Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
Tattoo removal with ingenol mebutate
Authors Cozzi SJ, Le TT, Ogbourne SM, James C, Suhrbier A
Received 27 February 2017
Accepted for publication 5 April 2017
Published 25 May 2017 Volume 2017:10 Pages 205—210
DOI https://doi.org/10.2147/CCID.S135716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Sarah-Jane Cozzi,1 Thuy T Le,1 Steven M Ogbourne,2 Cini James,1 Andreas Suhrbier1
1Inflammation Biology Laboratory, QIMR Berghofer Medical Research Institute, Brisbane, 2Genecology Research Center, Faculty of Science, Health, Engineering and Education, University of the Sunshine Coast, Maroochydore DC, QLD, Australia
Abstract: An increasing number of people are getting tattoos; however, many regret the decision and seek their removal. Lasers are currently the most commonly used method for tattoo removal; however, treatment can be lengthy, costly, and sometimes ineffective, especially for certain colors. Ingenol mebutate is a licensed topical treatment for actinic keratoses. Here, we demonstrate that two applications of 0.1% ingenol mebutate can efficiently and consistently remove 2-week-old tattoos from SKH/hr hairless mice. Treatment was associated with relocation of tattoo microspheres from the dermis into the posttreatment eschar. The skin lesion resolved about 20 days after treatment initiation, with some cicatrix formation evident. The implications for using ingenol mebutate for tattoo removal in humans are discussed.
Keywords: tattoo, ingenol mebutate, mouse
Introduction
Tattooing has increased in popularity in recent years among both women and men.1,2 In 2002, a Harris poll showed a tattoo prevalence of 16%, and in 2006, a North American survey found that 24% of 18–50-year olds had tattoos.3 A survey of 16–64-year olds (n=8,656) in Australia found that 14.5% of respondents had a tattoo.1 In the USA, ≈50,000 new tattoos are placed every year, and 24% of college students and 10% of adult males have been estimated to have tattoos.4 Unfortunately, up to 20%–50% of wearers regret obtaining their tattoos,4,5 with one survey reporting that 28% of tattooed individuals regretted the decision within 1 month.6 However, the percentage of individuals with tattoos who undergo tattoo removal treatment is reported to be around 6%–8%.5,7
Tattoo removal dates back to ancient Egyptian times, with ancient Greeks purporting to use inter alia, garlic mixed with cantharidin,4 an acantholytic vesicant (with a number of traditional dermatological uses) that can be obtained from beetles in the family Meloidae.8 Currently lasers, primarily Quality-switched lasers, are generally used for tattoo removal9 and can, for instance, achieve up to 95% clearance of black and blue tattoos.10,11 Laser treatment is believed to result in both chemical cleavage of pigment and/or fragmentation the pigment particles,2 with the fragments phagocytosed by the posttreatment inflammatory infiltrate. Subsequent local redistribution of fragments, and perhaps removal via the lymphatics, is believed to result in the tattoo becoming clinically inapparent.10,11 Laser treatments are typically spaced by 1–2 months, with the overall treatment course often prolonged and costly, requiring from 4 to more than 10 treatment sessions.2 Tattoo removal can also be incomplete, with tattoos containing a mixture of different colors presenting a particular challenge.2,10,11 However, picosecond laser technology continues to evolve, with new wavelength lasers improving the removal of difficult-to-remove colors.10,11
Ingenol mebutate is a topical therapeutic agent derived from the sap of Euphorbia peplus12,13 and is licensed for use in field-directed treatment of actinic keratosis, with treatment involving a daily application of 0.05% ingenol mebutate for 2 or 3 days.14,15 The treatment, by removing both actinic keratosis and surrounding mutated keratinocytes, seeks to reduce future risk of squamous cell carcinoma development.16,17 The drug (originally called PEP005) activates protein kinase C,18 and the treatment is associated with a transient inflammatory response,19 with a generally favorable cosmetic outcome in human studies.16,20,21 Studies in mice have shown that topical ingenol mebutate treatment results in 1) the loss of the epithelial layer within 1–2 days, with keratinocytes undergoing primary necrosis13 (or possibly pyroptosis22), 2) dermal hemorrhage,22,23 and 3) an acute inflammatory response17 involving a pronounced neutrophil infiltrate within 24 hours.13,24 This is followed by eschar formation and reepithelialization, which begins within 48 hours after treatment initiation.17,22 Here, we show that ingenol mebutate treatment of skin tattoos in SKH/hr hairless mice resulted in their complete removal. The dye-containing tattoo microspheres used herein were followed by histology and could clearly be seen to have relocated from the dermis into the posttreatment eschar.
Methods
Animal ethics statement
Mouse work was conducted in accordance with the “Australian code for the care and use of animals for scientific purposes” as defined by the National Health and Medical Research Council of Australia. The mouse work was approved by the QIMR Berghofer Medical Research Institute’s animal ethics committee (P891). Mice were euthanized using carbon dioxide.
SKH1/hr mice
Outbred SKH1/hr mice were obtained from Charles River Laboratories (Wilmington, NC, USA), and a breeding colony for outbred SKH1/hr mice was established at QIMR Berghofer Medical Research Institute.
Tattoo application and ingenol mebutate treatment
Mice were tattooed on their backs with one ≈1 cm × ≈1 cm cross using human-grade blue tattoo ink composed of a fluorescent dye encapsulated within polymethylmethacrylate microspheres (FireFly BMX1000, Marine Blue; Protat Tattoo Supplies, Adelaide, SA, Australia). The tattoos were applied using a Harvard Apparatus tattooing machine (Harvard Apparatus Ltd., Holliston, MA, USA). Two weeks later, the mice were randomly allocated into two groups, one received ingenol mebutate (0.1% in gel) and the other gel alone (placebo; both supplied by Peplin Ltd., Brisbane, QLD, Australia).17 Treatments were applied topically (50 µL) to the tattoo, daily for 2 days. The gels were spread to ensure that the gel covered the entire tattoo. The gel contains isopropyl alcohol, hydroxyethylcellulose, citric acid monohydrate, sodium citrate, benzyl alcohol, and purified water.
Histology
Skin samples at the tattoo site were cut out with a scalpel and fixed in 10% formalin and processed for paraffin embedding at the indicated times after ingenol mebutate treatment. Paraffin sections were stained with hematoxylin and eosin and were viewed with a Nikon Eclipse E800 fluorescent microscope and images taken with a Nikon DXM 1200F digital camera attachment (Nikon, Sydney, NSW, Australia). Slides were illuminated with normal bright light and UV light (DAPI setting).
Results
Tattoo removal with 0.1% ingenol mebutate
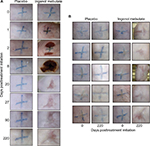
Tattooed crosses were placed on the backs of hairless SKH1/hr mice, and after 2 weeks, the tattooed areas (Figure 1A and B, Day 0) were treated once per day for 2 days with placebo gel or 0.1% ingenol mebutate gel. Two days after ingenol mebutate treatment initiation, an eschar began to form at the treatment site, which was fully formed by Day 8 (Figure 1A). Around Day 20, the eschar dropped off and no tattoo remained visible in the skin, with the site continuing to heal over the following weeks (Figure 1A). Placebo-treated tattoos showed no significant change over this period (Figure 1A). A cohort of five mice is shown to illustrate the consistency of the results obtained, with tattoos shown before treatment (Day 0) and 220 days after treatment (Figure 1B). Placebo treatment again had no significant effect (Figure 1B). Ingenol mebutate gel at 0.05% (a concentration used in humans16) was also partially effective at tattoo removal in SKH/hr mice (data not shown).
The topical ingenol mebutate treatment used herein for tattoo removal showed similar changes in the skin of SKH1/hr mice to those reported previously when ingenol mebutate was used for field-directed treatment of skin lesions.17,25 Inflammation (erythema) was evident within a day, and eschar formation began on Day 2, with the skin recovering around Day 20 (Figure 1A). Ingenol mebutate treatment (as reported previously in these mice17) resulted in some skin contraction (suggesting dermal disruption), with slightly darker pink irregular markings remaining within the treatment areas (Figure 1A and B, Day 220). Such changes are not seen in humans after ingenol mebutate treatment.16,20,21
Histological examination of ingenol mebutate-treated tattoos
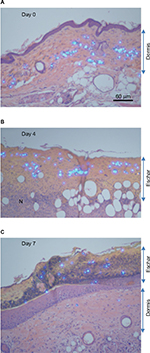
For the tattoos, a microsphere-based product was used, which contained a fluorescent dye that fluoresces under UV light. The light-blue colored tattoo microspheres could thus be clearly seen in hematoxylin and eosin sections illuminated with both white and UV light. The microspheres were clearly visible in the dermis prior to treatment (Figure 2A, dermis). After ingenol mebutate treatment, hemorrhage, mononuclear cell infiltrates (shown previously to be neutrophils13,24), and loss of epidermis were apparent within 1–2 days, as described previously in these mice.17 Importantly, by Day 4, the microspheres could be clearly seen located within the purulent exudate forming an eschar over the dermis (Figure 2B, eschar). A resolving mononuclear cell infiltrate could also be seen in the dermis (Figure 2B, N). By Day 7, the epithelium had fully reformed (slightly thickened26) and the eschar had darkened and crusted, with tattoo microspheres clearly located within the eschar (Figure 2C, eschar).
Discussion
Here, we show that 0.1% ingenol mebutate gel was able to remove efficiently and consistently 2-week-old tattoos from SKH/hr hairless mice. The mechanism of tattoo removal by ingenol mebutate appears to be quite distinct from that seen after laser treatment. The tattoo dye-containing microspheres remained intact and were exuded out of the dermis into an eschar, which then dropped off as the skin healed. This mechanism of action would suggest that tattoo removal facilitated by ingenol mebutate is likely to be independent of the tattoo ink color.
Ingenol mebutate treatment induces a complex series of changes in the skin, including epithelial and dermal disruption, hemorrhage, inflammation, and neutrophil infiltration, followed by eschar formation and ultimately some cicatrix formation (Figures 1 and 2).17 Imiquimod, which like ingenol mebutate is used for treating actinic keratoses, was also reported similarly to be able to remove tattoos in a guinea pig model, with evident epidermal and dermal necrosis, hemorrhage, inflammation, neutrophil infiltration, and eschar formation.27 Dermabrasion (to remove the epidermis) has also been used for tattoo removal, with tattoo pigment mobilized and extruded from the inflamed skin into a dressing covering the treatment site.28,29 Increased tattoo pigment phagocytosis has also been proposed as a mechanism for imiquimod and dermabrasion.29,30 However, ingenol mebutate activates PKC,18 which is reported to suppress phagocytic activity of neutrophils.31,32 One might therefore speculate that the ingenol mebutate treatment-induced inflammatory response mobilized the tattoo microspheres via edema formation and degradation of the extracellular matrix,33 with the microspheres (≈30 µm diameter) likely becoming encapsidated by connective tissue.34 Purulent exudation, driven by tissue pressure (≈10 mmHg), may then carry the microspheres out of the skin into an eschar. A weakness of the current study is that no time series was undertaken; the efficacy of ingenol mebutate for removing older tattoos (potentially with more mature encapsidation34) remains to be established.
Imiquimod has been reported to facilitate tattoo removal in conjunction with Quality-switched laser treatment in a guinea pig model.35 Human studies have shown inconsistent results, with a study in 3 patients suggesting that imiquimod facilitated tattoo removal in conjunction with laser treatment,30 whereas another study in 19 patients reported that imiquimod was ineffective in this setting.36 Whether ingenol mebutate could synergize with laser treatment to facilitate tattoo removal in humans without cicatrix formation clearly remains to be tested, given that there are substantial differences between mouse and human skin.37
Acknowledgments
S-J Cozzi was funded by an Industry Fellowship from the National Health and Medical Research Council (NHMRC), Australia. A Suhrbier is a Principal Research Fellow of the NHMRC. The research was conducted at QIMR Berghofer with consumables and research staff funded by Peplin Ltd. We thank the histopathology and animal house staff of QIMRB for excellent support.
Disclosure
A Suhrbier was a paid consultant for Peplin Ltd. and LEO Pharma. S-J Cozzi was an employee of LEO Pharma. SM Ogbourne was an employee of Peplin Ltd. A Suhrbier and S-J Cozzi are named inventors on a patent (WO/2012/176015) held by LEO Pharma, but receive no royalties or other remuneration. The authors report no other conflicts of interest in this work.
References
Heywood W, Patrick K, Smith AM, et al. Who gets tattoos? Demographic and behavioral correlates of ever being tattooed in a representative sample of men and women. Ann Epidemiol. 2012;22(1):51–56. | ||
Laux P, Tralau T, Tentschert J, et al. A medical-toxicological view of tattooing. Lancet. 2016;387(10016):395–402. | ||
Urdang M, Mallek JT, Mallon WK. Tattoos and piercings: a review for the emergency physician. West J Emerg Med. 2011;12(4):393–398. | ||
Adatto MA, Halachmi S, Lapidoth M. Tattoo removal. Curr Probl Dermatol. 2011;42:97–110. | ||
Armstrong ML, Roberts AE, Koch JR, Saunders JC, Owen DC, Anderson RR. Motivation for contemporary tattoo removal: a shift in identity. Arch Dermatol. 2008;144(7):879–884. | ||
Varma S, Lanigan SW. Reasons for requesting laser removal of unwanted tattoos. Br J Dermatol. 1999;140(3):483–485. | ||
Houghton SJ, Durkin K, Parry E, Turbett Y, Odgers P. Amateur tattooing practices and beliefs among high school adolescents. J Adolesc Health. 1996;19(6):420–425. | ||
Moed L, Shwayder TA, Chang MW. Cantharidin revisited: a blistering defense of an ancient medicine. Arch Dermatol. 2001;137(10):1357–1360. | ||
Ho SG, Goh CL. Laser tattoo removal: a clinical update. J Cutan Aesthetic Surg. 2015;8(1):9–15. | ||
Baumler W. Laser treatment of tattoos: basic principles. Curr Probl Dermatol. 2017;52:94–104. | ||
Jakus J, Kailas A. Picosecond lasers: a new and emerging therapy for skin of color, minocycline-induced pigmentation, and tattoo removal. J Clin Aesthet Dermatol. 2017;10:14–15. | ||
Ramsay JR, Suhrbier A, Aylward JH, et al. The sap from Euphorbia peplus is effective against human nonmelanoma skin cancers. Br J Dermatol. 2011;164(3):633–636. | ||
Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64(8):2833–2839. | ||
Alchin DR. Ingenol mebutate: a succinct review of a succinct therapy. Dermatol Ther. 2014;4(2):157–164. | ||
Berman B. Safety and tolerability of ingenol mebutate in the treatment of actinic keratosis. Expert Opin Drug Saf. 2015;14(12):1969–1978. | ||
Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366(11):1010–1019. | ||
Cozzi SJ, Ogbourne SM, James C, et al. Ingenol mebutate field-directed treatment of UVB-damaged skin reduces lesion formation and removes mutant p53 patches. J Invest Dermatol. 2012;132(4):1263–1271. | ||
Kedei N, Lundberg DJ, Toth A, Welburn P, Garfield SH, Blumberg PM. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004;64(9):3243–3255. | ||
Erlendsson AM, Karmisholt KE, Haak CS, Stender IM, Haedersdal M. Topical corticosteroid has no influence on inflammation or efficacy after ingenol mebutate treatment of grade I to III actinic keratoses (AK): a randomized clinical trial. J Am Acad Dermatol. 2016;74(4):709–715. | ||
Siller G, Rosen R, Freeman M, Welburn P, Katsamas J, Ogbourne SM. PEP005 (ingenol mebutate) gel for the topical treatment of superficial basal cell carcinoma: results of a randomized phase IIa trial. Australas J Dermatol. 2010;51(2):99–105. | ||
Lewohl M, Sohn A. Ingenol mebutate (ingenol 3-angelate, PEP005): focus on its uses in the treatment of nonmelanoma skin cancer. Expert Opin Drug Saf. 2012;7(2):121–128. | ||
Le TT, Skak K, Schroder K, et al. IL-1 contributes to the anti-cancer efficacy of ingenol mebutate. PloS One. 2016;11(4):e0153975. | ||
Li L, Shukla S, Lee A, et al. The skin cancer chemotherapeutic agent ingenol-3-angelate (PEP005) is a substrate for the epidermal multidrug transporter (ABCB1) and targets tumor vasculature. Cancer Res. 2010;70(11):4509–4519. | ||
Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177(11):8123–8132. | ||
Cozzi SJ, Le TT, Ogbourne SM, James C, Suhrbier A. Effective treatment of squamous cell carcinomas with ingenol mebutate gel in immunologically intact SKH1 mice. Arch Dermatol Res. 2013;305(1):79–83. | ||
Schroder WA, Anraku I, Le TT, et al. SerpinB2 deficiency results in a stratum corneum defect and increased sensitivity to topically applied inflammatory agents. Am J Path. 2016;186(6):1511–1523. | ||
Solis RR, Diven DG, Colome-Grimmer MI, Snyder Nt, Wagner RF Jr. Experimental nonsurgical tattoo removal in a guinea pig model with topical imiquimod and tretinoin. Dermatol Surg. 2002;28(1):83–86; discussion 86–87. | ||
Clabaugh WA. Tattoo removal by superficial dermabrasion. Five-year experience. Plast Reconstr Surg. 1975;55(4):401–405. | ||
Clabaugh W. Removal of tattoos by superficial dermabrasion. Arch Dermatol. 1968;98(5):515–521. | ||
Elsaie ML, Nouri K, Vejjabhinanta V, et al. Topical imiquimod in conjunction with Nd:YAG laser for tattoo removal. Lasers Med Sci. 2009;24(6):871–875. | ||
DeChatelet LR, Shirley PS, Johnston RB Jr. Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976;47(4):545–554. | ||
Wang-Iverson P, Pryzwansky KB, Spitznagel JK, Cooney MH. Bactericidal capacity of phorbol myristate acetate-treated human polymorphonuclear leukocytes. Infect Immun. 1978;22(3):945–955. | ||
Alfakry H, Malle E, Koyani CN, Pussinen PJ, Sorsa T. Neutrophil proteolytic activation cascades: a possible mechanistic link between chronic periodontitis and coronary heart disease. Innate Immun. 2016;22(1):85–99. | ||
Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. Migration studies and histology of injectable microspheres of different sizes in mice. Plast Reconstr Surg. 2004;113(5):1380–1390. | ||
Ramirez M, Magee N, Diven D, et al. Topical imiquimod as an adjuvant to laser removal of mature tattoos in an animal model. Dermatol Surg. 2007;33(3):319–325. | ||
Ricotti CA, Colaco SM, Shamma HN, Trevino J, Palmer G, Heaphy MR Jr. Laser-assisted tattoo removal with topical 5% imiquimod cream. Dermatol Surg. 2007;33(9):1082–1091. | ||
Sepehri M, Jorgensen B, Serup J. Introduction of dermatome shaving as first line treatment of chronic tattoo reactions. J Dermatolog Treat. 2015;26:451–455. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.