall et al41 published a literature review of perception and acceptability of the MN technology for vaccination, particularly in the pediatric population. In general, the review highlights the positive perceptions of the technology both in the general public and in health care professionals, listing a variety of advantages commonly associated with this approach. Nevertheless, concerns about unfamiliarity with the technology and inability to ensure accurate vaccine delivery were also highlighted and require further efforts by researchers and companies.
In contrast to taking medicine orally or via an injection whereby delivery is complete within minutes, DMN must be worn until the needles have dissolved within the skin. This is dependent upon the DMN formulation and can range from minutes to hours.19 Thus, an innovative means of monitoring DMN delivery may be beneficial to the end users, providing assurance of correct usage and assisting in the future translation of the technology to clinical use. In light of this, the use of a low-cost pressure-indicating sensor film to provide feedback upon MN application has been evaluated.42 To elaborate, human volunteers self-applied MNs, with and without a pressure-sensing film. Following this, optical coherence tomography was used to visualize MN insertion in skin and to monitor the insertion depth into the skin layers. The pressure-sensing film facilitated colorimetric analysis to provide feedback on MN insertion. Assessment of participants’ opinions indicated that 75% preferred the MNs with the pressure-sensing film. It must be noted that these experiments were conducted with hydrogel-forming MNs, rather than DMN, although the principles of insertion remain the same between both modalities. Another study by Norman et al37 assessed the usability and acceptability of stainless steel MN for vaccination against influenza utilizing a snap device as a force feedback to users. To elaborate, 91 subjects were recruited and received either placebo MN given by the investigator or by self-administration or an IM injection of saline. Of the MN group, MNs were given three times and 70 of the participants inserted MN with thumb pressure alone while the remainder used the snap-based device that closed shut at a certain force. Thereafter, skin staining and acceptability was measured with an adaptive-choice analysis. Results indicated that the best usability was evident with the snap device, with users inserting a median value of 93–96% of MN over three repetitions.
Overcoming pain and needle phobia
Needle-phobia can, in many cases, be an impediment to patient adherence to vaccination programs.2,3 Needle gauge and the mechanics of needle insertion, including force and mechanical workload of the hypodermic needle, have all been found to correlate with the frequency of pain.43 As DMNs are fabricated to be short and narrow enough to avoid stimulation of dermal nerves, there is no pain associated with vaccine administration via this route.20,44 Therefore, patient adherence to vaccination programs via DMN is likely to increase and as highlighted previously, DMN are, in general, well accepted by patients.20,45,46
Potential for reduced cost vaccination
It is anticipated that DMN will result in reduced vaccination costs due to the prospect of self-vaccination or vaccination by untrained personal, the elimination of hazardous sharps waste and simplification of the supply chain. It will be important to develop an understanding of DMN manufacturing requirements and subsequent costs, which are likely to be vaccine-specific, to fully compare DMN vaccination to other conventional approaches.24 It is also worth to consider that if applicator devices are used to facilitate DMN insertion, this will undoubtedly increase DMN vaccination costs.
Elimination of hazardous sharps waste
As DMN are fabricated from water-soluble, biocompatible materials that dissolve in the skin post-insertion, they overcome the generation of biohazardous sharps wastes and any material that remains on the skin may be discarded in non-sharps waste.47 Thus, this circumvents the risk of injury and disease transmission from used or contaminated needles.4
Improved thermostability, simplified supply chain and subsequent increased vaccine coverage
DMN are smaller in size than traditional hypodermic needles and syringes, thus offering simplified supply chains, storage and distribution.48 Most vaccines necessitate storage at specific temperatures from the point of manufacture, through transportation, storage and administration. This results in significant economic challenges, particularly in developing world countries where the infrastructure requirements for cold chain storage are usually difficult to meet. Moreover, failures in the cold chain and vaccine exposure to temperatures outside of recommended ranges can result in decreased vaccine potency and subsequent lack of protection against the vaccine-preventable illness.49 DMN are fabricated such that the vaccine is contained within the DMN in its dried form, in many cases in combination with suitable excipients to improve thermostability.50 Accordingly, because of their solid-state formulation, DMN can be stored at ambient temperatures, overcoming the requirement for cold chain storage completely or partially.50 In the case of the latter whereby only partial thermostability is achieved, the DMN may be refrigerated during storage but may not require cold chain storage during distribution to remote locations or during mass vaccination campaigns.
A number of reported studies have demonstrated the thermostability of vaccines in DMN. As an example of this, Mistilis et al51 developed a thermostable DMN for influenza vaccination. It was demonstrated that a number of DMN formulations were stable during storage at room temperature for up to 6 months. Following this, a more recent study reported by the same research team assessed the long-term stability of DMN containing influenza vaccine during storage outside the cold chain and when exposed to potential stresses found during manufacturing and storage. In short, it was demonstrated that influenza vaccine in DMN lost no significant activity during exposure to 60ºC for 4 months, multiple freeze-thaw cycles or electron beam irradiation.52 The thermostability of adenovirus-based vaccines53 and measles vaccines has also been demonstrated.54 Moreover, Kolluru and co-workers recently developed a thermostable DMN for administration of inactivated polio vaccine with improved thermostability, in comparison to that of the conventional liquid inactivated polio vaccine (IPV).50 A number of excipients were screened for their ability to improve stability and combinations of maltodextrin and D-sorbitol in histidine buffer were found to be effective in preserving activity of IPV. The resultant DMN maintained the stability of IPV in storage at up to 40ºC, with more than 40% of activity maintained post-storage for 2 months, and more than 20% after 1 year. The time required for a vaccine to maintain its thermostability in storage conditions depends both on the vaccine itself and on the location where it will be distributed and administered. For example, in Bihar, vaccines stable for 1 month were used in advance of expiry, whilst in Mozambique, vaccines required a longer time interval (2 months) to be used before expiry.48
DMN vaccination: trends, progress and recent applications
The first successful vaccination with DMN was reported by the Prausnitz group in 2010.55 In this study, DMN were fabricated from liquid vinyl pyrrolidone monomer, with needles of 650 µm in height, and containing 3 µg of lyophilized inactivated influenza virus vaccine. The DMN were inserted into mouse skin by hand pressure and dissolved within minutes. It was demonstrated that the DMN could induce protective immune responses, greater than those observed following IM vaccination with the same dose. Specifically, it was shown that DMN induced enhanced antibody and cellular responses, resulting in lung viral clearance post lethal influenza challenge. Following this initial study, Kendall and co-workers developed the NanopatchTM and demonstrated successful delivery of Quil-A adjuvanted ovalbumin and influenza vaccine.56 Concurrent with the reports by Prausnitz et al,55 Kendall and co-workers showed that DMN were more efficient at inducing antibody titers against ovalbumin in mice than the conventional IM injection. In the case of the influenza vaccine, authors report strong antibody responses generated in mice immunized with DMN, using a much lower dose than that of the IM injection control. Following these initial publications which evidenced the promising potential of the technology, a plethora of studies have reported the successful delivery of various vaccine antigens, with significant progress being made in the field of DMN vaccination. A summary of published preclinical studies utilizing DMN for vaccination is presented in Table 1. As shown, this has included a variety of viral, bacterial and model antigens, with overall promising results.15,19,52–55,57–78
 |  |  |  |
Table 1 Preclinical vaccine studies with DMN |
Viral vaccines
A wide range of publications have reported on the use of DMN for vaccination against polio, measles, influenza, HIV, hepatitis B and enterovirus 71 (EV71), the causative agent of hand-foot-and-mouth disease (HFMD), as highlighted above.
In 2015, Edens and colleagues developed DMN from gelatin/sucrose to deliver IPV to rhesus macaques (Macaca mulatta). DMN contained 100 needles, each 650 µm in height and rhesus macaques were vaccinated with DMN or via IM injection. DMN were well tolerated by the monkeys and neutralizing antibody titers were equivalent among both immunization strategies for IPV types 1 and 2. Following this work, the same group published results on the development of polymeric DMN for measles vaccination in rhesus macaques.54 In this case, DMN were fabricated from sucrose and carboxymethyl cellulose (CMC) and contained the standard dose of measles vaccine (1000 TCID50). The rhesus macaques were vaccinated with DMN or SC injection and results showed that both groups generated comparable levels of neutralizing antibody responses to measles. DMN were found to retain their potency post-storage at elevated temperatures suggesting improved thermostability in comparison to that of standard lyophilized vaccine. Subsequent studies by the same group further explored the stability of other vaccines in DMN and this included subunit and inactivated vaccines,50,80 with influenza receiving particular attention.51,52
Influenza has been indeed the focus of the majority of preclinical studies concerning the delivery of viral vaccines with DMN, mostly using mice as the animal model. In 2016, Vrdoljak and co-workers proposed the use of polyvinyl alcohol (PVA) and trehalose in DMN with different array geometries and needle heights (12×12, 280 µm long and 5×5, 550 µm long) for single-dose influenza immunization in mice.15 Using this approach, significant dose-sparing was observed in comparison with conventional IM injection, with broadly neutralizing antibody responses that were even able to tackle heterosubtypic viral strains and non-stalk regions of hemagglutinin. Similarly, another study reported protective neutralizing antibody responses against two influenza virus strains (PR8 and Vac-3) in mice immunized with DMN.59 In this case, one of the strains required a boost immunization to generate protective responses, evidencing the need for specific strategies to be developed for each particular vaccine. DMN have also been used as a boost strategy following priming with the inactivated virus58 or as a supplementary vaccination approach in combination with IM vaccine injection,61 with strong, longer-lasting, protective antibody responses being achieved in comparison with animals receiving IM-only immunization. Finally, some authors have also extended the application of this strategy, as is the case of Vassilieva and co-workers, who studied the effect of statin therapy in the immune response against DMN-based influenza vaccines.60 In this study, the authors observed a positive effect of DMN vaccination in overcoming the declining of antibody titers observed in animals treated with statins, in comparison with IM immunization. The same group has also explored the inclusion of adjuvants, such as the granulocyte-macrophage colony-stimulating factor (GM-CSF) in influenza-delivering DMN, with promising results.62
To move toward the evaluation of the suitability of DMN use in human subjects, this approach has also been investigated in higher animals. For example, Arya and co-workers assessed the safety and immunogenicity of DMN vaccination utilizing a rabies DNA vaccine in dogs, with the aim of preventing the complex and expensive post-exposure vaccination scheme in humans.70 The vaccine was stable during formulation and storage, for at least 3 weeks at 4ºC. DMN were applied to the ears of dogs by hand and while mild erythema was observed, complete resolution occurred within 7 days post-vaccination and no systemic adverse reactions occurred. Importantly, DMN were found to be as immunogenic as the IM vaccine injection, as seen by serum antibody titers. In another example, DMN were used for immunization of pigs against hepatitis B virus, with QS-21-loaded liposomes included in the formulation as an adjuvant.77 The antibody responses generated in this study were equivalent between groups receiving two doses of DMN vaccine, IM prime and DMN boost or two IM doses of the commercial vaccine, reinforcing the potential of this approach even in other animal models besides the common rodents.
Following the abovementioned studies, the clinical evaluation of these DMN vaccination approaches was published by a couple of research groups.18,81 In 2015, Hirobe et al reported a study with 40 healthy male volunteers, receiving trivalent seasonal influenza hemagglutinin antigens either with DMN or through SC injection.81 In this case, DMN were applied using a handheld applicator and the safety and efficacy of the immunization was evaluated only in subjects showing at least 50% of needle dissolution after application. Nevertheless, DMN immunization led to equivalent or superior immune responses against the antigens in comparison with the conventional SC injection, inducing activation of T cells and antigen-specific IFN-γ-producing cells.
On the other hand, the Prausnitz group has more recently published the results of a randomized, partly blinded, placebo-controlled, Phase 1 study on the safety, immunogenicity and acceptability of DMN vaccination against influenza.18 DMN delivery (20 mins) of influenza vaccine was compared to that of IM administration of the same vaccine. Both participants and health care workers administered the vaccine by DMN (Figure 3A and D). The mean geometric antibody titers, as determined by hemagglutination inhibition antibody assay, were similar at day 28 between the DMN and the IM treatment groups for the three virus strains employed (H1N1; H3N2 and B strain). Importantly, similar titers were observed for the DMN self-vaccination group, highlighting the simplicity of this vaccination approach. Post DMN vaccination, local reactions were evident in the skin (Figure 3E and F); however, these quickly resolved and participants were in favor of the DMN vaccination over IM vaccinations. Importantly, participants found DMN to be less painful than that of IM injection (Figure 3F).
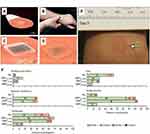 |
Figure 3 Representative images of the DMN containing influenza vaccine employed during the Phase I trial. (A) Each DMN contained 100 microneedles, 650 µm in height, mounted on an adhesive backing and (B) the DMN was manually administered to the wrist, enabling self-administration by participants in the study. (C–D) Post insertion in the skin, the DMN dissolved thus delivering the influenza vaccine in the skin layers, represented here by a blue dye. (E) Some local reactions were evident in the skin post DMN insertion. (F) Local reactions associated with vaccination in the different groups are shown. Adapted from The Lancet, vol 190 (10095), Nadine G Rouphael, Michele Paine, Regina Mosley et al, The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial, pages 649-658, Copyright (2017), with permission from Elsevier.18Abbreviations: IIV, inactivated influenza vaccine; MNP, microneedle patch; HCW, health care worker; IM, intramuscular; DMN, dissolving microneedles. |
Bacterial vaccines
While most published studies with DMN have focused on viral infections, a small number of studies have also investigated DMN vaccination against bacteria. As an example of this, Esser and co-workers tested the hypothesis that DMN could potentially overcome the immunotolerance induced during pregnancy and enhance protective immunity to tetanus in mothers and their newborns.79 DMN were prepared using a two-step fabrication process from a formulation of PVA, sucrose and CMC. The resultant DMN were 650 µm in height and 250 µm in diameter at the base and were used for the delivery of unadjuvanted tetanus toxoid to the skin of pregnant mice. The study demonstrated that the DMN were superior to IM injection, with mice born to DMN vaccinated mothers showing detectable tetanus-specific IgG antibodies for up to 12 weeks of age and complete protection to tetanus challenge up to 6 weeks of age. On the contrary, mice that were vaccinated via the IM route failed to survive challenge.
In an innovative strategy, Chen and co-workers approached the well-known local adverse reactions elicited by the commercial tuberculosis vaccine (BCG) by developing DMN with an internal “cave” where the powder BCG vaccine could be accommodated and released in the ID space.72 With this strategy, the authors were able to completely avoid the local reactions in mice, while inducing immune responses similar to those achieved with ID injection. Other studies looked at DMN-based immunization against Neisseria gonorrhoeae and Staphylococcus aureus, with promising results. In the first case, DMN-immunization of mice with whole-cell gonococci encapsulated in albumin microparticles led to higher antibody levels than an SC injection of the vaccine suspension.63 Similarly, Liu and co-workers reported extended antigen retention in mice, higher antibody levels and stronger protection against challenge using DMN for vaccination against S. aureus, in comparison with IM vaccination.71
More recently, our research group has reported the successful ID vaccination of mice using DMN fabricated from Gantrez® S-97 and containing heat-inactivated bacteria.10 Specifically, it was demonstrated that the incorporation of heat-inactivated Pseudomonas aeruginosa into DMN (500 µm in height) and subsequent vaccination of mice, resulted in these mice having a greater capability to control bacterial infection following challenge. This proof-of-concept work demonstrated the potential of DMN for ID vaccination against a bacterium for which there is currently no licensed vaccine. Moreover, this is a cost-effective approach that could easily be implemented in developing countries, allowing a rapid and simplified approach to anti-bacterial immunization.
Model and novel vaccines
In addition to DMN delivery of vaccines currently in use, researchers have also investigated the use of DMN for the delivery of alternative and model vaccine agents.28,68,82–84 In the field of model antigens, ovalbumin (OVA) is definitely the most common choice for vaccine delivery studies. In the last few years, several authors have reported on the development of DMN-based vaccination strategies with OVA as a model antigen, mainly in rodent models (mice and rats).19,64–66 In this scope, An and co-workers have published the development of polyacrylic acid DMN in a 10×10 array, with 500 µm-long needles and amphiphilic OVA, aiming at a lymph-node targeting vaccine.66 This approach led to high accumulation of the antigen in the lymph nodes and stronger humoral and cellular responses than those achieved with ID injection. Similarly, other authors have described the use of chitosan-based DMN for the immunization of rats with OVA, in two subsequent studies. In the first one, chitosan DMN acted as an implanted depot, with the needle tips left in the animals’ skin to release OVA for up to 28 days.64 This led a 2.5-fold dose-sparing effect observed with DMN immunization in a single-dose approach, in comparison with IM injection. Subsequently, the same group reported the modification of DMN to achieve a “prime-boost” effect, with antigen loaded into faster-dissolving sodium hyaluronate needle tips and slower-dissolving chitosan needle shafts.65 With this strategy, the immune responses elicited were stronger and longer-lasting than those obtained with chitosan-only DMN or repeated or double-dose SC OVA injection, thus reinforcing the potential of this novel DMN formulation.
Yan and colleagues recently evaluated the ability of DMN fabricated from sodium hyaluronate (HA) to vaccinate mice against tuberculosis (TB).73 These HA DMN had 500 µm in height and a base diameter of 250 µm, and were formulated to contain a DNA vaccine encoding the secreted protein Ag85B of Mycobacterium tuberculosis (Figure 4A–C). The developed DMN were successfully inserted in murine skin (Figure 4D), as confirmed also by histological analysis of the application site tissue (Figure 4E). DMN vaccination was compared to that of IM injection. While no significant difference was observed between DMN and IM groups with low dose (4.2 µg), DMN induced better antibody responses than those of IM injection at a higher dose (12.6 µg). Similar results were obtained in terms of cellular immune response, by measuring cytokines in splenocytes. Mice vaccinated either by DMN or IM injection were subsequently challenged by tail vein injection with 5×105 colony forming units (CFU) of the H37Rv strain of M. tuberculosis, 4 weeks post-vaccination. Analysis of CFU post-challenge demonstrated that the bacterial load in the lungs and spleens of the DMN group was significantly lower than that of the control groups (Figure 4F and G). Analysis of survival post-vaccination showed that the DMN group had prolonged survival, compared to that of the DMN without DNA (MNAWD), while the IM group did not elicit a significant change in survival rate. Collectively, these data demonstrate that DMN vaccination may provide more effective protection against this pathogen compared to that induced by IM vaccination.
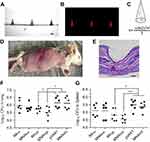 |
Figure 4 Representative images of DMN without dye (A) and with sulforhodamine B (B), observed using confocal microscopy. The theoretical DNA content per needle was calculated according to the volume of a conical shape, where R is the diameter and H is the height of the DMN (C). The DMN efficiently inserted in murine skin (D), as confirmed by histological analysis (E) (scale bar 100 µm). Bacterial counts in the lungs (F) and spleens (G) of immunized mice 4 weeks post challenge. Groups: Microneedle arrays without DNA (MNPWD), vector pVAX1 in saline as negative control, IMlow and IMhigh intramuscular injection groups given Ag85B DNA at a dose of 4.2 µg and 12.6 µg in 100-µL saline, MNPlow and MNP high given vaccine doses of 4.2 µg and 12.6 µg by administering one and DMN to each mouse. *p< 0.05, ***p< 0.001. Adapted from Vaccine, vol 36 (30), Qinying Yan, Zhigang Cheng, Houming Liu et al, Enhancement of Ag85B DNA vaccine immunogenicity against tuberculosis by dissolving microneedles in mice, pages 4471-4476, Copyright (2018), with permission from Elsevier.73 Abbreviation: DMN, dissolving microneedles. |
DMN have also been investigated for vaccination against Alzheimer’s disease. Matsuo et al75 used DMN for delivery of amyloid-beta peptide, composed of amino acid residues 1-41 (Aβ1-41), to a mouse model of Alzheimer’s disease. Amyloid-beta had previously shown therapeutic efficacy in mouse models but human clinical trials had to be stopped due to serious adverse reactions, specifically incidences of meningoencephalitis caused by Th1 cell activation and infiltration into the brain. Various studies have suggested that TC vaccination would likely trigger Th2-dominant immune responses and thus the research team in question hypothesized that DMN delivery of amyloid-beta could be effective at treating Alzheimer’s disease, potentially circumventing the previously encountered adverse reactions. Accordingly, the researchers fabricated DMN from hyaluronate as the base material (MicroHyala). The resultant DMN were cone-shaped and had needle lengths of 300 µm or 800 µm. While this approach induced little improvement in cognitive function and Th2-dominant immune responses, DMN vaccination did induce anti-Aβ1-41 immune responses but further modifications of the delivery platform were required.
DMN vaccination to other tissues
The studies described herein highlight the swathe of work that has been conducted to date using DMN for skin vaccination purposes. Inspired by such successes with DMN for ID vaccination, DMN have more recently been used for delivery at other tissues, including the oral and vaginal mucosa.85 As an example of this, Wang and co-workers combined different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate DMN as a vaccine adjuvant delivery system for the vaginal mucosa.86 To elaborate, mannosylated lipid A-liposomes (MLLs) and stealth lipid A-liposomes (SLLs) were loaded with a model antigen (OVA) and formulated with ammonium bicarbonate forming proSLL/MLL MN (proSMMA), that upon rehydration dissolved rapidly. Vaccination of mice with proSMMAs applied to the vaginal mucosa resulted in robust mucosal and systemic antigen-specific humoral and cellular immunity. The surface antigen gD of herpes simplex virus (HSV2) was also loaded in proSMMAs, and vaccination of mice resulted in successful protection against HSV2 virus challenge, a virus that infects over 20 million people annually. The authors concluded that the developed proSMMAs showed promising potential for the dual delivery of antigen and adjuvant to the vaginal mucosa, and could potentially be loaded with various antigens for the development of vaccines, particularly against sexually transmitted infectious agents.
The oral mucosa has also been investigated as a site for MN vaccination; however, such studies have focused on the use of coated MN rather than DMN.87 Moving forward, development of DMN for vaccination at other tissues will necessitate novel DMN designs to account for different tissue anatomy, physiology and biomechanics. Lessons learned from previous studies conducted with DMN for ID vaccination will undoubtedly prove valuable in this regard.
Safety considerations for adoption of DMN in a clinical setting
DMNs differ from conventional transdermal patches in that the needles of the array breach the SC barrier, penetrating to the viable epidermis and dermis. Thus, it is pivotal that DMN do not contain a microbial load capable of inducing local or systemic infection, or modulating the immune response to the antigen delivered.88 Consequently, research efforts have investigated the sterile manufacture of some DMN types, in anticipation of a sterility requirement being imposed to guarantee patient safety.89 Sterilization methods for DMN require careful attention to prevent modification of the DMN product and the components within. Additionally, the expenses related to such processes should also be taken into consideration. Aseptic manufacturing of DMN is likely to prove expensive; however, sterilization methods including gamma irradiation, moist heat or microwave heating could result in damages to DMN or degradation of vaccine antigens or adjuvants contained within the DMN matrix. It has been demonstrated that some components used for DMN fabrication exhibit inherent antimicrobial properties, demonstrating no microbial growth post-storage and as such are unlikely to cause skin or systemic infection.90 Accordingly, while it is unlikely that DMN will cause infection, it will be of pivotal importance to ensure the components within DMN do not alter or modulate the immune response to the antigen.
Information gained from research to date implies that appropriate use of DMNs is unlikely to result in skin infections. The skin, by virtue of its protective function against the external environment, is subjected to a multitude of microscopic insults as a result of injuries such as scratches or trauma, from which the skin repairs itself, without infection occurring. According to some published studies, the ability of microorganisms to traverse the holes created in the skin as a result of DMN insertion appears to be minimal. We have previously demonstrated that MNs allow lower microbial penetration than that of the traditional hypodermic needle in vitro. Specifically, by employing SilescolTM membranes, we demonstrated that the total numbers of Candida albicans, Pseudomonas aeruginosa and Staphlycoccus epidermidis crossing the membranes were significantly lower with solid silicon MN, in comparison with the use of a 21G hypodermic needle.91 In another study, using polymeric DMN, we have also showed that repeated application of these patches to mouse skin in vivo did not lead to any significant alteration in biomarkers of infection, immunity and inflammation.92 Given the antimicrobial properties of the skin, it is thus likely that the appropriate use of DMN would cause neither local nor systemic infection in immune-competent individuals. Concurrent with results in this study, another study by Wei-Ze et al93 reported that rats treated with solid silicon MNs did not become infected post incubation with Staphylococcus aureus. However, it should be highlighted that the needle length in this study was only 70–80 µm, leading to smaller pores formed in the skin and potentially less probability of microbial penetration.
Additional safety concerns include the biocompatibility of the materials selected for DMN formulation and fabrication. DMN may be fabricated from a range of polymeric materials (as reviewed in24,94). Of paramount importance will be an assurance that no local or systemic reactions occur in the skin as a result of the polymers used in the fabrication of DMN. Biocompatibility and safety studies are warranted to investigate this. To date, there is little knowledge on the long-term effects of repeatedly penetrating the skin with DMN. As previously described, DMN result in polymer deposition in the skin, due to dissolution of the vaccine-containing needles, the effects of which are currently unknown. The polymers used for DMN formulation should, therefore, be able to be eliminated from the body by polymer degradation if biodegradable or by excretion via glomerular filtration if non-degradable.95 For the latter, polymer molecular weight will be particularly important, as excretion will only be possible if the polymer size is below the glomerular filtration size threshold, as shown for polyvinylpyrrolidone.96 While polymers used in DMN fabrication have widespread use in other common pharmaceutical and cosmetic applications, they have never been used ID in the clinic. Accordingly, the long-term effects of polymer deposition in the skin are not fully understood. The deposition of polymers which are not excreted could result in polymer accumulation in tissue, potentially causing local erythema or granuloma formation or accumulation in clearance organs in the body. While it is anticipated that repeated long-term applications will be unnecessary for vaccine delivery, the impact of polymer deposition will still need to be fully elucidated. Moreover, while prime-boost regimes may be necessitated, it is unlikely that the DMNs would be inserted into the exact same insertion site on the skin’s surface.
Future perspectives
DMN have undoubted potential, as evidenced by the significant body of work published in the field, on the microfabrication, vaccine delivery and subsequent immunogenicity of vaccines delivered via this route. For adoption of DMN in clinical practice, a number of questions remain to be addressed. The scale-up of DMN production for industrialization and mass production will necessitate considerable thought. A wide range of different manufacturing methodologies for DMN fabrication in a small-scale laboratory setting have been reported; however, there remain significant barriers for adoption of these approaches on an industrial scale.97,98 Often, DMN fabrication requires multiple fabrication steps for localization of vaccine antigen and adjuvant in certain parts of the DMN array, for improved delivery efficacy and immunogenicity.99,100 Adoption of such methodologies to larger scale could pose significant challenges and it would be necessary for industry to make significant investments in both equipment and processing capabilities. Manufacturers will need guidance in terms of good manufacturing practices, pharmacopoeial standards and standardized tests for DMNs production and characterization.101 Of note, LTS Lohmann Therapie-Systeme AG, the world’s largest manufacturer of transdermal patches, holds now a manufacturing license for MN. Considering that DMN contain vaccine within the matrix of the array, each DMN may possess different characteristics and thus it will be required that each DMN be tested to ensure it is fit for purpose. The current lack of regulatory guidance in this area presents a problem for DMN product development. If DMN are to be implemented to clinical use, regulatory guidelines pertaining to patient use are warranted. The PATH Centre of Excellence for Microarray Technology aims to address regulatory issues and quality control tests in order to progress this technology. Such factors requiring consideration are packaging, disposal, ease of use, confirmation of insertion and subsequent delivery, in addition to the previously mentioned safety concerns.95
To date, there are contradictory opinions as to whether DMN will need to be used in combination with an applicator device. Several researchers and companies have reported the development of applicators for MN patches and the topic has been reviewed elsewhere.102–105 In the case of DMN, numerous studies have clearly demonstrated that DMN can be easily and reliably inserted into the skin by minimally trained personnel and patients, but further in-depth studies are required to ascertain if variability exists between users. This will undoubtedly have implications for vaccine delivery and the resultant immune response. Indeed, the introduction of an applicator device would ensure consistent application forces between end users but this would have also significant additional cost implications, above the previously reported acceptable estimates of $1 USD + API.39 The use of alternative feedback mechanisms, for example, a pressure-indicating film, would provide a less expensive alternative option.42 It will be of pivotal importance to assess the opinions of the end-users of DMN in this regard and to include their insights in the co-design of future MN devices.
An investigation into the potential implications of polymer deposition in the skin will also require consideration. Factors such as polymer-induced irritation, changes in skin barrier function and in-depth studies of microbial penetration must be conducted. Additionally, the biodistribution and subsequent polymer accumulation in the skin will need to be fully understood before DMN are routinely used in clinical settings. Similarly, studies on the antigen bioavailability following transdermal administration evaluating the actual antigen dose delivered to the skin and reaching the blood circulation could be interesting to broaden the knowledge on the mechanism of action and efficacy of this immunization approach.
A scheme of the mechanism of vaccine delivery using DMN as well as a list of the main advantages and challenges associated with this immunization approach are summarized in Figure 5.
Conclusion
Vaccination is considered to be one of the most significant health interventions for the control of infectious disease. The skin, being the largest organ of the human body, has garnered substantial interest as a site to facilitate ID vaccination via DMN. The recent Phase I clinical trial using DMN for influenza vaccine delivery published by the Prausnitz group, in combination with the breadth of ongoing work being conducted throughout the globe, exemplifies the tremendous potential DMN could have if brought to clinical use. Moving forward, there remain a number of questions that will need to be addressed if DMN are to be adopted for clinical use. To date, the commercial translation of DMN for ID vaccination has been limited by the relatively small number of clinical studies conducted in humans, in combination with implementation of large-scale, cost-effective manufacturing processes. Despite the aforementioned hurdles which must be addressed in advance of the large-scale clinical exploitation of DMN, the valuable impact these delivery devices could have, particularly in the developing world, can already be predicted.
Abbreviations
APC, antigen-presenting cell; dDC, dermal dendritic cell; CMC, carboxymethyl cellulose; DMN, dissolving microneedle array; EV71, Enterovirus 71; GM-CSF, granulocyte-macrophage colony-stimulating factor; HA, sodium hyaluronate; HFMD, hand-foot-and-mouth disease; HIV, human immunodeficiency virus; HSV2, herpes simplex virus; ID, intradermal; IPV, inactivated polio vaccine; IM, intramuscular; LC, langerhans cells; MLLs, mannosylated lipid A-liposomes; MN, microneedle array; OCT, optical coherence tomography; PVA, polyvinyl alcohol; SC, subcutaneous; SLLs, stealth lipid A-liposomes; TC, transcutaneous.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rodgers AM, Cordeiro AS, Kissenpfennig A, Donnelly RF. Microneedle arrays for vaccine delivery: the possibilities, challenges and use of nanoparticles as a combinatorial approach for enhanced vaccine immunogenicity. Expert Opin Drug Deliv. 2018. doi:10.1080/17425247.2018.1505860
2. Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg. 2003;68(3):341–344. doi:10.4269/ajtmh.2003.68.341
3. Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30(32):4807–4812. doi:10.1016/j.vaccine.2012.05.011
4. Arya JM, Prausnitz MR. Microneedle patches for vaccination in developing countries. J Control Release. 2016;240:135–141. doi:10.1016/j.jconrel.2015.11.019
5. Kaufmann JR, Miller R, Cheyne J. Vaccine supply chains need to be better funded and strengthened, or lives will be at risk. Health Aff. 2011;30(6):1113–1121. doi:10.1377/hlthaff.2011.0368
6. Ashok A, Brison M, LeTallec Y. Improving cold chain systems: challenges and solutions. Vaccine. 2017;35(17):2217–2223. doi:10.1016/j.vaccine.2016.08.045
7. Baicus A. History of polio vaccination. World J Virol. 2012;1(4):108. doi:10.5501/wjv.v1.i4.108
8. Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol. 2015;10(5):791–808. doi:10.2217/fmb.15.19
9. Davitt CJ, Lavelle EC. Delivery strategies to enhance oral vaccination against enteric infections. Adv Drug Deliv Rev. 2015;91:52–69. doi:10.1016/j.addr.2015.03.007
10. Rodgers AM, McCrudden MT, Vincente-Perez EM, et al. Design and characterisation of a dissolving microneedle patch for intradermal vaccination with heat-inactivated bacteria: a proof of concept study. Int J Pharm. 2018;549(1–2):87–95. doi:10.1016/j.ijpharm.2018.07.049
11. Du G, Hathout RM, Nasr M, et al. Intradermal vaccination with hollow microneedles: a comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J Control Release. 2017;266:109–118. doi:10.1016/j.jconrel.2017.09.021
12. Depelsenaire AC, Meliga SC, McNeilly CL, et al. Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J Invest Dermatol. 2014;134(9):2361–2370. doi:10.1038/jid.2014.174
13. Koutsonanos DG, Martin M, del P, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. Unutmaz D, ed. PLoS One. 2009;4(3):e4773. doi:10.1371/journal.pone.0004773
14. Courtenay AJ, Rodgers AM, McCrudden MT, McCarthy HO, Donnelly RF. Novel hydrogel-forming microneedle array for intradermal vaccination in mice using ovalbumin as a model protein antigen. Mol Pharm. 2019;16(1):118–127. doi:10.1021/acs.molpharmaceut.8b00895
15. Vrdoljak A, Allen EA, Ferrara F, Temperton NJ, Crean AM, Moore AC. Induction of broad immunity by thermostabilised vaccines incorporated in dissolvable microneedles using novel fabrication methods. J Control Release. 2016;225:192–204. doi:10.1016/j.jconrel.2016.01.019
16. Zhao J, Zhang Q-B, Liu B, et al. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int J Nanomedicine. 2017;12:4763–4772. doi:10.2147/IJN.S132456
17. González-Vázquez P, Larrañeta E, McCrudden MT, et al. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J Control Release. 2017;265:30–40. doi:10.1016/j.jconrel.2017.07.032
18. Rouphael NG, Paine M, Mosley R, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390(10095):649–658. doi:10.1016/S0140-6736(17)30575-5
19. Ono A, Ito S, Sakagami S, et al. Development of novel faster-dissolving microneedle patches for transcutaneous vaccine delivery. Pharmaceutics. 2017;9(4):27. doi:10.3390/pharmaceutics9030027
20. Ripolin A, Quinn J, Larrañeta E, Vicente-Perez EM, Barry JG, Donnelly RF. Successful application of large microneedle patches by human volunteers. Int J Pharm. 2017;521(1–2):92–101. doi:10.1016/j.ijpharm.2017.02.011
21. Lee I-C, Lin W-M-M, Shu J-C-C, Tsai S-W-W, Chen C-H-H, Tsai M-T-T. Formulation of two-layer dissolving polymeric microneedle patches for insulin transdermal delivery in diabetic mice. J Biomed Mater Res - Part A. 2017;105(1):84–93. doi:10.1002/jbm.a.35869
22. Chen H, Wu B, Zhang M, et al. A novel scalable fabrication process for the production of dissolving microneedle arrays. Drug Deliv Transl Res. 2019;9(1):240–248. doi:10.1007/s13346-018-00593-z
23. Nejad HR, Sadeqi A, Kiaee G, Sonkusale S. Low-cost and cleanroom-free fabrication of microneedles. Microsyst Nanoeng. 2018;4(1):17073. doi:10.1038/micronano.2017.73
24. Rodgers AM, Courtenay AJ, Donnelly RF. Dissolving microneedles for intradermal vaccination: manufacture, formulation, and stakeholder considerations. Expert Opin Drug Deliv. 2018;15(11):1039–1043. doi:10.1080/17425247.2018.1522301
25. Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng. 2017;8(1):
26. Kim Y-C, Prausnitz MR. Enabling skin vaccination using new delivery technologies. Drug Deliv Transl Res. 2011;1(1):7–12. doi:10.1007/s13346-010-0005-z
27. Hegde NR, Kaveri SV, Bayry J. Recent advances in the administration of vaccines for infectious diseases: microneedles as painless delivery devices for mass vaccination. Drug Discov Today. 2011;16(23–24):1061–1068. doi:10.1016/J.DRUDIS.2011.07.004
28. Zaric M, Lyubomska O, Touzelet O, et al. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D, L-Lactide-Co-Glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7(3):2042–2055. doi:10.1021/nn304235j
29. Glenn GM, Taylor DN, Li X, Frankel S, Montemarano A, Alving CR. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med. 2000;6(12):1403–1406. doi:10.1038/82225
30. Güereña-Burgueño F, Hall ER, Taylor DN, et al. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect Immun. 2002;70(4):1874–1880. doi:10.1128/IAI.70.4.1874
31. McKenzie R, Bourgeois AL, Frech SA, et al. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine. 2007;25(18):3684–3691. doi:10.1016/j.vaccine.2007.01.043
32. Frech SA, Dupont HL, Bourgeois AL, et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371(9629):2019–2025. doi:10.1016/S0140-6736(08)60839-9
33. Combadière B, Vogt A, Mahé B, et al. Preferential amplification of CD8 effector-t cells after transcutaneous application of an inactivated influenza vaccine: a randomized Phase I trial. Kallas EG, ed. PLoS One. 2010;5(5):e10818. doi:10.1371/journal.pone.0010818
34. Al-Zahrani S, Zaric M, McCrudden CM, Scott CJ, Kissenpfennig A, Donnelly RF. Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert Opin Drug Deliv. 2012;9(5):541–550. doi:10.1517/17425247.2012.676038
35. Quan F-S, Kim Y-C, Compans RW, Prausnitz MR, Kang S-M. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010;147(3):326–332. doi:10.1016/J.JCONREL.2010.07.125
36. Fernando GJ, Chen X, Primiero CA, et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J Control Release. 2012;159(2):215–221. doi:10.1016/J.JCONREL.2012.01.030
37. Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–1862. doi:10.1016/j.vaccine.2014.01.076
38. Donnelly RF, Moffatt K, Alkilani AZ, et al. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: a pilot study centred on pharmacist intervention and a patient information leaflet. Pharm Res. 2014;31(8):1989–1999. doi:10.1007/s11095-014-1301-y
39. Lee BY, Bartsch SM, Mvundura M, et al. An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine. 2015;33(37):4727–4736. doi:10.1016/j.vaccine.2015.02.076
40. Birchall JC, Clemo R, Anstey A, John DN. Microneedles in clinical practice – an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28(1):95–106. doi:10.1007/s11095-010-0101-2
41. Marshall S, Sahm LJ, Moore AC. Microneedle technology for immunisation: perception, acceptability and suitability for paediatric use. Vaccine. 2016;34(6):723–734. doi:10.1016/j.vaccine.2015.12.002
42. Vicente-Pérez EM, Quinn HL, McAlister E, et al. The use of a pressure-indicating sensor film to provide feedback upon hydrogel-forming microneedle array self-application in vivo. Pharm Res. 2016;33(12):3072–3080. doi:10.1007/s11095-016-2032-z
43. Gill HS, Prausnitz MR. Does needle size matter? J Diabetes Sci Technol. 2007;1(5):725–729. doi:10.1177/193229680700100517
44. Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human subjects. Clin J Pain. 2008;24(7):585–594. doi:10.1097/AJP.0b013e31816778f9
45. Mooney K, McElnay JC, Donnelly RF. Children’s views on microneedle use as an alternative to blood sampling for patient monitoring. Int J Pharm Pract. 2014;22(5):335–344. doi:10.1111/ijpp.12081
46. Quinn HL, Hughes CM, Donnelly RF. In vivo and qualitative studies investigating the translational potential of microneedles for use in the older population. Drug Deliv Transl Res. 2018;8(2):307–316. doi:10.1007/s13346-017-0393-4
47. Rodgers AM, McCrudden MT, Courtenay AJ, et al. Control of Klebsiella pneumoniae infection in mice using dissolving microarray patches containing gentamicin. Antimicrob Agents Chemother. 2019:1–11. doi:10.1128/AAC.02612-18
48. Wedlock PT, Mitgang EA, Elsheikh F, et al. The potential effects of introducing microneedle patch vaccines into routine vaccine supply chains. Vaccine. 2019;37(4):645–651. doi:10.1016/J.VACCINE.2018.12.008
49. Hanson CM, George AM, Sawadogo A, Schreiber B. Is freezing in the vaccine cold chain an ongoing issue? A literature review. Vaccine. 2017;35(17):2127–2133. doi:10.1016/J.VACCINE.2016.09.070
50. Kolluru C, Gomaa Y, Prausnitz MR. Development of a thermostable microneedle patch for polio vaccination. Drug Deliv Transl Res. 2019;9(1):192–203. doi:10.1007/s13346-018-00608-9
51. Mistilis MJ, Bommarius AS, Prausnitz MR. Development of a thermostable microneedle patch for influenza vaccination. J Pharm Sci. 2015;104(2):740–749. doi:10.1002/jps.24283
52. Mistilis MJ, Joyce JC, Esser ES, et al. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Transl Res. 2017;7(2):195–205. doi:10.1007/s13346-016-0282-2
53. Bachy V, Hervouet C, Becker PD, et al. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci. 2013;110(8):3041–3046. doi:10.1073/pnas.1214449110
54. Edens C, Collins ML, Goodson JL, Rota PA, Prausnitz MR. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine. 2015;33(37):4712–4718. doi:10.1016/J.VACCINE.2015.02.074
55. Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–920. doi:10.1038/nm.2182
56. Raphael AP, Prow TW, Crichton ML, Chen X, Fernando GJ, Kendall MA. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small. 2010;6(16):1785–1793. doi:10.1002/smll.201000326
57. Kommareddy S, Baudner BC, Oh S, Kwon S, Singh M, O’Hagan DT. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharm Sci. 2012;101(3):1021–1027. doi:10.1002/JPS.23019
58. Zhu W, Pewin W, Wang C, et al. A boosting skin vaccination with dissolving microneedle patch encapsulating M2e vaccine broadens the protective efficacy of conventional influenza vaccines. J Control Release. 2017;261:1–9. doi:10.1016/j.jconrel.2017.06.017
59. Nakatsukasa A, Kuruma K, Okamatsu M, et al. Potency of whole virus particle and split virion vaccines using dissolving microneedle against challenges of H1N1 and H5N1 influenza viruses in mice. Vaccine. 2017;35(21):2855–2861. doi:10.1016/j.vaccine.2017.04.009
60. Vassilieva EV, Wang S, Li S, Prausnitz MR, Compans RW. Skin immunization by microneedle patch overcomes statin-induced suppression of immune responses to influenza vaccine. Sci Rep. 2017;7(1):1–9. doi:10.1038/s41598-017-18140-0
61. Deng L, Chang TZ, Wang Y, et al. Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc Natl Acad Sci. 2018;115(33):E7758–E7767. doi:10.1073/pnas.1805713115
62. Littauer EQ, Mills LK, Brock N, et al. Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza. J Control Release. 2018;276:1–16. doi:10.1016/j.jconrel.2018.02.033
63. Gala R, Zaman R, D’Souza M, Zughaier S. Novel whole-cell inactivated neisseria gonorrhoeae microparticles as vaccine formulation in microneedle-based transdermal immunization. Vaccines. 2018;6(3):60. doi:10.3390/vaccines6030060
64. Chen M-C, Lai K-Y, Ling M-H, Lin C-W. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomater. 2018;65:66–75. doi:10.1016/j.actbio.2017.11.004
65. Chiu Y-H, Chen M-C, Wan S-W. Sodium hyaluronate/chitosan composite microneedles as a single-dose intradermal immunization system. Biomacromolecules. 2018;19(6):2278–2285. doi:10.1021/acs.biomac.8b00441
66. An M, Liu H. Dissolving microneedle arrays for transdermal delivery of amphiphilic vaccines. Small. 2017;13(26):1–8. doi:10.1002/smll.201700164
67. Edens C, Dybdahl-Sissoko NC, Weldon WC, Oberste MS, Prausnitz MR. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine. 2015;33(37):4683–4690. doi:10.1016/j.vaccine.2015.01.089
68. Liao JF, Lee JC, Lin CK, Wei KC, Chen PY, Yang HW. Self-assembly DNA polyplex vaccine inside dissolving microneedles for high-potency intradermal vaccination. Theranostics. 2017;7(10):2593–2605. doi:10.7150/thno.19894
69. Vreman S, McCaffrey J, Popma-de Graaf DJ, et al. Toll-like receptor agonists as adjuvants for inactivated porcine reproductive and respiratory syndrome virus (PRRSV) vaccine. Vet Immunol Immunopathol. 2019;212:27–37. doi:10.1016/j.vetimm.2019.04.008
70. Arya JM, Dewitt K, Scott-Garrard M, Chiang Y-W, Prausnitz MR. Rabies vaccination in dogs using a dissolving microneedle patch. J Control Release. 2016;239:19–26. doi:10.1016/j.jconrel.2016.08.012
71. Liu S, Zhang S, Duan Y, et al. Transcutaneous immunization of recombinant Staphylococcal enterotoxin B protein using a dissolving microneedle provides potent protection against lethal enterotoxin challenge. Vaccine. 2019;37(29):3810–3819. doi:10.1016/j.vaccine.2019.05.055
72. Chen F, Yan Q, Yu Y, Wu MX. BCG vaccine powder-laden and dissolvable microneedle arrays for lesion-free vaccination. J Control Release. 2017;255:36–44. doi:10.1016/j.jconrel.2017.03.397
73. Yan Q, Cheng ZZ, Liu H, et al. Enhancement of Ag85B DNA vaccine immunogenicity against tuberculosis by dissolving microneedles in mice. Vaccine. 2018;36(30):4471–4476. doi:10.1016/j.vaccine.2018.06.025
74. Matsuo K, Hirobe S, Yokota Y, et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J Control Release. 2012;160(3):495–501. doi:10.1016/J.JCONREL.2012.04.001
75. Matsuo K, Okamoto H, Kawai Y, et al. Vaccine efficacy of transcutaneous immunization with amyloid β using a dissolving microneedle array in a mouse model of Alzheimer’s disease. J Neuroimmunol. 2014;266(1–2):1–11. doi:10.1016/J.JNEUROIM.2013.11.002
76. Zhu Z, Ye X, Ku Z, et al. Transcutaneous immunization via rapidly dissolvable microneedles protects against hand-foot-and-mouth disease caused by enterovirus 71. J Control Release. 2016;243:291–302. doi:10.1016/J.JCONREL.2016.10.019
77. Poirier D, Renaud F, Dewar V, et al. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials. 2017;145:256–265. doi:10.1016/j.biomaterials.2017.08.038
78. Pattani A, McKay PF, Garland MJ, et al. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release. 2012;162(3):529–537. doi:10.1016/j.jconrel.2012.07.039
79. Esser ES, Romanyuk AA, Vassilieva EV, et al. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. J Control Release. 2016;236:47–56. doi:10.1016/j.jconrel.2016.06.026
80. Chu LY, Ye L, Dong K, Compans RW, Yang C, Prausnitz MR. Enhanced stability of inactivated influenza vaccine encapsulated in dissolving microneedle patches. Pharm Res. 2016;33(4):868–878. doi:10.1007/s11095-015-1833-9
81. Hirobe S, Azukizawa H, Hanafusa T, et al. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials. 2015;57:50–58. doi:10.1016/j.biomaterials.2015.04.007
82. Zaric M, Lyubomska O, Poux C, et al. Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and th1 immune responses by murine langerhans cells. J Invest Dermatol. 2015;135(2):425–434. doi:10.1038/jid.2014.415
83. Mönkäre J, Reza Nejadnik M, Baccouche K, Romeijn SG, Jiskoot W, Bouwstra JA. IgG-loaded hyaluronan-based dissolving microneedles for intradermal protein delivery. J Control Release. 2015;218:53–62. doi:10.1016/j.jconrel.2015.10.002
84. Leone M, Priester MI, Romeijn S, et al. Hyaluronan-based dissolving microneedles with high antigen content for intradermal vaccination: formulation, physicochemical characterization and immunogenicity assessment. Eur J Pharm Biopharm. 2019;134:49–59. doi:10.1016/j.ejpb.2018.11.013
85. Lee JW, Prausnitz MR. Drug delivery using microneedle patches: not just for skin. Expert Opin Drug Deliv. 2018;15(6):541–543. doi:10.1080/17425247.2018.1471059
86. Wang N, Zhen Y, Jin Y, et al. Combining different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate microneedle arrays as a vaginal mucosal vaccine adjuvant-dual delivery system (VADDS). J Control Release. 2017;246:12–29. doi:10.1016/J.JCONREL.2016.12.009
87. Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS. Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm Res. 2014;31(9):2393–2403. doi:10.1007/s11095-014-1335-1
88. González García LE, MacGregor MN, Visalakshan RM, et al. Self-sterilizing antibacterial silver-loaded microneedles. Chem Commun. 2019;55(2):171–174. doi:10.1039/C8CC06035E
89. McCrudden MT, Alkilani AZ, Courtenay AJ, et al. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv Transl Res. 2014;5(1):3–14. doi:10.1007/s13346-014-0211-1
90. Donnelly RF, Singh TRR, Alkilani AZ, et al. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: potential for enhanced patient safety. Int J Pharm. 2013;451(1–2):76–91. doi:10.1016/j.ijpharm.2013.04.045
91. Donnelly RF, Singh TRR, Tunney MM, et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26(11):2513–2522. doi:10.1007/s11095-009-9967-2
92. Vicente-Pérez EM, Larrañeta E, McCrudden MT, et al. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. Eur J Pharm Biopharm. 2017;117:400–407. doi:10.1016/j.ejpb.2017.04.029
93. Wei-Ze L, Mei-Rong H, Jian-Ping Z, et al. Super-short solid silicon microneedles for transdermal drug delivery applications. Int J Pharm. 2010;389(1–2):122–129. doi:10.1016/j.ijpharm.2010.01.024
94. Leone M, Mönkäre J, Bouwstra JA, Kersten GF. Dissolving microneedle patches for dermal vaccination. Pharm Res. 2017;34(11):2223–2240. doi:10.1007/s11095-017-2223-2
95. Quinn HL, Larrañeta E, Donnelly RF. Dissolving microneedles: safety considerations and future perspectives. Ther Deliv. 2016;7(5):283–285. doi:10.4155/tde.14.82
96. Nair B. Final report on the safety assessment of polyvinylpyrrolidone (PVP). Int J Toxicol. 1998;17(4_suppl):95–130. doi:10.1177/109158189801700408
97. Indermun S, Luttge R, Choonara YE, et al. Current advances in the fabrication of microneedles for transdermal delivery. J Control Release. 2014;185:130–138. doi:10.1016/j.jconrel.2014.04.052
98. Donnelly RF, Singh TRR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010;17(4):187–207. doi:10.3109/10717541003667798
99. Kennedy J, Larrañeta E, McCrudden MT, et al. In vivo studies investigating biodistribution of nanoparticle-encapsulated rhodamine B delivered via dissolving microneedles. J Control Release. 2017;265:57–65. doi:10.1016/j.jconrel.2017.04.022
100. Chu LY, Choi S-O, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci. 2010;99(10):4228–4238. doi:10.1002/jps.22140
101. Lutton RE, Moore J, Larrañeta E, Ligett S, Woolfson AD, Donnelly RF. Microneedle characterisation: the need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv Transl Res. 2015;5(4):313–331. doi:10.1007/s13346-015-0237-z
102. Park JH, Choi SO, Seo S, Bin CY, Prausnitz MR. A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm. 2010;76(2):282–289. doi:10.1016/j.ejpb.2010.07.001
103. Leone M, van Oorschot BH, Nejadnik MR, et al. Universal applicator for digitally-controlled pressing force and impact velocity insertion of microneedles into skin. Pharmaceutics. 2018;10(4). doi:10.3390/pharmaceutics10040211
104. Singh TR, Dunne NJ, Cunningham E, Donnelly RF. Review of patents on microneedle applicators. Recent Pat Drug Deliv Formul. 2011;5(1):11–23.
105. Sausse Lhernould M, Delchambre A. Innovative design of hollow polymeric microneedles for transdermal drug delivery. Microsyst Technol. 2011;17(10–11):1675–1682. doi:10.1007/s00542-011-1355-2
106. Leone M, Mönkäre J, Bouwstra JA, Kersten GF. Dissolving Microneedle Patches for Dermal Vaccination. Pharm Res. 2017;34(11):2223–2240. doi:10.1007/s11095-017-2223-2
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

