Back to Journals » ClinicoEconomics and Outcomes Research » Volume 8
Targeting improved patient outcomes using innovative product listing agreements: a survey of Canadian and international key opinion leaders
Authors Thompson M, Henshall C, Garrison LP, Griffin A, Coyle D, Long S, Khayat Z, Anger D, Yu R
Received 18 September 2015
Accepted for publication 31 March 2016
Published 26 August 2016 Volume 2016:8 Pages 427—433
DOI https://doi.org/10.2147/CEOR.S96616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Melissa Thompson,1 Chris Henshall,2 Louis P Garrison,3 Adrian D Griffin,4 Doug Coyle,2,5 Stephen Long,6 Zayna A Khayat,7 Dana L Anger,1 Rebecca Yu8
1Cornerstone Research Group Inc., Burlington, ON, Canada; 2Health Economics Research Group, Brunel University London, London UK; 3Pharmaceutical Outcomes Research and Policy Program, School of Pharmacy, University of Washington, Seattle, WA, USA; 4Government Affairs & Policy, Johnson & Johnson, High Wycombe, UK; 5School of Epidemiology, Public Health and Preventive Medicine, University of Ottawa, Ottawa, ON, Canada; 6Health and Life Sciences, Global Public Affairs, Calgary, AB, 7Health Systems Innovation at MaRS Discovery District, Toronto, ON, Canada; 8Strategic Health Technology Assessment, Government Affairs & Market Access, Janssen Inc., Toronto, ON, Canada
Objectives: To address the uncertainty associated with procuring pharmaceutical products, product listing agreements (PLAs) are increasingly being used to support responsible funding decisions in Canada and elsewhere. These agreements typically involve financial-based rebating initiatives or, less frequently, outcome-based contracts. A qualitative survey was conducted to improve the understanding of outcome-based and more innovative PLAs (IPLAs) based on input from Canadian and international key opinion leaders in the areas of drug manufacturing and reimbursement.
Methods: Results from a structured literature review were used to inform survey development. Potential participants were invited via email to partake in the survey, which was conducted over phone or in person. Responses were compiled anonymously for review and reporting.
Results: Twenty-one individuals participated in the survey, including health technology assessment (HTA) key opinion leaders (38%), pharmaceutical industry chief executive officers/vice presidents (29%), ex-payers (19%), and current payers/drug plan managers/HTA (14%). The participants suggested that ~80%–95% of Canadian PLAs are financial-based rather than outcomes-based. They indicated that IPLAs offer important benefits to patients, payers, and manufacturers; however, several challenges limit their use (eg, administrative burden, lack of agreed-upon endpoint). They noted that IPLAs are useful in rapidly evolving therapeutic areas and those associated with high unmet need, a quantifiable endpoint, and/or robust data systems. The Canadian Agency for Drugs and Technologies in Health, the pan-Canadian Pharmaceutical Alliance, and other arms-length organizations could play important roles in identifying uncertainty and endpoints and brokering pan-Canadian PLAs. Industry should work collaboratively with payers to identify uncertainty and develop innovative mechanisms to address it.
Conclusion: The survey results indicated that while challenging, use of IPLAs may be associated with various benefits. Collaboration among stakeholders remains key: Canadian agencies could play an important role in the success of these agreements, while industry should be proactive in offering solutions that will help improve outcomes across the entire health care system.
Keywords: product listing agreement, survey, innovative, Canada, CADTH, pCPA
Introduction
Historically, the majority of the risk associated with uncertainty in making drug-coverage decisions has been borne by government payers and insurance companies.1,2 That is, after regulatory approval, manufacturers of pharmaceutical products have traditionally received a fixed price per unit sold, regardless of the health outcomes associated with the product or its total sales volume.3 However, in a time of high competition for limited health care resources, growing availability of efficacious yet high-cost therapies, and heightened patient awareness and expectations, payers are setting stricter clinical outcome targets and demanding clearer evidence of value before funding new health care technologies.1,4 Manufacturers seeking reimbursement must therefore be able to clearly demonstrate the added clinical benefit and value for money of their products versus currently available options to obtain funding.4
Despite the recent focus on more stringent clinical and economic evidence, new health care technologies frequently remain associated with uncertainty in terms of their likely effectiveness, cost effectiveness, and overall budget impact at the time they receive regulatory approval.1,2,5 Importantly, such clinical and financial uncertainty can result in the delayed availability of therapies that may provide meaningful effects on patient survival and/or quality of life.4 In addition to the potential for poorer health outcomes, such delays or nonlistings impede the strengthening of a product’s evidence base in the real world.2,4 Regardless of the level of clinical uncertainty, there is often significant public pressure from patients, industry, and sometimes government to enable rapid access to new products of potential value.6 Payers must therefore balance the timely entry of promising new therapies with the responsible management of health care budgets.7
To address the uncertainties related to health benefits and/or costs that are associated with new health care technologies, product listing agreements (PLAs) – known by a broad range of taxonomies and formats (eg, risk-sharing or managed entry agreements, performance-based contracts, coverage with evidence development, etc) – are increasingly being used.1,4,8,9 These agreements represent contracts or arrangements between payers and manufacturers that attempt to balance the benefits of timely patient access to new health care technologies while addressing the clinical uncertainties and/or economic risks associated with their use.2,5,9
In Canada and many other developed countries worldwide, PLAs have traditionally been financially based (eg, rebating, and price/volume agreements), providing coverage while limiting the overall financial liability related to uncertainties regarding a product’s cost effectiveness or budget impact. However, to move beyond this financial focus – in particular, that of rebating – there appears to be increasing interest in the use of agreements that can “guarantee” the health outcomes of patients. Evidence suggests that use of more innovative financial/and outcomes-based PLAs (collectively referred to here as IPLAs) may facilitate increased evidence development. The capture of such evidence may in turn support better informed funding decisions, whether adoption or rejection, and thus improve both patient outcomes and the value achieved from health care spending.1,2
In Canada, public drug programs fund the largest portion of spending on prescription pharmaceuticals (42% in 2014),10 with federal, provincial, and territorial agencies providing coverage through their own specific formularies.11 The Canadian Agency for Drugs and Technologies in Health (CADTH; includes the Common Drug Review [CDR] for noncancer drugs and the pan-Canadian Oncology Drug Review [pCODR] for cancer drugs), Canada’s national health technology assessment (HTA) agency, provides standardized clinical and cost-effectiveness evaluations of drugs that help inform reimbursement decisions within these jurisdictions.12,13 In an attempt to consolidate the public sector’s purchasing power for drug therapies, the pan-Canadian Pharmaceutical Alliance (pCPA) was established in August 2010.14 After review of final CADTH listing recommendations, the pCPA acts as a provincial/territorial price negotiator for selected drugs with the goal of achieving lower costs and consistent pricing across all Canadian jurisdictions. Such negotiations may involve the use of PLAs; if used, the public payer in each jurisdiction signs the PLA with the manufacturer. The manufacturer and public payer have joint responsibilities in terms of the implementation of the PLA.
In this study, a structured, scoping literature review was conducted and two surveys were developed to understand the following information from the perspective of Canadian and international key opinion leaders (KOLs):
- the current landscape, evolution, and future of PLA and IPLA use for drug technologies within the context of the Canadian market;
- the roles, responsibilities, opportunities, and challenges associated with the use of such agreements;
- the potential role of national agencies such as CADTH and the pCPA as intermediaries in the design and implementation of IPLAs;
- initiatives that could be undertaken by manufacturers to make the use of IPLAs more acceptable to payers.
Methods
A structured scoping review of the published literature was conducted using Medline (PubMed) to identify information related to the use of PLAs and IPLAs in Canada and worldwide. Search terms included the following: “product listing agreement”, “managed entry agreement”, “risk-sharing agreement”, “performance-based contract”, “patient access scheme”, and “coverage with evidence development”. Similar terms were used to search gray literature for relevant web pages, presentations, and reports. Citations relevant to understanding PLA and IPLA types, their attributes, and their use in Canada and worldwide were identified through title/abstract review and selected for full-text review. Findings from the literature review were used to develop a short preread for the survey participants. This document summarized the types and characteristics of PLAs and IPLAs and key issues surrounding their development and use in Canada/internationally. The results of the literature review were also used to inform two qualitative surveys: a 16-question survey targeting current payers, ex-payers/consultants, and drug plan managers and a five-question survey targeting representatives from the pharmaceutical industry (ie, chief executive officers [CEOs] and public affairs vice presidents [VPs]) (Supplementary material). Two surveys were created to allow flexibility in survey duration and focus and to increase the ability to engage with various KOLs.
Potential survey participants were purposefully selected to represent payers, drug plan managers, and manufacturers from across Canada and with international expertise. These individuals were invited via email to partake in the survey. Those who agreed to participate were provided with the preread and survey for review and preparation. The surveys were conducted over the telephone or during in-person interviews by one person, with a second person present to record responses. Informed consent was not obtained for participation in the survey, as the participants were KOLs; however, non-industry participants did sign a contract and received an honorarium for their participation. Ethical approval was not required by a review board for the conduct of the survey.
All survey responses were anonymously compiled in terms of key learnings, areas of consensus and disagreement, and key points of interest. An analysis of the survey’s findings was conducted and its results were shared with all interested participants during two web-based meetings.
Results
Participant demographics
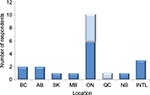
A total of 21 individuals participated in the surveys: 15 in the 16-question survey and six in the five-question survey. Of the total participants, 38% were HTA KOLs, 29% were pharmaceutical industry CEOs or VPs, 19% were ex-payers, and 14% were current payers/drug plan managers/HTA agency leaders (Figure 1). Canadian participants were located across seven different provinces, including British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, and Nova Scotia; most participants (n=11) were from Ontario (Figure 2). The three international participants were from the UK (n=2) and the US (n=1).
  | Figure 1 Distribution of survey participants by occupation Abbreviations: CEOs, chief executive officers; HTA, health technology assessment; KOLs, key opinion leaders; VPs, vice presidents. |
Survey responses
The majority of surveys were conducted via 30 minute (five-question version) or 90 minute (16-question version) phone calls; only two surveys were conducted in person. Not all participants chose to answer all questions because of variability in their experience and/or expertise.
Current landscape
Most survey participants believed that PLAs are being contracted at an increasing frequency for pharmaceutical products both in Canada and worldwide. They noted that these agreements are most commonly executed as a strategy to create expenditure and drug budget certainty, seemingly becoming “business as usual” in many reimbursement negotiations.
The participants indicated that although there is continued interest in IPLAs, such interest has diminished over time in favor of more convenient volume-based rebate agreements, with some use of per-patient caps. In Canada, participant-estimated use of PLAs ranged from 80%–95% of all agreements established between payers and manufacturers compared with 5% to 20% for IPLAs. Coverage with evidence development-type agreements were suggested to be more commonly used for medical devices than pharmaceutical products.
According to the participants, the introduction of the pCPA has encouraged the use of rebate-based PLAs as a result of the agency’s objective to provide value and consistent pricing among participating provinces. Some participants noted that the routine nature of rebating may be affecting the ability to achieve “value-based” reimbursement.
Benefits
The survey participants suggested that patients, payers, and manufacturers may attain a broad range of benefits from the use of PLAs and IPLAs. As noted in Table 1, similar benefits may be realized by some or all of these stakeholders. The survey participants specifically linked outcomes-based PLAs to broad-reaching benefits, such as improving patient health, increasing knowledge regarding a drug or therapeutic area, and providing savings across the health care system as a whole (eg, efficient resource use, reduced complications and hospitalizations).
Challenges
Numerous challenges to the use of IPLAs were noted by survey participants, with several common themes:
- High administrative burden, resource demands, and costs to execute and manage the agreements.
- Difficulty achieving agreement on suitable clinical outcomes to measure.
- Siloed nature of
- policies and capabilities across provinces/drug funding practices;
- budgets within and across sectors and institutions within the health service;
- manufacturer budgets.
- Difficulty adapting to or changing existing health care delivery practices (eg, patient monitoring and follow-up).
- Limited data collection systems within and across provinces; for example, across-province variability in coding and collecting outcomes and lack of cross-country connectivity to share and compare data.
Other challenges associated with the use of IPLAs included unwillingness to take on risk and a lack of trust among stakeholders. Some participants additionally noted that IPLAs can sometimes increase budgetary uncertainty. That is, the number of patients who will require coverage (eg, based on response criteria) may remain unclear, and/or determination of a product’s financial impact may be prolonged outside the timeframe of the payer’s regular budget cycle.
Roles and responsibilities: design, implementation, management, and funding
The participants indicated that all stakeholders who ultimately hold budgetary and/or administrative responsibilities related to IPLAs should be involved in their design, implementation, and management. At a minimum, such stakeholders include the manufacturer and payer. Clinicians, pharmacy leaders, patients, hospital system administrators, researchers, statisticians, and health economists may additionally be consulted or responsible, particularly in terms of study design and implementation. Many participants stressed the importance of involving unbiased third parties in design, data collection, analysis, and interpretation to ensure study robustness/validity in agreements that are seeking to reduce uncertainty about clinical outcomes.
The survey participants offered a variety of opinions when asked about the funding of IPLAs. Some suggested that industry should be required to pay for additional real-world evaluations, as certain provinces (eg, smaller ones) may not be able to fund them. Others indicated that payers – the ultimate “buyers” – should finance the cost to develop and implement the agreements, as the outcomes of the IPLA may offer benefit or reduce costs for the entire health care system. Some participants believed that funding responsibilities should be shared.
Roles of CADTH and the pCPA
The responses of the participants were highly variable when asked about the potential roles of CADTH and the pCPA in the design, implementation, and management of PLAs and IPLAs. A key driver of this variability was each participant’s perception of each agency’s capacity/resources based on the current operating model or alignment with current missions or mandates. That is, some participants suggested that CADTH is unlikely to have the resources needed to play a role in the development of IPLAs and should remain focused on its current mandate. Conversely, others noted that CADTH (and in particular, pCODR) already engages in processes that identify evidence gaps and could therefore help pinpoint product uncertainty. Further, the participants suggested that CADTH could potentially take proposed agreements into consideration at the time of product submission for HTA review. For the pCPA, some participants thought that it is too early in the group’s development for it to take on more than its current role. Further, they felt that the pCPA is currently too focused on rebates, and/or that the challenges of navigating different provincial policies are too great for it to establish IPLAs. However, others indicated that the pCPA may be able to facilitate communication among provinces to share data and broker pan-Canadian PLAs and IPLAs. Overall, the participants indicated that if CADTH or the pCPA were to be involved in PLAs or IPLAs, the role of each agency should fit within (or require little alteration of) their existing mandate or framework.
Success factors
Several factors were seen to contribute to the success of IPLAs. The participants noted that consultation and collaboration need to occur among all relevant stakeholders beginning in the design stage and continuing throughout the life of the IPLA. Furthermore, a clear understanding of all clinical and economic uncertainty should be obtained before IPLA development. Almost unanimously, the participants stated that outcome-based IPLAs should include agreed-upon outcome measures that are quantitative, open to little interpretation, and suitable for collection within a realistic timeframe (generally no more than 3 years). It was additionally noted that a trusted third party should be involved in structuring the agreement, collecting data, and/or interpreting outcomes.
Scenarios and therapeutic areas in which IPLAs may be most valuable
Participants were queried regarding the types of scenarios or therapeutic areas in which the use of IPLAs could be most valuable (Table 2). They suggested that scenarios with clinical or economic uncertainty in which relevant treatment outcomes can be easily identified, agreed upon, and examined in a timely manner are most amenable to IPLAs. Similarly, situations in which databases are readily available or there would be minimal burden in terms of implementation (eg, minimal burden on patient experience hospital administration) may help facilitate their conduct. In some cases, an IPLA may be a useful way to reach agreement on reimbursement when a competitor is shortly expected to enter the market. Cancer and biologic therapies were frequently highlighted as areas in which the use of IPLAs might be most useful. This was primarily because of the rapid development occurring in these areas and the considerable uncertainty associated with their cost (eg, high price, potential for indication/dose creep over time) and clinical effects (eg, variable patient response).
Role of manufacturers: initiatives to increase acceptability
The participants suggested a variety of initiatives that could be undertaken by manufacturers to increase the uptake of IPLAs. Most commonly, it was noted that manufacturers could cover the costs of additional resource requirements required within the agreement. They could also increase their use of due diligence during all stages of product development, identifying sources of clinical and economic uncertainty, seeking opportunities to improve outcomes, and proactively offering creative solutions to mitigate risk. Manufacturers could contribute to real-world data collection initiatives and allow increased data sharing to improve the understanding of both drug- and nondrug-related health outcomes. Further still, industry could implement “appropriate use” projects involving collaboration among industry, patients, and providers. They could educate health ministers, encouraging them to advocate for health outcomes-based agreements, or spearhead the development of PLA guidelines or best practices. Manufacturers could additionally increase the flexibility of their own budgets to reduce their “siloed” nature and enable internal negotiations with other related business units. It was also suggested that manufacturers could work together to collectively benefit from research related to particular therapeutic areas, for example, by cosupporting a patient registry for a disease targeted by numerous treatment types. Finally, the importance of acting reliably and in a trustworthy manner was underscored: manufacturers should ensure clarity in regard to the terms of their agreements, follow through on these terms, and refrain from attempting to change their interpretation at a later date.
Outlook of IPLAs in Canada
Overall, the survey participants showed an interest in increasing the use of IPLAs across the health care system; however, they indicated that much work is still required to ensure successful implementation. The drug plan managers, HTA representatives, and current/ex-payers appeared to be more optimistic about the expansion of IPLA use than industry CEOs/VPs, perhaps as result of prior unsuccessful experiences among the latter in the agreement on or execution of IPLAs. Several participants suggested that there needs to be a shift away from the rebate mindset of reimbursement, and that progress will depend on a lead or government support at the payer level and above. In general, the participants indicated that Canada may be in a unique position to pursue IPLAs, having a national HTA review agency and the pCPA, as well as robust health databases in some provinces. Support from these groups could provide important opportunities to broker national agreements or initiate a structured implementation of IPLAs (ie, experiment within certain provinces and gauge results) that would benefit all Canadians.
Discussion
The survey results indicate that, while challenging, the use of IPLAs is seen to be associated with various benefits and remains of interest to various Canadian and international stakeholders. Its conclusions are similar to those in a recent survey regarding risk-sharing agreements in the US private sector.15 Canadian agencies such as CADTH and the pCPA could play a key role in the success of these agreements by providing feedback or considering IPLAs within their current frameworks; however, the required resources, timing, and process by which this may occur remain unclear. Industry should be proactive in terms of proposing and supporting solutions that will improve patient outcomes and product knowledge across the entire health care system. Where possible, manufacturers should draw from their experiences in other jurisdictions where products have launched using IPLA schemes.
Limitations
Limitations of this study include those frequently encountered in qualitative survey-based investigations. There is potential for bias related to participant experience, employment, and geographical location and the qualitative nature of the questions. The perspectives of patients and private Canadian payers were not available for inclusion but should be explored in future study. Presentation of the results by stakeholder group would be of interest but was not undertaken because of the small number of participants. Finally, the total number of survey participants was relatively small and approximately half were from Ontario. While this may suggest an overrepresentation of this province, Ontario is home to ~39% of Canadians, CADTH, and numerous drug manufacturers.
Summary
The use of more innovative financial- and outcomes-based PLAs remains of interest to payers, manufacturers, and HTA leaders across Canada. Collaboration between these groups, among others, will be critical to overcome the many challenges associated with IPLAs and ensure their long-term success.
Acknowledgment
Funding for this project was provided by Janssen Inc.
Disclosure
C Henshall, LP Garrison, and Z Khayat received consultancy fees from Janssen Inc. for their input into the survey and manuscript. C Henshall performs paid consulting work for various companies, not-for-profit organizations, health systems, and governments on topics similar or related to this manuscript. S Long performs paid consulting work for various companies, not-for-profit organizations, and health systems. The authors report no other conflicts of interest in this work.
References
Adamski J, Godman B, Ofierska-Sujkowska G, et al. Risk sharing agreements for pharmaceuticals: potential considerations and recommendations for European payers. BMC Health Serv Res. 2010;10:153–168. | ||
Stafinski T, McCabe CJ, Menon D. Funding the unfundable: mechanisms for managing uncertainty in decisions on the introduction of new and innovative technologies into healthcare systems. Pharmacoeconomics. 2010;28:113–142. | ||
Rodriguez-Ibeas R, Arizti I, Antonanzas F. PHP150 Pharmaceutical pricing under uncertainty: risk-sharing contracts. Value Health. 2011;14:A233–A510. | ||
Ferrario A, Kanavos P. Managed Entry Agreements for Pharmaceuticals: the European Experience. Brussels, Belgium: EMiNet; 2013. Available from: http://eprints.lse.ac.uk/50513. Accessed May 14, 2014. | ||
Levin L, Goeree R, Levine M, et al. Coverage with evidence development: the Ontario experience. Int J Technol Assess Health Care. 2011;27:159–168. | ||
Henshall C. Overview of Current Trends in HTA. Presented at Forum 6 (HTA), European Health Forum, Gastein; 2011. Available from: http://www.ehfg.org/fileadmin/ehfg/Website/Archiv/2011/Presentations/F6/f6-s1-Henshall.pdf. Accessed May 14, 2014. | ||
Trueman P, Grainger DL, Downs KE. Coverage with evidence development: applications and issues. Int J Technol Assess Health Care. 2010;26:79–85. | ||
Carlson JJ, Sullivan SD, Garrison LP, Neumann PJ, Veenstra, DL. Linking payment to health outcomes: a taxonomy and examination of performance-based reimbursement schemes between healthcare payers and manufacturers. Health Policy. 2010;96(3):179–190. | ||
Garrison LP Jr., Towse A, Briggs A, et al. Performance-based risk-sharing arrangements-good practices for design, implementation, and evaluation: report of the ISPOR good practices for performance-based risk-sharing arrangements task force. Value Health. 2013;16:703–719. | ||
Canadian Institute for Health Information (CIHI). Prescribed Drug Spending in Canada, 2013: A Focus on Public Drug Programs; 2013. Available from: https://secure.cihi.ca/free_products/Prescribed%20Drug%20Spending%20in%20Canada_2014_EN.pdf. Accessed March 11, 2016. | ||
Health Canada. Health Care System: Access to Insurance Coverage for Prescription Medicines. Ottawa, Ontario; 2004. Available from: http://www.hc-sc.gc.ca/hcs-sss/pharma/acces/index-eng.php. Accessed March 11, 2016. | ||
Canadian Agency for Drugs and Technologies in Health (CADTH). CADTH Common Drug Review (CDR). Ottawa, Ontario; 2016. Available from: https://www.cadth.ca/about-cadth/what-we-do/products-services/cdr. Accessed March 11, 2016. | ||
Canadian Agency for Drugs and Technologies in Health (CADTH). CADTH pan-Canadian Oncology Drug Review (pCODR). Ottawa, Ontario; 2016. Available from: https://www.cadth.ca/about-cadth/what-we-do/products-services/cdr. Accessed March 11, 2016. | ||
Council of the Federation Secretariat. Canada’s Premiers. The pan-Canadian Pharmaceutical Alliance. Ottawa, Ontario; 2013. Available from: http://www.pmprovincesterritoires.ca/en/initiatives/358-pan-canadian-pharmaceutical-alliance. Accessed March 11, 2016. | ||
Garrison LP Jr, Carlson JJ, Bajaj PS, et al. Private sector risk-sharing agreements in the United States: trends, barriers, and prospects. Am J Manag Care. 2015;21(9):632–640. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.