Back to Journals » OncoTargets and Therapy » Volume 12
Targeting ZEB2 By microRNA-129 In Non-Small Cell Lung Cancer Suppresses Cell Proliferation, Invasion And Migration Via Regulating Wnt/β-Catenin Signaling Pathway And Epithelial–Mesenchymal Transition
Authors Li X, Li C, Bi H, Bai S, Zhao L, Zhang J, Qi C
Received 29 May 2019
Accepted for publication 2 October 2019
Published 5 November 2019 Volume 2019:12 Pages 9165—9175
DOI https://doi.org/10.2147/OTT.S217536
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Xingtao Li,1 Chunhong Li,2 Hongmei Bi,3 Shufang Bai,4 Lin Zhao,5 Jing Zhang,6 Chunhui Qi7
1Department of Clinical Laboratory, Jinan City People’s Hospital, Laiwu 271100, People’s Republic of China; 2Department of Public Health, Jinan Zhangqiu District Hospital of TCM, Jinan 250200, People’s Republic of China; 3Department of Respiratory Medicine, The Third People’s Hospital of Qingdao, Qingdao 266041, People’s Republic of China; 4Department of Ultrasound, The People’s Hospital of Zhangqiu Area, Jinan 250200, People’s Republic of China; 5Department of Respiratory Medicine, People’s Hospital of Rizhao, Rizhao 276826, People’s Republic of China; 6Department of Cardiothoracic Vascular Surgery, People’s Hospital of Rizhao, Rizhao 276826, People’s Republic of China; 7Department of Pharmacy, Weifang People’s Hospital, Weifang 261041, People’s Republic of China
Correspondence: Chunhui Qi
Department of Pharmacy, Weifang People’s Hospital, 151 Guangwen street, Weifang 261041, People’s Republic of China
Tel +8600634-6279088
Email [email protected]
Introduction: Non-small cell lung cancer (NSCLC) is a common cause of deaths all over the world. Emerging evidence has indicated that microRNA (miR) play key roles in NSCLC progression. We aimed to determine the functions of miR-129 in NSCLC. miR-129 was dramatically downregulated in NSCLC tissue samples and cells. The decreased miR-129 was found to be associated with poorer prognosis and malefic phenotype of NSCLC patients. We demonstrated that miR-129 upregulation could inhibit NSCLC cell growth. Furthermore, we also sought the molecular mechanism by which miR-129 repressed NSCLC development.
Methods: QRT-PCR was applied to detect the expressions of miR-129 in 51 pairs of NSCLC tissue samples. We further performed the Kaplan–Meier analysis to determine the association between miR-129 expressions and the survival rate of NSCLC patients. We then measured the expression levels of miR-129 in NSCLC cell lines. After that, MTT assays were performed to determine the influence of miR-129 on A549 cell proliferation. Transwell assay was then conducted to explore the biological functions of miR-129 in invasion and migration of NSCLC cells.
Results: Results showed that ZEB2 was directly targeted by miR-129 in NSCLC cell lines. Moreover, miR-129 restoration could inhibit EMT and Wnt/β-catenin in NSCLC cell lines.
Conclusion: In short, all these results indicated that miR-129/ZEB2 axis maybe a useful diagnostic and prognostic biomarker for NSCLC treatment.
Keywords: miR-129, ZEB2, NSCLC, Wnt/β-catenin, EMT
Introduction
Lung cancer has the highest morbidities and mortalities worldwide.1 Particularly, 85% of the cases with lung cancer are non-small cell lung cancer (NSCLC), which is the leading cause for lung cancer deaths.2 Currently, surgery is the main treatment for NSCLC, and adjuvant chemotherapy has gradually become common in patients with proper indications post-operation.3 Moreover, after surgical resections and other interventions, the 5-year survival rate for NSCLC remains below 50%.4 NSCLC is still one of the most challenging cancers in the clinic, despite the emergence of targeted biological agents and novel cytotoxic drugs.5 Therefore, it is necessary to search the factors of NSCLC pathogenesis for the improvement of clinical therapies.
MicroRNAs (miRNAs/miRs) could regulate gene expressions by targeting the 3ʹ UTRs in different kinds of cellular processes.6 It has been shown that miRNA plays key roles in tumorigenesis. For example, miR-411 was found to inhibit the malignant behaviors in colorectal carcinoma via regulation of PIK3R3;7 dysregulation of miR-567 could contribute to carcinogenesis of breast cancer;8 miR-544 promoted colorectal cancer progression by targeting forkhead box O1.9 Moreover, functional research of miRNAs shows that miRNAs are almost involved in all biological processes, such as cell metastasis, growth, differentiation and apoptosis.10–12 Therefore, in cancer progression, over-expressed or down-regulated miRNAs may be potential candidates for therapeutic interventions. In addition, miRNA, which regulates the responses of tumor cells to chemotherapy, could be inhibited or over-expressed as an adjuvant for tumor therapy. Importantly, recent studies have indicated the underlying significance of miR-129 in diagnosis and prognosis predictions of NSCLC.13,14 However, the mechanism of miR-129 remains largely unknown. In the current study, we explored the bio-functions of miR-129 in NSCLC to identify new biomarkers for effective diagnosis and prediction of prognosis in tumor treatments, which may exhibit significant implications in the clinic.
Zinc finger E-box binding homeobox 2 (ZEB2) is a member of the ZEB family of transcription factors.15 Studies have reported that ZEB2 was a regulator of epithelial-to-mesenchymal transition (EMT).16 In EMT, cells in epithelial phenotypes are converted into mesenchymal phenotypes with increased invasion and migration capacities. In this process, mesenchymal marker is upregulated while E-cadherin marker is downregulated.17 The overexpression of ZEB2 has been reported in different cancer types and has been suggested as a candidate biomarker for poor prognosis.18,19 Therefore, suppressing ZEB2 activation is a promising approach for suppressing cancer by inhibiting EMT. The signaling pathways known to be activated in NSCLC included Wnt/β-catenin signaling pathway, which regulated multiple processes involved in tumor growth, survival, migration, differentiation, and apoptosis.20–22 Consequently, this study investigated the roles of miR-129 in NSCLC Wnt/β-catenin and EMT, to provide new ideas for the efficacious developments of clinical therapy of NSCLC.
Materials And Methods
Tissue Samples
From October 2011 to June 2012, 51 pairs of NSCLC tissue samples and matched para-carcinoma tissue samples were collected from the Jinan City People’s Hospital after receiving written informed consent. All enrolled patients underwent no prior radiation therapy or chemotherapy. The collected tissue samples were frozen in liquid nitrogen and reserved at −80°C. Our study gains approval from the Ethics Committee of Jinan City People’s Hospital. All patients provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Cell Lines
Human NSCLC cells (NCI-H460, NCI-H1299, and A549) and normal bronchial epithelium cell line BEAS-2B were obtained from American Type Culture Collection (ATCC). The spca1 cell line was obtained from Shanghai Sure Shengwu Technology Co., LTD (Shanghai, China). All the cells were maintained in RPMI-1640 (Gibco; Thermo Fisher Scientifc, Inc., Waltham, MA, USA) including 10% FBS (Invitrogen, Carlsbad, CA, USA) at 37°C.
Cell Transfections
MiR-129 mimics, inhibitor or negative controls (NC) used in current study were obtained from GenePharma (Shanghai, China). miRNAs were transfected into NSCLC cells by Lipofectamine® 2000 (Invitrogen) following the manufacturer’s protocols.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from cells and tissues with TRIzol (Invitrogen, Carlsbad, CA, USA), and the PrimeScript reverse transcription reagent kit (Thermo Fisher Scientific) was utilized for cDNAs synthesis. qPCR was conducted with SYBR® Premix Ex Taq™ II (Takara, Dalian, China) using a 7500 Fast Real-Time PCR detection system (Thermo Fisher Scientifc). 2−ΔΔCt method was utilized to calculate relative expressions. U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were internal controls. The primer sequences are list in Table 1.
 |
Table 1 Primer Sequences For qRT-PCR |
MTT Assay
The cell proliferation ability was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays. In brief, NSCLC cells with different transfections (miR-129 mimics/inhibitor) were plated into 96-well plates and incubated for 0, 24, 48, 72hrs. Then, MTT (5 mg/mL) solution was appended into each well and incubated for another 4hrs. After that, 150 μL dimethyl sulfoxide (DMSO) was added. The OD490 values were determined by a microplate (BioTek, Winooski, VT, USA).
Transwell Assays
Transwell assay was carried out to explore the roles of miR-129 in invasive and migratory capacities of NSCLC cells with 8-µm pore sized transwell chamber (BD Biosciences, San Jose, CA, USA). For invasion assay, the upper chambers were precoated with a matrigel (BD Biosciences). The transfected cells at 1 × 105 cells/well resuspended in FBS-free medium were plated into the top chamber. Medium containing 10% FBS was added to the bottom chambers. After incubated for 48 hrs, the non-invading or -migrating cells were scraped whereas cells adhering to the bottom surface of the upper chamber were fixed and stained. Finally, the results were quantified by counting five independent visual fields under the microscope (Olympus).
Western Blot
The cell lysates were prepared with radioimmunoprecipitation assay (RIPA) buffer (Thermo Scientific) and a BCA Protein Assay Kit (Pierce, Rockford, IL, USA) was used to examine the protein concentrations. Then, the protein was loaded on 10% SDS-PAGE for separation and then transferred onto polyvinylidene fluoride (PVDF) membranes (Invitrogen) which were blocked with 5% skim milk in TBST at room temperature for 2hrs. Subsequently, the membrane was incubated overnight with primary first antibodies at 4°C. After that, the membranes were then incubated with HRP-labeled secondary antibody (1:3000, Abcam) at room temperature for 2hrs. The protein band was analyzed with ECL reagent (Beyotime). The following primary antibodies were used: antibodies against cyclin D1 (1:1000, Abcam Cambridge, MA, USA), c-Myc (1:1000, Abcam), β-catenin (1:1000, Abcam), p-GSK3β (1:1000, Abcam), total GSK3β (1:1000, Abcam), E-cadherin (1:2000, Abcam), N-cadherin (1:2000, Abcam), Vimentin (1:1000, Abcam), ZEB2 (1:1000, Abcam) and GAPDH (1:1000, Abcam). GAPDH was an internal reference.
Luciferase Reporter Assay
The mutant (MUT) or wild-type (WT) sequences containing the predicted target site of miR-129 in the 3ʹUTRs of ZEB2 mRNA were inserted into the pmir-GLO vectors (Promega Corporation, Madison, WI, USA). Then, 50 nM miR-129 mimics and the ZEB2 −3ʹUTR-MUT or ZEB2 −3ʹUTR-WT were cotransfected into NSCLC cells using Lipofectamine 2000; 5 ng of pRL‐SV40 was added per 80 ng of plasmid; 48 hrs after transfections, luciferase activity was detected by the Dual-Luciferase Reporter Assay system (Promega) in line with the manufacturers’ proposals.
Statistical Analysis
All data were from at least 3 independent experiments. Statistical analysis was performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL). Differences between two groups were analyzed by using the Student’s t-test. Comparison between groups was done using one-way ANOVA test followed by Post Hoc Test (Least Significant Difference). The survival rate was analyzed by Kaplan–Meier analysis and log-rank test. P<0.05 was statistically significant difference.
Results
Downregulated miR-129 In NSCLC Tissue Samples Indicated A Poor Survival Rate
QRT-PCR was applied to detect the expressions of miR-129 in NSCLC tissue samples. As shown in Figure 1A, miR-129 expression in NSCLC tissues was dramatically decreased compared to the adjacent non-tumor tissue samples. We then explored the correlation between miR-129 level and clinical characteristics of NSCLC patients. As itemized in Table 2, the downregulated miR-129 was associated with the adverse clinicopathologic parameters of NSCLC patients. We further performed the Kaplan–Meier analysis to determine the association between miR-129 expressions and the survival rate of NSCLC patients. Results indicated that patients with low miR-129 group had a significantly poor overall survival (OS) than those in high miR-129 group (Figure 1B).
 |
Table 2 Correlation Of miR-129 Expression With The Clinicopathological Characteristics Of The NSCLC Patients |
MiR-129 Overexpression Repressed NSCLC Cell Proliferation
We then measured the expression levels of miR-129 in NSCLC cell lines. As expected, miR-129 was prominently downregulated in NSCLC cells in comparison with that in bronchial epithelium cell (Figure 2A). The effects of miR-129 on the biological phenotypes were investigated; 48hrs after transfection of miR-129 mimics or inhibitors, the transfection efficiencies in A549 cells were detected by qRT-PCR analysis. miR-129 expression was evidently increased by miR-129 mimics whereas decreased by miR-129 inhibitor in A549 cells (Figure 2B and C). After that, MTT assays were performed to determine the influence of miR-129 on A549 cell proliferation. miR-129 overexpression significantly inhibited A549 cell proliferation (Figure 2D). On the other hand, results also shown that A549 cell proliferation ability was dramatically promoted by miR-129 inhibition (Figure 2E).
MiR-129 Upregulation Suppressed NSCLC Cell Invasion And Migration
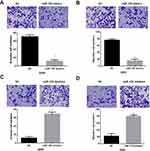
Transwell assay was then conducted to explore the biological functions of miR-129 in invasion and migration of NSCLC cells. Results indicated that miR-129-overexpressed A549 cells showed remarkably decreased invasion and migration abilities (Figure 3A and B). In contrast, the invasion and migration abilities were significantly promoted by miR-129 inhibition in A549 cells (Figure 3C and D). The above findings suggested that miR-129 served as an anti-tumor miRNA in NSCLC.
ZEB2 Was A Target Of miR-129 In NSCLC Cell Lines
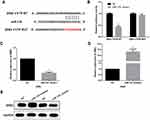
To investigate the potential mechanisms underlying miR-129-induced regulation of NSCLC biology, TargetScan was utilized to explore the targets of miR-129. ZEB2 was one candidate target of miR-129 (Figure 4A). Next, we performed luciferase reporter assays to confirm the above hypothesis. A549 cell lines were cotransfected with ZEB2-3ʹUTR-WT or -MUT and miR-129 mimics. As shown in Figure 4B, the luciferase activity was significantly decreased by miR-129 mimics in the ZEB2-3ʹUTR-WT group, while luciferase activity of ZEB2-3ʹUTR-MUT exhibited no obvious changes. Next, the regulatory functions of miR-129 in ZEB2 expressions were investigated. Results demonstrated that miR-129 upregulation led to a prominent repression of ZEB2 expressions, and on the other hand, transfection of miR-129 inhibitor remarkably promoted the ZEB2 expression (Figure 4C–E). Therefore, ZEB2 was a potential target of miR-129 in NSCLC cell lines.
MiR-129 Inhibited NSCLC Cell EMT And Wnt/β-Catenin Signaling Pathway
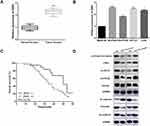
We then performed qRT-PCR to determine the expressions of ZEB2 in NSCLC tissues. Results indicated that ZEB2 in NSCLC tissues was mainly located in the nucleus (Figure 5A). Positive rates of ZEB2 protein expressions in adjacent normal tissue samples were evidently lower than that in NSCLC tissue samples (Figure 5B). Subsequently, we analyzed the influence of ZEB2 on the OS of NSCLC patients. It was found that patients with higher ZEB2 expression level presented lower OS than patients with lower ZEB2 expression level (Figure 5C). Next, Western blot analysis was used to detect the functions of miR-129 in NSCLC cell EMT and Wnt/β-catenin. As shown in Figure 5D, miR-129 upregulation significantly promoted E-cadherin expression while inhibited Vimentin and N-cadherin expressions in A549 cells, and on the other hand, miR-129 inhibition exhibited the opposite functions. Moreover, Western blotting results showed that the expressions of activated β-catenin, c-Myc, cyclin D1, and p-GSK3β were significantly decreased by miR-129 overexpression and were notably increased in cells with low miR-129 expressions (Figure 5D). Findings indicated that miR-129 participated in regulating NSCLC cell EMT and Wnt/β-catenin signaling pathway.
MiR-129 Inhibited NSCLC Cell Progression Via Regulating ZEB2
Since above results have indicated ZEB2 was direct target of miR-129 in NSCLC, ZEB2 might take part in miR-129-mediated inhibition of NSCLC cells. Therefore, to detect whether ZEB2 affects miR-129-induced function, we used miR-129 mimic to over express miR-129 in A549 cells and then transfect with ZEB2 plasmid in these cells to reverse ZEB2 expression. The mRNA level of ZEB2 was reduced by miR-129 mimic, and then increased after transfection with ZEB2 plasmid in A549 cells (Figure 6A). As estimated, miR-129 up-regulation inhibited NSCLC cells proliferation, migration and invasion, and ZEB2 plasmid evidently reversed miR-129 inhibited cell proliferation, migration and invasion as shown in Figure 6B–D. Overall, we deducted that ZEB2 was involved in miR-129-mediating inhibition of NSCLC progression.
Discussion
NSCLC remains one of the main causes for cancer deaths all around the world due to a lack of understanding about the mechanism of NSCLC tumorigenesis.23 Novel biomarkers which may effectively assess the risks of tumor metastasis and recurrence, are worthy of exploration for NSCLC patients. Emerging studies have confirmed the dysregulation of miRNAs in NSCLC, indicating that aberrantly expressed miRNAs (or the target genes) play key roles in NSCLC progression.24 Therefore, to seek more effective treatments; contemporary research is centralized on mechanism of epigenetic or genetic NSCLC processes. For instance, Liao XH et al found that miR-500a-3p suppressed NSCLC cell invasion and proliferation by regulating LY6K.25 Chen Y et al reported that miR-148a served as a prognostic factor and suppressed migration and invasion through Wnt1 in NSCLC.26 Peng X et al revealed that miR-19 promoted NSCLC cell proliferation via inhibiting CBX7 expression.27
Our study provided functional evidence that miR-129 was important in NSCLC tumorigenesis. In particular, miR-129 expression was obviously decreased in NSCLC. Moreover, low miR-129 expression was related to malignant phenotypes and poor prognosis of patients with NSCLC. Findings also indicated that miR-129 overexpression prominently repressed NSCLC cell proliferation, invasion and migration capacities. Moreover, upregulation of miR-129 repressed EMT and Wnt/β-catenin in NSCLC cells. Additionally, miR-129 overexpression decreased the in vivo NSCLC growth. Altogether, these results demonstrated miR-129 exerted tumor-suppressive role in NSCLC by regulating EMT and Wnt/β-catenin.
The dysregulation of miR-129 is frequently identified in multiple types of human cancers, playing important roles in tumor occurrence and development.miR-129 was involved in the occurrence of uterine fibroid through inhibiting TET1.28 In colorectal cancer, miR-129 enhanced chemosensitivity and promoted apoptosis to 5-fluorouracil.29 miR-129 potentiated chemosensitivity and inhibited growth in neuroblastoma via regulation of MYO10.30 miR-129 inhibited prostate cancer development via PI3K/AKT/mTOR and targeting ETS1.31 These findings suggested that miR-129 can be developed into an effective therapeutic target for these types of cancer. In addition, multiple targets of miR-129 have been validated, including CDK1 and iASPP in Burkitt lymphoma,32 PAK5 in hepatocellular carcinoma,33 and MAL2 in papillary thyroid carcinoma.34 These studies suggested that miR-129 exhibited tissue-specific functions. ZEB2 confirmed as a functional target of miR-129 in NSCLC. Furthermore, ZEB2 was involved in miR-129-mediating inhibition of NSCLC progression. Given the important roles of ZEB2 in NSCLC, regulation of the miR-129/ZEB2/Wnt/β-catenin axis is a potential novel therapeutic strategy for NSCLC patients.
Conclusion
In conclusion, miR-129 was significantly down-regulated in NSCLC. Elevated miR-129 inhibited tumorigenesis and malignant progression of NSCLC. Moreover, we confirmed that the anti-NSCLC roles of miR-129 were regulated by ZEB2 and Wnt/β-catenin. Therefore, our findings suggest that miR-129 is a potential molecular target in NSCLC treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi:10.4065/83.5.584
3. Zhou YW, Li R, Duan CJ, Gao Y, Cheng YD, Zhang CF. Overexpressed C14orf166 associates with disease progression and poor prognosis in non-small-cell lung cancer. Biosci Rep. 2018;38(5). doi:10.1042/BSR20180479
4. Giaccone G. Clinical impact of novel treatment strategies. Oncogene. 2002;21(45):6970–6981. doi:10.1038/sj.onc.1205565
5. Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer Am Cancer Soc. 2015;121(5):664–672. doi:10.1002/cncr.29098
6. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321–333. doi:10.1038/nrc3932
7. Zhao J, Xu J, Zhang R. MicroRNA-411 inhibits malignant biological behaviours of colorectal cancer cells by directly targeting PIK3R3. Oncol Rep. 2018;39(2):633–642. doi:10.3892/or.2017.6135
8. Bertoli G, Cava C, Diceglie C, et al. MicroRNA-567 dysregulation contributes to carcinogenesis of breast cancer, targeting tumor cell proliferation, and migration. Breast Cancer Res Treat. 2017;161(3):605–616. doi:10.1007/s10549-016-4079-2
9. Yao GD, Zhang YF, Chen P, Ren XB. MicroRNA-544 promotes colorectal cancer progression by targeting forkhead box O1. Oncol Lett. 2018;15(1):991–997. doi:10.3892/ol.2017.7381
10. Chen G, Zhou H. MiRNA-708/CUL4B axis contributes into cell proliferation and apoptosis of osteosarcoma. Eur Rev Med Pharmacol Sci. 2018;22(17):5452–5459. doi:10.26355/eurrev_201809_15805
11. Zhang R, Li F, Wang W, Wang X, Li S, Liu J. The effect of antisense inhibitor of miRNA 106b approximately 25 on the proliferation, invasion, migration, and apoptosis of gastric cancer cell. Tumour Biol. 2016;37(8):10507–10515. doi:10.1007/s13277-016-4937-x
12. Yu Y, Luo W, Yang ZJ, et al. miR-190 suppresses breast cancer metastasis by regulation of TGF-beta-induced epithelial-mesenchymal transition. Mol Cancer. 2018;17(1):70. doi:10.1186/s12943-018-0818-9
13. Li J, Wang H, Ke H, Ni S. MiR-129 regulates MMP9 to control metastasis of non-small cell lung cancer. Tumour Biol. 2015;36(8):5785–5790. doi:10.1007/s13277-015-3247-z
14. Liu MX, Zhou KC, Cao Y. MCRS1 overexpression, which is specifically inhibited by miR-129*promotes the epithelial-mesenchymal transition and metastasis in non-small cell lung cancer. Mol Cancer. 2014;13:245. doi:10.1186/1476-4598-13-245
15. Santiago JJ, Dangerfield AL, Rattan SG, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239(6):1573–1584. doi:10.1002/dvdy.22280
16. Jacob S, Nayak S, Fernandes G, et al. Androgen receptor as a regulator of ZEB2 expression and its implications in epithelial-to-mesenchymal transition in prostate cancer. Endocr Relat Cancer. 2014;21(3):473–486. doi:10.1530/ERC-13-0514
17. Zhang Z, Yang C, Gao W, et al. FOXA2 attenuates the epithelial to mesenchymal transition by regulating the transcription of E-cadherin and ZEB2 in human breast cancer. Cancer Lett. 2015;361(2):240–250. doi:10.1016/j.canlet.2015.03.008
18. Li MZ, Wang JJ, Yang SB, et al. ZEB2 promotes tumor metastasis and correlates with poor prognosis of human colorectal cancer. Am J Transl Res. 2017;9(6):2838–2851.
19. Dai YH, Tang YP, Zhu HY, et al. ZEB2 promotes the metastasis of gastric cancer and modulates epithelial mesenchymal transition of gastric cancer cells. Dig Dis Sci. 2012;57(5):1253–1260. doi:10.1007/s10620-012-2042-6
20. Zhang W, Duan N, Zhang Q, et al. DNA methylation mediated down-regulation of miR-370 regulates cell growth through activation of the Wnt/beta-catenin signaling pathway in human osteosarcoma cells. Int J Biol Sci. 2017;13(5):561–573. doi:10.7150/ijbs.19032
21. Li X, Lu Q, Xie W, Wang Y, Wang G. Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/beta-Catenin signaling. Biochem Biophys Res Commun. 2018;496(2):443–449. doi:10.1016/j.bbrc.2018.01.052
22. Yang Z, Ji L, Jiang G, et al. FL118, a novel camptothecin analogue, suppressed migration and invasion of human breast cancer cells by inhibiting epithelial-mesenchymal transition via the Wnt/beta-catenin signaling pathway. Biosci Trends. 2018;12(1):40–46. doi:10.5582/bst.2017.01288
23. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
24. Wang K, Chen M, Wu W. Analysis of microRNA (miRNA) expression profiles reveals 11 key biomarkers associated with non-small cell lung cancer. World J Surg Oncol. 2017;15(1):175. doi:10.1186/s12957-017-1244-y
25. Liao XH, Xie Z, Guan CN. MiRNA-500a-3p inhibits cell proliferation and invasion by targeting lymphocyte antigen 6 complex locus K (LY6K) in human non-small cell lung cancer. Neoplasma. 2018;65(5):673–682. doi:10.4149/neo_2018_170516N355
26. Chen Y, Min L, Ren C, et al. miRNA-148a serves as a prognostic factor and suppresses migration and invasion through Wnt1 in non-small cell lung cancer. PLoS One. 2017;12(2):e171751. doi:10.1371/journal.pone.0171751
27. Peng X, Guan L, Gao B. miRNA-19 promotes non-small-cell lung cancer cell proliferation via inhibiting CBX7 expression. Onco Targets Ther. 2018;11:8865–8874. doi:10.2147/OTT.S181433
28. Lu JL, Zhao L, Han SC, et al. MiR-129 is involved in the occurrence of uterine fibroid through inhibiting TET1. Eur Rev Med Pharmacol Sci. 2018;22(14):4419–4426. doi:10.26355/eurrev_201807_15492
29. Karaayvaz M, Zhai H, Ju J. miR-129 promotes apoptosis and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cell Death Dis. 2013;4:e659. doi:10.1038/cddis.2013.193
30. Wang X, Li J, Xu X, Zheng J, Li Q. miR-129 inhibits tumor growth and potentiates chemosensitivity of neuroblastoma by targeting MYO10. Biomed Pharmacother. 2018;103:1312–1318. doi:10.1016/j.biopha.2018.04.153
31. Xu S, Ge J, Zhang Z, Zhou W. MiR-129 inhibits cell proliferation and metastasis by targeting ETS1 via PI3K/AKT/mTOR pathway in prostate cancer. Biomed Pharmacother. 2017;96:634–641. doi:10.1016/j.biopha.2017.10.037
32. Zou H, Zou R, Chen K, et al. miR-129 targets CDK1 and iASPP to modulate Burkitt lymphoma cell proliferation in a TAp63-dependent manner. J Cell Biochem. 2018;119(11):9217–9228. doi:10.1002/jcb.27189
33. Zhai J, Qu S, Li X, et al. miR-129 suppresses tumor cell growth and invasion by targeting PAK5 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;464(1):161–167. doi:10.1016/j.bbrc.2015.06.108
34. Gao X, Chen Z, Li A, Zhang X, Cai X. MiR-129 regulates growth and invasion by targeting MAL2 in papillary thyroid carcinoma. Biomed Pharmacother. 2018;105:1072–1078. doi:10.1016/j.biopha.2018.06.050
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.