Back to Journals » Drug Design, Development and Therapy » Volume 17
Tebentafusp in the Treatment of Metastatic Uveal Melanoma: Patient Selection and Special Considerations
Authors Montazeri K , Pattanayak V, Sullivan RJ
Received 14 November 2022
Accepted for publication 10 January 2023
Published 7 February 2023 Volume 2023:17 Pages 333—339
DOI https://doi.org/10.2147/DDDT.S368954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Kamaneh Montazeri,1 Vikram Pattanayak,2 Ryan J Sullivan1
1Division of Hematology/Oncology, Massachusetts General Hospital, Boston, MA, USA; 2Department of Pathology, Massachusetts General Hospital, Boston, MA, USA
Correspondence: Ryan J Sullivan, Massachusetts General Hospital Cancer Center, 55 Fruit Street, Boston, MA, 02114, USA, Tel +1 617-724-4000, Email [email protected]
Abstract: Uveal melanoma (UM) is a rare type of melanoma with distinct features from cutaneous melanoma, low response rates to immune checkpoint inhibition, and poor survival rates. Tebentafusp, a bispecific antibody engaging T cells with gp 100 on HLA-A*02:01, was recently approved by the FDA as the first drug of its class and the first treatment approved by the FDA to treat UM. In this review, we summarize the preclinical and clinical data on tebentafusp for UM. We additionally discuss patient selection and the relevant challenges. For the literature search, PubMed search and relevant articles presented at international conferences were used.
Keywords: metastatic uveal melanoma, T cell engaging agents, T cell receptor, gp 100, HLA-A*02:01
Introduction
Uveal melanoma (UM) accounts for over 95% of intra-ocular malignancies.1 Unfortunately, despite effective local treatment of the primary tumor, nearly 50% of UM patients develop metastatic disease.2 The prognosis in the metastatic setting remains very poor with median overall survival (OS) of less than one year.3–5 UM comprises less than 5% of all melanomas and is molecularly distinct from cutaneous melanoma (CM).6,7
Unlike CM, checkpoint immunotherapy (CPI) has modest efficacy in metastatic UM (mUM) with response rates of less than 20% and median overall survival (OS) rates of up to 19 months.8,9 There are no approved targeted therapies in UM as BRAF mutations are not present in these tumors.10 Several trials testing the activity of MEK inhibitor selumetinib alone or in combination with chemotherapy failed to show any significant benefit in mUM.11–13 Despite the high prevalence of GNAQ and GNA11 mutations in UM, there are no approved treatments that target these mutations. In January 2022, tebentafusp, a bispecific antibody engaging T cells with glycoprotein 100 (gp100) on melanoma cells, received Food and Drug Administration (FDA) approval for the treatment of HLA-A*02:01-positive unresectable or metastatic UM (mUM).14 The European Medicines Agency (EMA) approved tebentafusp for the same indication in April 2022. Tebentafusp was the first T cell receptor (TCR)-based therapeutic that received approval by the FDA for cancer treatment and was the first drug to receive FDA approval for the treatment of mUM. In this review, we summarize the clinical data evaluating the efficacy and safety of tebentafusp in unresectable or mUM patients (Table 1). Additionally, we discuss patient selection criteria and the related restrictions and challenges.
 |
Table 1 Examples of Clinical Trials of Tebentafusp |
Tebentafusp
Tebentafusp Development and Mechanism of Action
To improve immune response against cancer antigens, a novel class of immunotherapy agents has been developed called immune-mobilizing monoclonal TCR against cancer (ImmTAC). ImmTACs are bi-specific molecules with an anti-CD3 single-chain antibody fragment (scFv) that can engage polyclonal T cells bound to a monoclonal high affinity TCR (mTCR), targeting a specific cancer-related antigen.15,16 Tebentafusp (formerly known as IMCgp100) is an ImmTAC comprising a mTCR targeting glycoprotein 100 (gp100) presented by HLA-A*02:01 on melanoma cells, fused to an anti-CD3 scFv (Figure 1). GP100 is highly expressed in UM and CM.17 The high affinity binding of tebentafusp to gp100 allows the engagement of polyclonal CD8+ and CD4+ T cells specifically into the tumor microenvironment. The preclinical studies of tebentafusp demonstrated cytotoxic activity by CD8+ T cells and CD4+ effector and memory T cells as well as pro-inflammatory cytokine and chemokine release, including TNF-α, IL-6, IL-2, resulting in broad immune responses.15 The in vitro preclinical study of tebentafusp indicated correlation between tebentafusp potency and HLA-A*02:01 expression.18
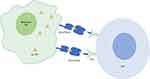 |
Figure 1 Schematic overview of mechanism of action of tebentafusp. |
Clinical Trials
Phase I/II Trial
Tebentafusp was initially assessed for safety and tolerability in a first-in-human multicenter open-label phase I/II study (NCT01211262), which enrolled a total of 84 HLA-A*02 positive adult patients with advanced melanoma, including 19 with mUM and 61 with mCM.19 Two different dosing regimens were assessed, weekly dosing from 5 to 900 ng/kg in Arm 1 and once daily dosing from 10 to 50 mcg on days 1–4 every 3 weeks. No dose limiting toxicity (DLT) was reported at 600 ng/kg weekly dosing. At 900 ng/kg, two patients developed hypotension considered to be a DLT. The recommended Phase II dose (RP2D) for weekly dosing was set as 68 mcg.19 The treatment was well tolerated overall. CRS is graded according to the American Society for Transplantation and Cellular Therapy (ASTCT) to different categories including grade 1 (fever), grade 2 (mild hypotension or hypoxia, with low-flow oxygen requirement), grade 3 (hemodynamic instability requiring a vasopressor, worsening hypoxia or requiring high-flow oxygen), and grade 4 (requiring multiple steroids and positive pressure oxygen). CRS was observed in 60% of patients, which was mild in 45% of patients, moderate in 10.7%, and severe in 3.6%.19,20 There was no grade 4 or higher toxicity. Rash and lymphopenia were the most common grade 3 or higher adverse events (AEs) present in 26% and 10% of patients, respectively, and thought to be on-target effect related to the drug mechanism.19 The presence of rash within the first 7 days from the first tebentafusp dose was associated with better overall survival (OS) rate 83% (95% CI 68–98) vs 44% (95% CI, 21 to 627).
The one-year OS rate was 65% (95% CI: 48–78%), equal in both mUM and mCM. Overall response rate (ORR) was 8.7% (n = 6), all of which were partial responses (PRs) and half (3) had UM, and 55% of the participants had stable disease based on RECIST criteria.19
Tebentafusp was noted to induce the IFN-γ pathway markers in the serum including an increase in IL-6, IL-2, IL-10 as well as CXCL11 and CXCL10, which are T cell attractants. Most of the cytokines showed a transient rise with a maximum level between 8 and 24 hours following the infusion, whereas CXCL10 and CXCL11 levels remained elevated. In addition, tissue biopsy showed increased CD3+, CD4+, and CD8+ T cells.19
A phase II study was then conducted treating a total of 127 HLA-A*02:01 mUM patients using the RP2D regimen using the weekly dosing regimen of tebentafusp which included 20 mcg for the first dose, 30 mcg for the second dose, and 68 mcg for the third dose thereafter. The most common AEs include pyrexia (80%), pruritus (67%), chills (64%). The most common grade 3 and higher AE was maculopapular rash (16%) followed by hypotension and increase in AST, most of which subsided following the third dose.21 Similar to the phase I/II trial, the ORR was low, around 5%, although a reduction in target lesion was present in 44% of patients.21 The OS at 12 months was 62% (CI 53–70%). Circulating tumor DNA (ctDNA) levels, measured at weeks 0, 5, 9 using a panel from Natera Inc was associated with overall survival (p value 0.0001).22 It is noteworthy that amongst those with radiographic disease progression, >0.5 log reduction in ctDNA was associated with OS improvement compared to <0.5 log reduction.22
Phase III Trial
The results of the phase III study of tebentafusp were published in September 2021.23 This open-label, multicenter, randomized, clinical trial compared tebentafusp with the investigator’s choice in 378 previously untreated HLA-A*02:01-positive mUM patients.23 Patients were randomized 2:1 to receive either tebentafusp or investigator’s treatment choice (control group), including single-agent pembrolizumab, ipilimumab or dacarbazine. Patients were stratified by LDH level. Cross-over was not initially permitted per the original trial design. Nevertheless, given the evidence for survival benefit with tebentafusp during the interim analysis, patients in the control arm ultimately were allowed to cross over to tebentafusp arm.23 The initial interim analysis performed at a median follow-up of 14.1 months demonstrated a one-year OS of 73% with tebentafusp compared to 59% with the investigator’s treatment choice, with a median OS of 21.7 months versus 16 months, respectively, and hazard ratio of death at 0.51 (95% CI 0.37–0.71, P < 0.001). Disease control, defined as complete response, PR, stable disease for 12 or more weeks was achieved by 46% (95% CI 39–52) of patients in the tebentafusp group vs 27% (95% CI 20–36) in the control group. The study-reported 6-month progression-free survival (PFS) benefit, a secondary endpoint, also was significant 31% vs 19% (HR 0.73, 95% CI 0.58–0.94, P = 0.01). The trial did not show an ORR benefit; ORR 9% (95% CI 6–13) vs 5% (95% CI 2–10) in the tebentafusp group versus the control group, respectively, indicating a discordance between radiographic ORR and OS benefit. The most common adverse events (AEs) of tebentafusp were cytokine-related, including fever, chills, and hypotension, followed by skin reactions such as rash, pruritus and erythema. Approximately 44% of the patients in the tebentafusp group experienced grade 3 or higher toxicities. The majority of these side effects happened during the first 4 weeks of therapy. There were no deaths related to AEs in the tebentafusp group.
HLA Allelic Variants, HLA Subtype Testing, Role in Patient Selection
Tebentafusp has been shown to have anti-tumor activity in cells expressing the HLA-A*02:01 allele of HLA-A2, which is present in approximately 50% of patients of European descent.24 Cells expressing other HLA-A*02 alleles, such as HLA-A*02:06 are not efficiently targeted by tebentafusp.23
The HLA-A gene is ~3200 bp in length, and differences between various HLA types (ie, A1 vs A2 vs A3) can be determined by dozens of polymorphic nucleotides scattered along those 3200 base pairs. Therefore, HLA antigens are expressed with a multi-field nomenclature. Antigens that are identical at the one or two field level (ie, A*02 or A*02:01) imply strong similarity of sequence at both the DNA and protein level. The A*02:01:01:01 and A*02:06:01:01 alleles have a high degree of sequence similarity and differ at only two out of 3200 base pairs. Given the sequence similarity, to fully assess a patient’s ability to respond to tebentafusp, high-resolution typing of the HLA-A locus is required.
High-resolution HLA typing is typically done using next-generation sequencing (NGS), which usually provides three- to four-field resolution of HLA types and provides more than sufficient resolution to distinguish between HLA-A*02 alleles. However, not all clinical labs perform HLA typing by NGS, and high-resolution typing by NGS can sometimes have relatively long turnaround times (7–10 days from sample receipt to report). To balance the needs of high resolution and turnaround, other methods for high-resolution HLA typing can be considered, such as A*02-specific SSO (sequence-specific oligonucleotide), higher resolution SSO, or real-time PCR.
Patient Selection, Challenges, and Perspectives
UM is a rare type of melanoma with grim prognosis upon metastasis. The OS rate remains poor leaving the mUM patients with unmet clinical needs. Tebentafusp was recently approved by the FDA, as the first therapy of its kind to receive FDA approval and as the first treatment to show OS benefit in mUM. Tebentafusp is a bispecific fusion protein engaging polyclonal CD-3 T cells with gp100-HLA-A*:02:01 bound antigen on melanoma cells.
Given the turnaround time to receive the HLA typing results, it may make sense to test all mUM and perhaps all high-risk UM patients for HLA-A*02:01 at the time of initial diagnosis. Direct consultation with the HLA lab is recommended to confirm both the resolution of typing and turnaround time. It is noteworthy that if the resolution of typing obtained only identifies HLA-A*02 at the single field level, it is still possible that a patient has an allele other than HLA-A*02:01, particularly if the patient is not of European ancestry. This has been summarized in Table 2.
 |
Table 2 HLA Allele Frequencies of Various Populations, Among All Individuals (Left) and Among HLA-A*02 Positive Individuals (Right) |
The highly selective design of the ImmTAC molecule as well as its high affinity to the tumor antigen, presented by the HLA-A*02:01, allows binding to specific cancer cells and attracting polyclonal cells to the cancer environment. However, this equally prevents approximately half of the patients with mUM from getting access to the solely available effective therapy for this aggressive disease. Designing similar agents that target less common HLA subtypes in mUM will expand access to treatment for a larger population. In a disease such as UM, which predominantly affects people of European background, there may be less of an issue, but for other diseases with a more diverse demographic affected, the development of ImmTACs against HLA-subtypes most commonly seen in Whites will become a major equity issue that companies, investigators, and regulatory authorities will need to sort out.
Discussion
The safety profile of tebentafusp is overall favorable with the majority of significant AEs occurring with the first few infusions, allowing transition to treatment as an outpatient after the 3rd infusion in most patients. Only 2% of patients receiving tebentafusp discontinued the treatment due to treatment-related AEs.23 Most patients experience some degree of CRS with tebentafusp, the majority of which are grade 1 or 2. These symptoms are usually manageable using antipyretics, intravenous hydration or holding the antihypertensive mediations one day prior to treatment. Patients may occasionally need steroids or vasopressor agents if not responsive to IV fluids. Skin reaction is the next most common AE, which usually presents as rash and erythema associated by edema particularly periorbital edema. Most of the skin reactions are limited to the first hours to days after the drug administration, and are generally controlled using antihistamines, topical steroids, or occasionally systemic steroids in case of severe symptoms. The use of systemic corticosteroids for the management of AEs was not associated with worsening of OS.25 Premedication was not required on the clinical trials, although premedication with antihistamines, H2 blockers and IV fluids seems to be helpful. Those patients who have developed significant CRS symptoms or skin reactions may benefit from one or two doses of corticosteroids prior to their first 3 or 4 infusions.
The assessment of clinical efficacy is challenging with tebentafusp. The development of week one rash was associated with strong OS benefit,26 likely due to gp100 expression on skin melanocytes targeted by tebentafusp. Rash, however, is not an independent predictor of OS and some patients without rash may also benefit from tebentafusp; therefore, its use is not appropriate for clinical decision-making. Although ctDNA levels were associated with OS in the clinical trial, the use of ctDNA in the real world setting may be more challenging given the lack of one diagnostic companion for ctDNA and the use of different commercial companies with variable sensitivities to detect ctDNA, which may affect the results. The OS benefit with tebentafusp was less among patients with larger metastatic lesions or worse performance status in the subgroup analyses.23 Recent data from the post-hoc analysis of OS among those with disease progression showed longer OS when continuing treatment beyond progression, and was not associated with new safety issues.27
Although statistically not significant, patients treated with ipilimumab appeared to do better compared to tebentafusp in the subgroup analysis of the phase III trial.23 This may be even more relevant, as the interim data from the trials of dual checkpoint inhibition in mUM, ie, PROSPER and GEM-1402, were not available at the time of the trial design and dual checkpoint blockade was not included in the control arm. Given the higher response rate of dual checkpoint versus single-agent checkpoint inhibitors in mUM, it is unknown whether tebentafusp is superior to this alternative standard of care regimen for mUM patients.
Moving forward, given that single-agent PD-1/PD-L1 inhibitors are well tolerated by most CM and UM patients with significant AEs in approximately 10–15% of patients, there likely is a potential role for the combination of T cell engagers such as tebentafusp and immune checkpoint inhibitors. There are currently ongoing clinical trials investigating the safety and efficacy of the combination. In addition, other ImmTAC agents are being used in clinical trials. The results of the Phase I trial of IMC-F106C, an ImmTAC targeting PRAME, was presented at the European Society of Medical Oncology (ESMO) in September 2022.28 Another trial includes a planned expansion arm to investigate dual ImmTAC combination of tebentafusp and PRAME-targeting therapy. Additionally, since the liver is the major site of disease progression in mUM, liver-directed therapy (LDT) may have a role in disease control benefit in combination with tebentafusp, although the best timing of LDT and the type of treatment is to be determined.
Finally, there may be an opportunity to explore the use of ImmTACs in the adjuvant or neoadjuvant setting for high-risk UM patients with the hopes to improve their outcomes.
Disclosure
Kamaneh Montazeri reports no conflicts of interest in this work. Ryan J Sullivan reports accepting fees for consulting/advisory board participation from BMS, Merck, Novartis, Eisai, Iovance, and Pfizer and has received grant funding from Merck. Vikram Pattanayak has a financial interest in SeQure, Dx, Inc., a company developing technologies for gene editing target profiling. Vikram Parranayak’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. He also reports royalty payments from Harvard University and Massachusetts General Hospital for the IP license in the gene editing space. The authors report no other conflicts of interest in this work.
References
1. Jager MJ, Shields CL, Cebulla CM, et al. Uveal melanoma. Nat Rev Dis Primers. 2020;6(1):24. doi:10.1038/s41572-020-0158-0
2. Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol. 2017;101(1):38–44. doi:10.1136/bjophthalmol-2016-309034
3. Khoja L, Atenafu EG, Suciu S, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019;30(8):1370–1380. doi:10.1093/annonc/mdz176
4. Lane AM, Kim IK, Gragoudas ES. Long-term risk of melanoma-related mortality for patients with uveal melanoma treated with proton beam therapy. JAMA Ophthalmol. 2015;133(7):792–796. doi:10.1001/jamaophthalmol.2015.0887
5. Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–4659. doi:10.1167/iovs.03-0538
6. Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
7. Chacón M, Pfluger Y, Angel M, Waisberg F, Enrico D. Uncommon subtypes of malignant melanomas: a review based on clinical and molecular perspectives. Cancers. 2020;12(9):2362. doi:10.3390/cancers12092362
8. Pelster MS, Gruschkus SK, Bassett R, et al. Nivolumab and ipilimumab in metastatic uveal melanoma: results from a single-arm phase II study. J Clin Oncol. 2021;39(6):599–607. doi:10.1200/jco.20.00605
9. Piulats JM, Espinosa E, de la Cruz Merino L, et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J Clin Oncol. 2021;39(6):586–598. doi:10.1200/jco.20.00550
10. Robertson AG, Shih J, Yau C, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2017;32(2):204–220.e15. doi:10.1016/j.ccell.2017.07.003
11. Carvajal RD, Piperno-Neumann S, Kapiteijn E, et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: a phase iii, multicenter, randomized trial (SUMIT). J Clin Oncol. 2018;36(12):1232–1239. doi:10.1200/jco.2017.74.1090
12. Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311(23):2397–2405. doi:10.1001/jama.2014.6096
13. Nathan P, Needham A, Corrie PG, et al. LBA73 - SELPAC: a 3 arm randomised phase II study of the MEK inhibitor selumetinib alone or in combination with paclitaxel (PT) in metastatic uveal melanoma (UM). Ann Oncol. 2019;30:v908–v910. doi:10.1093/annonc/mdz394.070
14. FDA, Resources for information on approved drugs. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tebentafusp-tebn-unresectable-or-metastatic-uveal-melanoma.
15. Boudousquie C, Bossi G, Hurst JM, Rygiel KA, Jakobsen BK, Hassan NJ. Polyfunctional response by ImmTAC (IMCgp100) redirected CD8(+) and CD4(+) T cells. Immunology. 2017;152(3):425–438. doi:10.1111/imm.12779
16. Oates J, Hassan NJ, Jakobsen BK. ImmTACs for targeted cancer therapy: why, what, how, and which. Mol Immunol. 2015;67(2Pt A):67–74. doi:10.1016/j.molimm.2015.01.024
17. Crabb JW, Hu B, Crabb JS, et al. iTRAQ quantitative proteomic comparison of metastatic and non-metastatic uveal melanoma tumors. PLoS One. 2015;10(8):e0135543. doi:10.1371/journal.pone.0135543
18. Harper J, Adams KJ, Bossi G, et al. An approved in vitro approach to preclinical safety and efficacy evaluation of engineered T cell receptor anti-CD3 bispecific (ImmTAC) molecules. PLoS One. 2018;13(10):e0205491. doi:10.1371/journal.pone.0205491
19. Middleton MR, Steven NM, Evans TJ, et al. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific t cell redirector with solid tumour activity: results from the FIH study in melanoma. J Clin Oncol. 2016;34(15_suppl):3016. doi:10.1200/JCO.2016.34.15_suppl.3016
20. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi:10.1182/blood-2014-05-552729
21. Sacco J, Carvajal R, Butler M, et al. 64MO - a phase (ph) II, multi-center study of the safety and efficacy of tebentafusp (tebe) (IMCgp100) in patients (pts) with metastatic uveal melanoma (mUM). Ann Oncol. 2020;31:S1441–S1451. doi:10.1016/j.annonc.2020.10.552
22. Shoushtari A, Collins L, Espinosa E, et al. Early reduction in ctDNA, regardless of best RECIST response, is associated with overall sur vival on tebentafusp in previously treated metastatic uveal noma patients. Ann Oncol. 2021;32(Suppl 5). doi:10.1016/j.annonc.2021.08.1702
23. Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196–1206. doi:10.1056/NEJMoa2103485
24. Hurley CK, Kempenich J, Wadsworth K, et al. Common, intermediate and well-documented HLA alleles in world populations: CIWD version 3.0.0. Hla. 2020;95(6):516–531. doi:10.1111/tan.13811
25. Ikeguchi A, Sacco JJ, Luke JJ, et al. Analysis of the effect of systemic corticosteroids on survival from tebentafusp in a Phase 3 trial of metastatic uveal melanoma. J Clin Oncol. 2022;40(16_suppl):9584. doi:10.1200/JCO.2022.40.16_suppl.9584
26. Hassel JC, Rutkowski P, Baurain J-F, et al. Co-primary endpoint of overall survival for tebentafusp (tebe)-induced rash in a phase 3 randomized trial comparing tebe versus investigator’s choice (IC) in first-line metastatic uveal melanoma. J Clin Oncol. 2021;39(15_suppl):9527. doi:10.1200/JCO.2021.39.15_suppl.9527
27. Sullivan RJ, Milhem MM, Demidov LV, et al. Treatment with tebentafusp beyond radiographic progressive disease (PD) in metastatic uveal melanoma (mUM). J Clin Oncol. 2022;40(16_suppl):9585. doi:10.1200/JCO.2022.40.16_suppl.9585
28. Hamid OST, Davar D, Davar D, et al. Results from phase I dose escalation of IMC-F106C, the first PRAME × CD3 ImmTAC bispecific protein in solid tumors. Ann Oncol. 2022;33(suppl_7):S331–S355. doi:10.1016/annonc/annonc1058
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
