Back to Journals » Clinical Ophthalmology » Volume 10
Tear volume estimation using a modified Schirmer test: a randomized, multicenter, double-blind trial comparing 3% diquafosol ophthalmic solution and artificial tears in dry eye patients
Authors Miyake H, Kawano Y, Tanaka H, Iwata A, Imanaka T, Nakamura M
Received 28 January 2016
Accepted for publication 2 April 2016
Published 13 May 2016 Volume 2016:10 Pages 879—886
DOI https://doi.org/10.2147/OPTH.S105275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hideki Miyake,1 Yuri Kawano,2 Hiroshi Tanaka,2 Akihiro Iwata,3 Takahiro Imanaka,1 Masatsugu Nakamura1
1Ophthalmic Disease Area Strategy Department, 2Clinical Operations Department, 3Data Science Department, R&D Division, Santen Pharmaceutical Co., Ltd. Osaka, Japan
Purpose: We aimed to evaluate the feasibility of using a modified Schirmer test to determine the increase in tear volume after administration of 3% diquafosol ophthalmic solution (diquafosol 3%) in dry eye patients.
Patients and methods: A randomized, multicenter, prospective, double-blind clinical study recruited 50 qualified subjects. They received diquafosol 3% in one eye and artificial tears in the other eye. The study protocol comprised a screening and treatment procedure completed within 1 day. The Schirmer test was performed on closed eyes three times a day. The primary efficacy end points were the second Schirmer test scores 10 minutes after the single dose. Secondary end points were the third Schirmer test scores 3 hours and 40 minutes after the single dose and the symptom scores prior to the second and third Schirmer tests.
Results: According to the Schirmer test, 10 minutes after administration, diquafosol 3% significantly increased tear volume compared to artificial tears. Diquafosol 3% and artificial tears both showed significant improvements in the symptom scores compared to baseline. However, there was no significant difference in the symptoms score between diquafosol 3% and artificial tears.
Conclusion: The modified Schirmer test can detect a minute change in tear volume in dry eye patients. These findings will be useful in the diagnosis of dry eye, assessment of treatment benefits in daily clinical practice, and the development of possible tear-secreting compounds for dry eye.
Keywords: P2Y2, efficacy, Diquas®
Introduction
One of the characteristic signs of dry eye patients is low levels of tear production. Lower tear volume leads to tear film instability, superficial punctate keratitis, and dry eye symptoms, such as dryness, foreign body sensation, and ocular discomfort.1 Drugs that enable an increase in tear volume are used for the treatment of dry eye patients.2 Estimating the precise tear volume allows the clinician to not only diagnose dry eye but also develop dry eye drugs more efficiently.
More than a century ago, Schirmer3 reported a method to detect tear volume. The device is made of filter paper strips and is able to be performed at any medical facility due to its low cost and lack of special equipment. The Schirmer test is one of the most important tests used to diagnose dry eye disease and clinical end points.4 A Schirmer test score >10 mm in 5 minutes is widely accepted as the normal value, whereas a score <5 mm is indicative of tear deficiency.5,6
Although the Schirmer test has been available for more than a century, clinical studies have shown that it does not properly detect the efficacy of drugs in patients with dry eye and that variability is one of the potential causes. The methodology of the test has changed over the years because of its variability. Some reports have investigated various factors, such as whether the test should be performed with eyes open or eyes closed,7,8 eye position,9 measurement time,8,10 and with or without anesthesia.11
Some new tests are now emerging, such as the tear meniscus height by the optical coherence tomography (OCT) test,12,13 the radius curvatures by the meniscometer test,14,15 and the tear volume by the strip meniscometry test.16 The OCT and meniscometer tests can precisely measure the tear meniscus height and radii of curvature, respectively. However, the equipment would not be appropriate for the subset of dry eye patients who do not form a normal tear meniscus, eg, patients with an irregular lid margin, conjunctivochalasis, and severely low tear production.
The 3% diquafosol ophthalmic solution (diquafosol 3%) has been launched as a drug for dry eyes in some Asian countries. Diquafosol stimulates the P2Y2 receptors on the ocular surface. A previous report concluded that diquafosol 3% increases the tear meniscus height determined by OCT in dry eye patients after a long period of treatment17 and the tear meniscus radius of curvature determined by meniscometry in healthy subjects18 and Sjögren’s syndrome patients.19 Diquafosol 3% also stimulates the secretion of sialic acid, which is a mucin-like substance, in tears in healthy subjects after a single dosing.20 Diquafosol 3% improved dryness after 4 weeks of treatment in a Phase II clinical trial.21 To our knowledge, there are no reports using the Schirmer test to detect a significant increase in tear volume and an improvement in symptoms after a single dosing of diquafosol 3% in dry eye patients.
The question whether the Schirmer test is a useful way to evaluate the efficacy of drugs that produce tears in dry eye patients remains unanswered. The purpose of this study was to use a modified Schirmer test to investigate the increase in tear volume after a single administration of diquafosol 3% in dry eye patients.
Patients and methods
Subjects
One hundred and two eligible eyes from 51 Japanese patients with dry eye (≥20 years) were enrolled in this clinical study. The inclusion criteria were as follows: 1) a definitive diagnosis of dry eye based on the 2006 Diagnostic Criteria for Dry Eye of the Japanese Dry Eye Society6 (subjective symptoms, abnormal tear state, and superficial punctate keratitis) in both eyes; and 2) a score of <10 mm (average of the longest part and the shortest part) for the Schirmer test conducted without anesthesia for 5 minutes in both eyes. Patients who had had an intraocular operation within 90 days before the screening or had undergone treatment to close the punctal within 30 days before the screening were excluded. Patients with ocular disease requiring treatment other than for dry eye, allergic conjunctivitis that worsened during the study period, contact lens use, or ophthalmic solution use on the study drug dosing day were also excluded.
Study materials
Diquafosol 3% (Diquas® ophthalmic solution 3%; Santen Pharmaceutical Co., Ltd., Osaka, Japan) and artificial tears (Soft Santear®; Santen Pharmaceutical Co., Ltd.: as the control) were used as study drugs.
Randomization
The subjects were randomized corresponding to allocation codes generated for group A (right eye: diquafosol 3%, left eye: artificial tears) and group B (right eye: artificial tear, left eye: diquafosol 3%) by a randomization manager using the permuted block method.
Study design and treatment
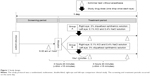
This study was a randomized, multicenter, double-blind, right-eye and left-eye comparison clinical study from November 2014 to February 2015. The study protocol comprised screening and treatment periods, both of which occurred on the same day (Figure 1). All patients (66 in total) who agreed to participate in this study provided written informed consent. After informed consent, subjects received the first Schirmer test without anesthesia (9.30 am ±1 hour). Eligible subjects were randomized into two groups and administered one drop of each study drug in each eye at 3 hours 20 minutes after the first Schirmer test. Ten minutes after administration, the second Schirmer test was implemented because diquafosol 3% significantly increases tear volume within 15 minutes.18 Finally, the third Schirmer test was performed 3 hours 40 minutes after administration. The Schirmer test was performed at intervals of >3 hours to confirm the disappearance of efficacy of diquafosol 3%.22 The subjective symptoms associated with dry eye were assessed by interviewing prior to each Schirmer test.
The primary end point was the amount of change in tear secretion from baseline at the time of the second Schirmer test. The secondary end points were as follows: 1) the change in tear secretion from baseline at the time of the third Schirmer test and 2) subjective symptoms. The presence of ocular symptoms was evaluated by conducting interviews; 12 items evaluated subjective symptoms. The severity of each ocular symptom was assessed on a four-point scale ranging from 0 to 3 as follows: 0= no symptoms; 1= mild; 2= moderate; and 3= severe.21
This study was performed in four sites in Japan, was conducted in compliance with the Declaration of Helsinki, and was approved by the Ethical Review Board of Yokohama Minoru Clinic. This study was also registered at www.clinicaltrials.jp under the identifier JapicCTI-142713 (Web site registration date: November 19, 2014).
In addition, we received an external audit by the Intellim Corporation (Osaka, Japan) regarding the data collection, data management, and statistical analysis.
Schirmer test procedure
Schirmer test strips (Shirumeru test paper; Showa Yakuhin Kako Co, Ltd, Tokyo, Japan) were used in this study. The examiner held the tip of the Schirmer test strip in a hook-like conformation to the lower eyelid of the outside one-third of the conjunctival sac of each subject without anesthesia. Subjects closed their eyelids during the test. After 5 minutes, the investigators removed the test strip and traced the tear edge with a pen, measuring the shortest and longest parts with an electronic caliper. The mean value between the longest and shortest parts was used for the statistical analysis. The Schirmer test, administered three times a day, was implemented by the same investigator.
Evaluation of ocular symptoms
Prior to each Schirmer test, the effects of a single dose of diquafosol 3% and artificial tears on subjective ocular symptoms were evaluated by conducting interviews regarding symptoms, such as foreign body sensation, photophobia, itching, eye pain, dryness, heavy eye feeling, blurred vision, asthenopia, eye discomfort, eye discharge, and tearing. The severity of ocular symptoms was assessed on a four-point scale from 0 to 3 (0= no symptoms; 1= mild; 2= moderate; 3= severe).
Statistical analyses
Data are shown as the mean ± standard error. The change in tear secretion from baseline was compared between the study drug groups by paired t-tests. SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
Results
Patients
Fifteen subjects who did not meet the criteria of the Schirmer test value were removed during the screening period. Finally, 51 subjects were randomized. All the randomized 51 subjects completed the schedule without discontinuation. One subject was excluded from the efficacy analysis set because a protocol deviation affecting the efficacy evaluation occurred.
Table 1 shows that there were no differences between groups A and B with regard to the demographic data of the efficacy analysis set. At baseline (first test), the Schirmer test scores of the diquafosol 3% group and artificial tear group were 2.886±0.399 mm and 3.010±0.387 mm, respectively (Table 2). There was no significant difference between the study drug groups.
  | Table 1 Demographic data |
  | Table 2 Actual measured value of the Schirmer test score at each point |
Primary end point
The change in the amount of tear secretion from baseline is shown in Figure 2. The measured value and the change in tear secretion from baseline are shown in Tables 2 and 3, respectively. In the second Schirmer test, the diquafosol 3% group showed a statistically significant increase in tear volume compared to baseline (1.131±0.470 mm; P=0.020), whereas the artificial tear group showed the same tear fluid level as baseline (0.083±0.442 mm; P=0.852). In addition, the difference in the change between the study drug groups (diquafosol 3% group – artificial tear group) was 1.048±0.449 mm. This difference was statistically significant (P=0.024). In terms of the change ratio in tear volume, the diquafosol 3% group showed an increase of 39.2% in tear volume. The artificial tear group showed an increase of 2.8%.
  | Figure 2 Change in tear secretion from baseline. |
Secondary end point
As shown in Table 3, in the third Schirmer test, the diquafosol 3% group showed the same tear fluid level as at baseline (0.055±0.448 mm; P=0.902), as did the artificial tear group (0.240±0.477 mm P=0.617).
In addition, the difference in the change between the study drug groups (diquafosol 3% group – artificial tear group) was –0.185±0.436 mm. This difference was also not statistically significant (P=0.673).
The diquafosol 3% and artificial tear groups showed significant improvement in the total dry eye symptom scores compared to the baseline score. However, there was no significant difference between the diquafosol 3% group and the artificial tear group in the total symptom scores from baseline (Figure S1).
Discussion
The Schirmer test is one of the most important tests used to evaluate the potential for tear production and to diagnose dry eye disease.5,6 The test can be performed at any medical facility because of its simplicity and lack of special equipment. Although the Schirmer test has been available for more than a century,3 in clinical studies, the Schirmer test does not properly evaluate the efficacy of drugs in patients with dry eye due to its variability. Improvements in the method and study design allow more reproducible and reliable tear volumes to be measured.7,9,11 In this study, we investigated whether a modified Schirmer test and study design were useful for evaluating tear secretion drugs, such as diquafosol 3%.
Fifty-one dry eye patients were enrolled, and 86% were female. The average age of the subjects was 58.7 years. This population’s characteristics coincide with those of actual dry eye patients, who tend to be postmenopausal women.
The original Schirmer test was described to be performed with the patient seated and with eyes open, blinking normally.3 Some authors have reported clinical studies using the test with eyes closed.7,8 The test performed with eyes closed could result in less variation in humidity, evaporation, reflex tearing, and illumination. The 2007 Report of the International Dry Eye Workshop suggested performing the Schirmer test with closed eyes.23
Schirmer strips are printed with a scale ranging from 0 mm to 35 mm. However, the scale is not sufficiently fine for a precise measurement. In this study, the investigators measured the distance from the shortest wet part to the longest part using an electronic caliper and calculated the average. Moreover, the same investigator performed the Schirmer test three times a day to diminish variability among individuals.
Tear production volume is diurnal24 and is also influenced by environmental factors. Execution times were defined in our protocol to eliminate the influence of diurnal changes and daily variation. Moreover, tear production can be influenced by intrinsic causes. Examples of intrinsic causes are aging, sex hormone balance, and systemic disease (eg, Sjögren’s syndrome, diabetes, and rheumatoid arthritis). To avoid the effects of intrinsic causes, one eye was treated with diquafosol 3%, while the other eye was treated with artificial tears.
Soft Santear was selected for comparison because it is widely used as an artificial tear in Japan. Diquafosol 3% includes preservatives, whereas Soft Santear does not have preservatives. In the study by Yokoi et al,18 a topical single application of diquafosol 3% significantly increased tear volume compared with application of Soft Santear, as measured using reflective meniscometry. The tear meniscus measurements were significantly higher after the single application of diquafosol 3% than after the application of saline, as determined using OCT.25 Our results are consistent with these previous observations. In the study by Takaoka-Shichijo et al,26 diquafosol resulted in a significant dose-dependent increase in tear volume according to the Schirmer strip test (compared with vehicle) in normal rabbits. Therefore, preservatives are unlikely to have an impact on the efficacy of diquafosol 3% on tear volume.
Our results show that diquafosol 3% significantly increased tear volume (by 1.048 mm) 10 minutes after a single administration compared to artificial tears, and the effect disappeared after 3 hours and 40 minutes. At its peak, diquafosol 3% resulted in an increase of ~30% in tear volume compared to the baseline score. Thus, diquafosol 3% improves ocular surface conditions by increasing tear volume in dry eye patients. To our knowledge, this is the first study to use the Schirmer test in dry eye patients to detect a significant increase in tear volume after a single dose of diquafosol 3%. Yokoi et al18 also reported that diquafosol 3% shows a peak in tear volume 10 minutes after administration, which disappeared by 60 minutes according to meniscometry. Our results correspond with this previous report.
This study was performed at four sites in Japan. The results from all sites show the increase and disappearance of the effect in terms of tear volume. These results suggest that our study design could be acceptable in multicenter clinical trials. Regarding symptoms, diquafosol 3% improved dryness after 4 weeks of treatment in a Phase II clinical trial.21 Our results showed that both diquafosol 3% and artificial tears significantly improve symptoms compared to baseline. There was no significant difference between the groups regarding the degree of improvement in symptoms. Further studies should be conducted to evaluate the effects on symptoms after single dosing.
One of the limitations of this study is that we did not account for the drainage and evaporation of tears. Tear volume in the conjunctival sac is determined by the balance between tear production and tear elimination. The influence of tear efflux should be studied further.
Conclusion
The modified Schirmer test can detect even a minute change in tear volume in dry eye patients. This study documents the ability to precisely measure tear volume in various types of dry eye patients, within any area or clinical setting, in a single day and at low cost. Therefore, we believe that this method will be useful for the diagnosis of dry eye, the assessment of treatment benefits in daily clinical practice, and the development of possible tear-secreting compounds for the treatment of dry eye disease.
Acknowledgments
The authors thank Yukie Migita and Keiko Okamoto for their support in the clinical operation. The following principal investigators participated in this study: Hiroshi Otake and Hideyo Shin (Kanagawa), Tamotsu Seki (Tokyo), and Hiroharu Shiba (Osaka). This study was sponsored by Santen Pharmaceutical Co., Ltd., Osaka, Japan.
Disclosure
All authors are employees of Santen Pharmaceutical Co., Ltd. The formulations used in this study are marketed products of Santen Pharmaceutical Co., Ltd. The authors report no other conflicts of interest in this work.
References
Versura P, Bavelloni A, Grillini M, Fresina M, Campos EC. Diagnostic performance of a tear protein panel in early dry eye. Mol Vis. 2013;19:1247–1257. | ||
Koh S. Clinical utility of 3% diquafosol ophthalmic solution in the treatment of dry eyes. Clin Ophthalmol. 2015;9:865–872. | ||
Schirmer O. Studien zur Phisiologie und Pathologie der Traenenabsonderung und Traenenabfuhr [Studies on physiology and pathology of the tear secretion and outflow]. Graefes Arch Klin Exp Ophthalmol. 1903;56:197–291. German. | ||
Alves M, Fonseca EC, Alves MF, et al. Dry eye disease treatment: a systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul Surf. 2013;11(3):181–192. | ||
[No authors listed]. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5(2):75–92. | ||
Shimazaki J. Definition and diagnosis of dry eye 2006. Atarashii Ganka. 2007;24:181–184. | ||
Serruya LG, Nogueira DC, Hida RY. Schirmer test performed with open and closed eyes: variations in normal individuals. Arq Bras Oftalmol. 2009;72(1):65–67. | ||
Kashkouli MB, Pakdel F, Amani A, Asefi M, Aghai GH, Falavarjani KG. A modified Schirmer test in dry eye and normal subjects: open versus closed eye and 1-minute versus 5-minute tests. Cornea. 2010;29(4):384–387. | ||
Bitton E, Wittich W. Influence of eye position on the Schirmer tear test. Cont Lens Anterior Eye. 2014;37(4):257–261. | ||
Bawazeer AM, Hodge WG. One-minute Schirmer test with anesthesia. Cornea. 2003;22(4):285–287. | ||
Li N, Deng X-G, He M-F. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int J Ophthalmol. 2012;5(4):478–481. | ||
Savini G, Barboni P, Zanini M. Tear meniscus evaluation by optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2006;37(2):112–118. | ||
Wang J, Palakuru JR, Aquavella JV. Correlations among upper and lower tear menisci, non-invasive tear break-up time and Schirmer’s test. Am J Ophthalmol. 2008;145(5):795–800. | ||
Yokoi N, Bron AJ, Tiffany JM, Kinoshita S. Reflective meniscometry: a new field of dry eye assessment. Cornea. 2000;19(3 Suppl): S37–S43. | ||
Bandlitz S, Purslow C, Murphy PJ, Pult H. Comparison of a new portable digital meniscometer and optical coherence tomography in tear meniscus radius measurement. Acta Ophthalmol. 2014;92(2): e112–e118. | ||
Ibrahim OM, Dogru M, Ward SK, et al. The efficacy, sensitivity, and specificity of strip meniscometry in conjunction with tear function tests in the assessment of tear meniscus. Invest Ophthalmol Vis Sci. 2011;52(5):2194–2198. | ||
Koh S, Ikeda C, Takai Y, Watanabe H, Maeda N, Nishida K. Long-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eye. Jpn J Ophthalmol. 2013;57(5):440–446. | ||
Yokoi N, Kato H, Kinoshita S. Facilitation of tear fluid secretion by 3% diquafosol ophthalmic solution in normal human eyes. Am J Ophthalmol. 2014;157(1):85–92.e1. | ||
Yokoi N, Sonomura Y, Kato H, Komuro A, Kinoshita S. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjögren’s syndrome. Eye. 2015;29(9):1204–1212. | ||
Shigeyasu C, Hirano S, Akune Y, Yamada M. Diquafosol tetrasodium increases the concentration of mucin-like substances in tears of healthy human subjects. Curr Eye Res. 2015;40(9):878–883. | ||
Matsumoto Y, Ohashi Y, Watanabe H, Tsubota K. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology. 2012;119(10):1954–1960. | ||
Mundasad MV, Novack GD, Allgood VE, Evans RM, Gorden JC, Yerxa BR. Ocular safety of INS365 ophthalmic solution: a P2Y(2) agonist in healthy subjects. J Ocul Pharmacol Ther. 2001;17(2):173–179. | ||
Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):108–152. | ||
Shen M, Wang J, Tao A, et al. Diurnal variation of upper and lower tear menisci. Am J Ophthalmol. 2008;145(5):801–806. | ||
Akiyama-Fukuda R, Usui T, Yoshida T, Yamagami S. Evaluation of tear meniscus dynamics using anterior segment swept-source optical coherence tomography after topical solution instillation for dry eye. Cornea. 2016;35(5):654–658. | ||
Takaoka-Shichijo Y, Murakami T, Nakamura M. [Stimulatory effect of diquafosol tetrasodium on tear fluid secretion in normal rabbits]. J Eye. 2011;28(7):1029–1033. Japanese. |
Supplementary material
  | Figure S1 Change in total symptom score from baseline. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.