Back to Journals » OncoTargets and Therapy » Volume 12
Targeted Toxin Gene Therapy Of Breast Cancer Stem Cells Using CXCR1 Promoter And bFGF 5′UTR
Authors Moradian C , Rahbarizadeh F
Received 28 June 2019
Accepted for publication 3 September 2019
Published 25 October 2019 Volume 2019:12 Pages 8809—8820
DOI https://doi.org/10.2147/OTT.S221223
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Cobra Moradian, Fatemeh Rahbarizadeh
Department of Medical Biotechnology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
Correspondence: Fatemeh Rahbarizadeh
Department of Medical Biotechnology, Faculty of Medical Sciences, Tarbiat Modares University, Jalal AleAhmad Highway, Tehran 14115-111, Iran
Tel +98 21 82883884
Fax +98 21 82884555
Email [email protected]
Background: Breast cancer stem cells (BCSCs) are cells with a higher ability to metastasis and resistance to conventional treatments. They have a phenotype of (CD44high/CD24low) and the unlimited ability for proliferation. Development of strategies to target the BCSC population may lead to the establishment of more effective cancer therapies. Pseudomonas exotoxin A (PE) is a potent cytotoxic protein. CXCR1 promoter provides BCSC and HER2 specificity on transcription level. 5′UTR of the basic fibroblast growth factor-2 (bFGF 5ʹUTR) provides tumor specificity on translation level. Here, we utilized a mutant form of PE encoding DNA “PE38”, CXCR1 promoter and bFGF 5ʹUTR to target BCSCs.
Methods: The stemness of SK-BR-3, MDA-MB-231 and MCF10A cell lines were evaluated based on the expression of the CD44high/CD24low stem cell signature and the ability to form mammospheres. Then, the cell lines were transfected with constructs encoding luciferase/PE38 under the control of the CMV/CXCR1 promoter with or without bFGF 5′UTR. Luciferase protein expression was evaluated using dual-luciferase reporter assay. PE38 transcript expression was measured by real-time PCR, and the cytotoxic effect of PE38 protein expression was determined by MTT assay.
Results: The percentage of CD44high/CD24low population did not correlate to mammosphere forming efficiency (MFE). Given that the percentage of CD44 high/CD24 low is not a conclusive BCSC profile, we based our work on the mammosphere assay. However, in comparison with MCF10A, the two tumorigenic cell lines had higher MFE, probably due to their higher BCSC content. Reporter assay and real-time PCR results demonstrated that CXCR1 promoter combined with bFGF 5ʹUTR increased BCSC-specific gene expression. Meanwhile, tightly regulated expression of PE38 using these two gene regulatory elements resulted in high levels of cell death in the two tumorigenic cell lines while having little toxicity toward normal MCF10A.
Conclusion: Our data show that PE38, CXCR1 promoter and bFGF 5ʹUTR in combination can be considered as a promising tool for killer gene therapy of breast cancer.
Keywords: breast cancer stem cell, PE38, CXCR1 promoter, bFGF-2, HER2, mammosphere
Introduction
A sub-population of tumor cells called cancer stem cells (CSCs) have tumor-initiating potential. They are defined by expression of the CD44high/CD24low stem cell signature, and the ability to generate mammospheres in non-adherent/serum-free conditions. Experiments have suggested that BCSCs are relatively resistant to both radiation and chemotherapy.1,2
Pseudomonas exotoxin A (PE) is a multi-domain protein. The N-terminal domain Ia (aa1–252) is required for recognition and binding to the cell. DomainII (aa253–364) is responsible for the translocation of the toxin across cellular membranes. The exact function of domainIb (aa365–404) has not been investigated yet, domainIII (aa405–613) with last 4 residues (aa400–404) of domainIb together form the catalytic subunit.3,4 The natural killing ability of PE makes it an attractive candidate for eradicating tumor cells. The mechanism of cell killing by PE is through ADP-ribosylation of eukaryotic elongation factor 2 (eEF-2) by transferring ADP-ribose from NAD+ to diphthamide residue on eEF-2, and subsequent inhibition of the protein synthesis, which leads to apoptosis of the host cells.5–7 PE38 is a 38kDa truncated form of PE that contains extensive deletions in Domain Ia (Δ1–250) and Ib (Δ365–380).7 Cell killing provoked by PE38 has successfully confirmed the cytotoxic potential of this toxin. To date, many PE38-based immunotoxins have been developed and they have been proved to efficiently kill cancer cells both in vitro and in vivo.8–12 There are also several reports on successful PE38-based cancer gene therapy.13,14 Here, we employed BCSC cell-specific expression of PE38 as a tool for breast cancer gene therapy.
The use of tumor-specific promoters is a promising strategy to restrict transcription of transgenes to tumor cells.15 IL-8 and the cognate CXCR1 receptor are overexpressed in BCSCs and HER2+ breast cancer cells compared to normal cells.16,17 In addition, highly structured, GC-rich 5ʹUTR of the bFGF-2 has been reported to provide tumor specificity both in vitro and in vivo18–21 probably due to the eukaryotic translation initiation factor 4E (eIF4E) abundancy in tumor cells. Translation initiation is largely dependent on eukaryotic translation initiation factor 4E (eIF4E) availability. eIF4E unwinds the secondary structure in the 5′UTR of mRNAs to form eIF4E complex resulting in subsequent cap-dependent translation of the mRNA. mRNAs with short unstructured 5ʹUTR are less dependent on the unwinding activity of the eIF4F complex. In contrast, the GC-rich region of mRNAs with long and highly structured 5ʹUTRs are translated less efficiently. eIF4E is expressed at a low level in most cell types.22,23 Meanwhile, it is frequently overexpressed in some carcinomas including breast cancer. Increased level of eIF4E in cancer cells facilitates the translation of mRNAs that are repressed in normal cells because of having an extensive secondary structure in their 5′ UTR.19,24
Here, we constructed vectors containing CXCR1 promoter and bFGF 5′UTR regulatory element for controlled regulation of PE38 expression, to limit the toxin expression to BCSCs and minimize its off-target effects. However, effective delivery of gene constructs to cells is essential for gene therapy approaches. Polyamidoamine (PAMAM) dendrimers are highly efficient carriers in gene delivery.25 They possess cationic primary amine groups on their branched surface, which electrostatically bound to the negatively charged nucleic acids and compact them to form dendriplexes. PAMAM dendrimers promote the cellular uptake of nucleic acids, and after cellular entry, protect them from degradation by nucleases and facilitate their endosomal escape by the proton sponge effect.26 In this study, we proposed a novel tripartite gene construct to target the BCSC populations of the breast cancer cells, as an exciting new toxin gene therapy approach.
Materials And Methods
Gene Constructs
CMV promoter was PCR amplified from pGL4.50 (Promega, WI) using the forward primer 5ʹ- AAGGGGTACCGCAGGTGCCAGAACATTTC -3ʹ and the reverse primer 5ʹ- CTAGCTAGCGATCTGACGGTTCACTAAACG -3ʹ with the following cycle profile: 95ºC for 5 min, 95ºC for 30 s, 56ºC for 40 s, 72ºC for 50 s for 30 cycles with a 7-min final extension at 72ºC. This fragment then digested by KpnI/NheI (Roche, Switzerland) and subcloned into the pGL4.14 vector (Promega) to make pGL4.14-CMV (pG-CM).
For cloning of CXCR1 promoter, upstream of CXCR1 gene was analyzed by promoter prediction bioinformatics tools and 1123 bp fragment surrounding the putative promoter region of this gene was amplified by PCR from peripheral lymphocyte DNA. This fragment was then digested by KpnI/NheI (Roche) and inserted into upstream of the luciferase gene in pGL4.14 vector to make pGL4.14- CXCR1 (pG-CX).
Human bFGF mRNA sequences (chromosome 4q26; NG_029067.1; Gene ID: 2247) were analyzed and 400-bp fragment of its 5ʹ region was selected. This fragment, flanked by NheI and BglII sites, was synthesized by Life Technology (Invitrogen, CA, USA) and received in pMK-RQ, then it was sub-cloned into the NheI and BglII sites of both pG-CM and pGCXvectors to make pG-CM-bFGF 5ʹUTR(pG-CM-bF) and pG-CX- bFGF 5ʹUTR (pG-CX-bF) constructs, respectively.
pBR391 (plasmid encoding PE38) was kindly provided by Prof. Ira Pastan (NIH) and the PE38 gene amplified from it by PCR using the forward primer 5ʹ- AGGAAGATCTATGGACTGGTACTTCGATG -3ʹ and the reverse primer 5ʹ- CTAGTCTAGAGCGTTACTTCAGGTCCTCG -3ʹ. Due to high GC content of the PE38 region, HotStarTaq DNA Polymerase and Q-Solution (Qiagen), with the cycle profile recommended by the manufacturer, were used for successful amplification. The PCR product was then substituted for luciferase gene between BglII and XbaI recognition sites of pG-CM, pG-CM-bF, pG-CX and pG-CX-bF vectors, to make pG-CM-PE, pG-CM-bF-PE, pG-CX-PE and pG-CX-bF-PE constructs, respectively. All the constructs were purified using the endotoxin-free plasmid DNA purification kit (MACHEREY-NAGEL), confirmed by sequencing and then used for cell transfections.
Cell Culture
Human breast cancer cell lines SK-BR-3 and MDA-MB-231 were purchased from DSMZ (Braunschweig, GERMANY). MCF-10A cell lines were obtained from ATCC (Manassas, VA). SK-BR-3 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) with 10% (v/v) FBS and penicillin/streptomycin. MDA-MB-231 cell lines were maintained in RPMI-1640 medium with 10% (v/v) FBS and penicillin/streptomycin. MCF-10A cell lines were cultured in DMEM-F12 medium (Invitrogen) containing horse serum 5% (v/v) (Gibco), hydrocortisone (0.5 µg/mL), insulin (10 µg/mL), EGF (20 ng/mL) and penicillin/streptomycin. All cell lines were maintained in a humidified incubator at 37 ºC and 5% CO2.
FACS
Flow cytometry was used to evaluate SK-BR-3, MDA-MB-231 and MCF-10A cell lines, for the expression of the stem cell markers CD44 and CD24. For this purpose, 70–80% confluent cells were washed twice with phosphate-buffered saline and then harvested with trypsin 0.05% (Thermo Fisher Scientific, USA). Detached cells were pelleted and re-suspended in phosphate-buffered saline supplemented with 0.5% fetal bovine serum (1×106 cells/50 μL). Monoclonal antibodies against human CD44 (FITC-conjugated) and CD24 (PE-conjugated) (Abcam, UK) were added to the cell suspension and incubated at 4ºC in the dark for 30 mins. The labeled cells were analyzed on a FACS Aria II Calibur (BD Biosciences). Data were analyzed with the Flowjo software version 7.2.4 (Tree Star Inc).
Mammosphere Culture
Mammospheres were generated from 2×104 single cells seeded in 6-well tissue culture plates coated with 1.5% agarose, containing 2 mL DMEM/F12 (GIBCO) without serum and supplemented with B27 (1:50, Invitrogen), 20 ng/mL EGF (R&D), 20 ng/mL bFGF (R&D) and 5 mg/mL insulin (Sigma). Cultures were incubated in a humidified atmosphere at 37°C and 5% CO2 for 3 days without moving or disturbing the plates. After 3 days, 400 µL of fresh media was added to each well (without removing the old media). To assess self-renewal ability of mammosphere derived cells, after 7 days in culture, mammospheres were collected by gentle centrifugation and dissociated to single cells enzymatically with a trypsin–EDTA solution (GIBCO) and mechanically by pipetting through a 200 µL pipet tip. The obtained single cells were replated at a density of 10,000 cells/mL for subsequent passages to obtain the next generation of mammospheres. The number of mammospheres (diameter >50 μm) for each well was evaluated under a microscope on day 7. MFE was calculated using the following equation: (number of mammospheres per well/number of cells seeded per well) × 100. Differentiation was induced by culturing cells dissociated from mammospheres in DMEM/F12 supplemented with serum, without growth factors. Experiments were done in triplicate.
Dendriplex Formation
HEPES-buffered glucose (HBG; HEPES 20 mM, Glucose 5% w/w, pH 7.4) was used to prepare plasmid DNA and PAMAM solutions. Equal volumes of pDNA and PAMAM (PM) solutions were mixed to achieve the N/P ratios of 5 (N denotes the number of residual primary amines on the PAMAM dendrimer, while P represents the number of phosphate groups in the plasmid DNA backbone). The mixture was immediately vortexed for 30 s and left for 30 mins at room temperature to form the dendriplexes (PAMAM dendrimer and plasmid DNA complexes).
Dendriplex Characterization
Gel Retardation Assay
The dendriplexes of PAMAM dendrimer and DNA were formed by using pG-CX-bF-PE as a model of DNA. The binding of pG-CX-bF-PE with PAMAM was confirmed by agarose gel retardation assay. PAMAM complexed with 1 μg plasmid DNA at N/P ratio of 5, and an amount of 1 μg free pG-CX-bF-PE as a control was loaded onto the 1% agarose gel. A 1:10 dilution of loading dye was added to each well and electrophoresis was carried out at a constant voltage of 100 V for 45 mins in the presence of ethidium bromide in 1 M Tris–acetate–EDTA (TAE) buffer solution.
Dynamic Light Scattering And Zeta Potential Measurements
Size and zeta potential of the PM/pG-CX-bF-PE dendriplexes at N/P ratio of 5 were determined by Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). The dendriplexes were prepared according to the method mentioned in Dendriplex formation section. They were then diluted by adding 800 μL of Milli-Q water.
Transmission Electron Microscopy
Transmission electron microscopy (TEM) was performed using a CEM 902A ZEISS (Jena, Germany) transmission electron microscope with an accelerating voltage of 80 kV, to investigate the size and morphology of the PAMAM/pG-CX-bF-PE dendriplexes at N/P ratio of 5. TEM images were analyzed with ImageJ software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/).
Transfection Procedure
Cells were grown to about 70% confluence. The freshly prepared dendriplexes were added to the cells in serum-free media (0.25 nm PAMAM/mL media). After 4 hrs, the media were replaced with serum-containing media.
PAMAM Gene Transfection Studies
To evaluate gene transfection efficiency of PAMAM dendrimers, dendriplexes of PAMAM and pEGFPN1 (PM/GFP) were prepared as described in the “Dendriplex formation” section. SK-BR-3, MDA-MB-231 and MCF-10A cell lines were transfected with PM/GFP dendriplexes. Forty-eight hours after transfection, fluorescent images of the transfected cells were taken using a fluorescence microscope (Carl Zeiss, NY). Transfection efficiency was quantitatively measured by flow cytometry using FACS Aria II Calibur (BD Biosciences) and the data were analyzed by Flowjo software version 7.2.4 (Tree Star Inc).
Co-Transfection And Reporter Assay
SK-BR-3, MDA-MB-231 and MCF-10A were co-transfected using PM/pG-CM, PM/pG-CM-bF, PM/pG-CX and PM/pG-CX-bF dendriplexes, with PM/pGL4.74 (Promega) expressing Renilla luciferase as an internal control to normalize cell viability and transfection efficiency. PM/pGL4.14 were used as the negative control. The ratio of firefly luciferase to Renilla luciferase was 2:1. After 48 hrs, luciferase assay was performed using the dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions and analyzed on a luminometer (AutoBio, China).
Analysis Of PE38 mRNA Expression
Sixteen hours after transfection with PM/pG-CM-PE, PM/pG-CM-bF- PE, PM/pG-CX- PE and PM/pF-CX-bF- PE dendriplexes, cells were collected, their total RNA was extracted using High Pure RNA Isolation kit (Roche), and cDNA was synthesized using M-MuLV reverse transcriptase and oligo-dT (Fermentas). Beta-actin was used as an internal control to normalize the level of the PE38 expression. Real-time PCR was performed by Rotor-Gene 6000 cycler (Corbett Life Science, Sydney, Australia) using RealQ Plus 2x Master Mix Green (Amplicon, Denmark). The primers for amplifying PE38 and beta-actin were as follows: PE38 for: 5ʹ AGGACCTCGACGCGATCTG, PE38 Rev: 5ʹ TCAGGCTGGTGCGGTAGAAG, beta-actin for: 5ʹ TCC CTGGAGAAGAGCTACG and beta-actin Rev: 5ʹ GTAGTTTCGTGGATGCCACA. The relative gene expression was determined by Pfaffl analysis.27 The experiment was repeated three times.
Cytotoxicity Study
To evaluate PE38 cytotoxicity, cell viability of transfected cells versus non-transfected cells was compared using the MTT assay. Briefly, cells were seeded in 96-well plates (2×104 cells/well) in complete medium, at the following day, cells were transfected with the PM/pG-CM-PE, PM/pG-CM-bF- PE, PM/pG-CX- PE and PM/pF-CX-bF- PE dendriplexes in triplicates. After 48 hrs, the medium was replaced by a fresh medium without serum and antibiotics. For MTT assay, 1 mg/mL MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma) was added to each well and followed as the manufacturer recommended.
Statistical Analysis
All statistical analyses were performed using GraphPad PRISM v.6.01 (GraphPad Software) with p < 0.05 taken as significant. Unpaired Student’s t-test was used to determine significant differences between each group. Two-way ANOVA with Tukey’s multiple comparison post hoc test was used to determine significant differences between different groups.
Results
Percentage Of CD44high/CD24low Population In SK-BR-3 And MCF-10A Does Not Correlate With MFE
SK-BR-3, MCF-10A and MDA-MB-231cell lines were analyzed using flow cytometry for CD44 and CD24 stem cell markers, and the result showed that SK-BR-3 cell line had 0.34% CD44high/CD24low sub-population (Figure 1A). For the MDA-MB-231 cell line, 80.23% of the cells were CD44+CD24– (Figure 1B) and the immortalized non-tumorigenic human breast epithelial cell line MCF-10A contained 74.31% CD44high/CD24low sub-population (Figure 1C).
In addition, mammosphere assay was performed to validate the stem property of the above cell lines. Mammospheres were photographed on day 7. As it can be seen in Figure 2, all the three cell lines were able to form mammospheres in non-adherent/serum-free conditions. However, size, morphology and mammosphere forming efficiency differed in different cell lines. SK-BR-3 formed small irregular structures; MDA-MB-231 produced the largest mammospheres, which were compact and rather irregular; MCF-10A generated large round and tightly aggregated structures. AftFer 1-week culture under mammosphere culture condition, MFE of SK-BR-3, MDA-MB-231 and MCF-10A were (2.5 ± 0.5, 1.7 ± 0.16 and 0.6 ± 0.09)%, respectively. With the aim of confirming the presence of self-renewing cells, the mammospheres were passaged on day 7 and could produce the second generation (data not shown). Lastly, cells dissociated from mammospheres differentiated and grew as adherent monolayer when shifted in serum-containing medium (data not shown).
 |
Figure 2 Optical microscope images showing different cell lines growing as non-adherent mammospheres after 7 days of cultivation. Images were analyzed with ImageJ software (NIH). |
Dendriplex Characterization Results
Gel electrophoresis experiment was carried out using PM\pG-CX-bF-PE at N/P ratio of 5. Retardation of pG-CX-bF-PE migration during the electrophoresis procedure indicates appropriate dendriplex complexation and condensation (Figure 3).
The size and zeta potential of the polyplexes at N/P ratio of 5 measured by Zetasizer Nano ZS are presented in Figure 4. PAMAM and pG-CX-bF-PE formed stable dendriplexes with a dendriplex size of 117.2±3 nm (Figure 4A) and zeta-potential value of +16.4±2 mV (Figure 4B). Low polydispersity index (0.14) indicated the formation of homogeneous and aggregate-free dendriplexes.
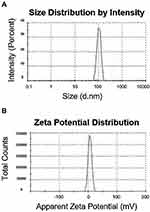 |
Figure 4 Size distribution (A) and zeta potential graphs (B) of the PM/pG-CX-bF-PE dendriplexes at N/P ratio of 5 measured by Malvern Zetasizer Nano ZS. |
Transmission electron microscopy was performed to investigate the size and morphology of the PM/pG-CX-bF-PE dendriplexes at N/P ratio of 5. TEM image showed spherical structures with an average particle size of 104±5 nm (calculated by measuring 50 particles using ImageJ software). The particles were found to have a narrow size distribution, demonstrating homogeneous dendriplex formation (Figure 5). The result of the TEM agreed with the DLS outcomes. However, the particle size visualized by TEM was smaller than those determined by DLS. This result could be explained by the fact that the particle size determined by DLS was the hydrodynamic size, whereas TEM determined the dry particle size.
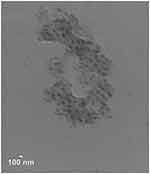 |
Figure 5 Transmission electron microscopy. The TEM image of dendriplexes formed with PAMAM and pG-CX-bF-PE. |
PAMAM Gene Transfection Efficiency
pEGFPN1 plasmid was used as a reporter gene to evaluate gene transfection efficiency of PAMAM dendrimers. The fluorescence images of GFP-expressing cells are shown in Figure 6. As it can be seen, PAMAM gene delivery efficiency in the three cell lines follows the order of SK-BR-3> MCF10A> MDA-MB-231. Also, GFP expression was quantified by flow cytometry and the outcome agreed with the fluorescence microscopy result. As it can be seen in Figure 7, transfection capabilities of PM/GFP polyplexes were 29.6%, 15% and 23.4% in SK-BR-3 (Figure 7A), MDA-MB-231 (Figure 7B) and MCF-10A (Figure 7C), respectively.
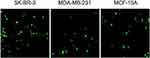 |
Figure 6 Representative fluorescence images of cell lines transfected with PM/GFP at N/P ratio of 5. |
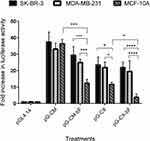
BCSC-Specific Expression Of Luciferase Protein
Co-transfections and reporter assay were applied to investigate the effectiveness of CXCR1 promoter and the bFGF 5ʹUTR regulatory element on targeting cancerous cells. The results of the reporter assay are represented in Figure 8.
By comparing the relative luciferase expression ratio in transfected cells, we found that the luciferase reporter gene expression under the control of CMV promoter was almost equally high in all cell lines compared to the pGL4.14 empty vector, ranging from 37 in SK-BR-3 to 33 in MDA-MB-231 and 36 in MCF-10A (p = ns for all).
When the cells were transfected with the pG-CM-bF, luciferase activity was 29 in SK-BR-3 and 25 in MDA-MB-231, while in MCF-10A it was 12, that was significantly lower from that seen in the cancerous cell lines (p < 0.001 compared to both).
Under the control of CXCR1 promoter in pG-CX construct, the luciferase reporter gene expression ratios were almost the same in SK-BR-3 and MDA-MB-231 (23 and 22, respectively; p = ns), while in MCF-10A it was 11, which was significantly lower than the two tumorigenic cell lines (p < 0.05 compared to both).
In the case of pG-CX-bF construct, the difference between luciferase expression in the two tumorigenic cell lines and the non-tumorigenic MCF-10A was statistically significant (p < 0.0001 compared to both), ranging from 22 in SK-BR-3 to 19 in MDA-MB-231 and 4 in MCF-10A.
It is noticeable that addition of bFGF 5ʹUTR to both CMV and CXCR1 did not decrease luciferase expression significantly in SK-BR-3 and MDA-MB-231 (p = ns for all), whilst the expression was down-regulated significantly in MCF-10A (p < 0.001 when comparing pG-CM with pG-CM-bF; p < 0.05 when comparing pG-CX with pG-CX-bF.
Luciferase expression differences between the two tumorigenic cell lines and the non-tumorigenic MCF-10A were more significant when they were transfected by pG-CX-bF (p < 0.0001 for both) compared to pG-CM-bF (p < 0.001 for both).
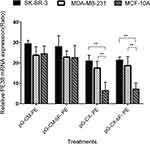
BCSC-Specific Expression Of PE38 mRNA
SK-BR-3, MDA-MB-231 and MCF-10A were transfected with the PM/pG-CM-PE, PM/pG-CM-bF- PE, PM/pG-CX- PE and PM/pG-CX-bF- PE dendriplexes and their respective PE38 mRNA expression pattern were analyzed by real-time PCR.
As it can be seen in Figure 9, the real-time PCR result showed that when transfected with pG-CM-PE, all the three cell lines exhibited a high expression level of PE38 transcripts, with no significant differences among the cell lines (p = ns for all). However, the level of PE38 mRNA was lower and exhibited variation between cell lines when the CXCR1 promoter was substituted for the CMV promoter (pG-CX- PE). Under the control of CXCR1promoter in the pG-CX- PE construct, the quantities of PE38 mRNA were 21 and 17 in SK-BR-3 and MDA-MB-231, respectively (p = ns for both), while in MCF-10A, it was 6, that was significantly lower than the two tumorigenic cell lines (p < 0.01 compared to both). However, by addition of the bFGF 5′UTR to the CMV or CXCR1 promoter, a slight elevation or reduction of the transcript level was observed in the three cell lines (p = ns for all). As bFGF 5′UTR influences the gene expression specifically on translation level, the observed fluctuation might be the consequence of the promoter displacement.
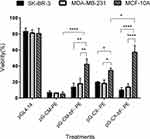
BCSC-Specific Cytotoxicity Of PE38
PE38 is a potent bacterial toxin and induces massive cell death. To evaluate the PE38 expression, the three cell lines were transfected with the PM/pG-CM-PE, PM/pG-CM-bF- PE, PM/pG-CX- PE and PM/pG-CX-bF- PE dendriplexes. The cytotoxic effect of the PE38 protein on the cancerous and normal cell lines was determined by MTT assay.
The results of the MTT assay are represented in Figure 10. It should be noted that pGL4.14 empty vector showed about 20% loss in viability of the cells (p = ns for all). Since all the plasmids were purified using the endotoxin-free plasmid purification kit (MACHEREY-NAGEL), the observed loss of cell viability could be assigned to the toxicity of the transfection process. The cell viabilities observed with pG-CM-PE were about 6% and not significantly different between the different cell lines (p = ns for all). Cell viability of SK-BR-3 and MDA-MB-231 transfected with the pG-CM-bF-PE were 13% and 17%, respectively, while in the MCF-10A, where eIF4E is not abundant, cell viability was recorded at 42% (p < 0.01 compared to both). In the case of pG-CX- PE construct, 20% and 18% cell viability for SK-BR-3 and MDA-MB-231 were detected, respectively, but it was 34% for MCF-10A (p < 0. 05 compared to both). When transfected with pG-CX-bF- PE, SK-BR-3 and MDA-MB-231 cells exhibited 10% and 14% viability, respectively. But MCF-10A showed 57% recovery of the cells (p < 0.0001 compared to both).
It is noticeable that addition of bFGF 5ʹUTR to both CMV and CXCR1 significantly increased MCF-10A viability (p < 0.0001 when comparing pG-CM- PE with pG-CM-bF- PE and p < 0.05 when comparing pG-CX- PE with pG-CX-bF- PE), whilst viability elevation was not significant in SK-BR-3 and MDA-MB-231 (p = ns for all). Cell viability differences between the two tumorigenic cell lines and the non-tumorigenic MCF-10A were more significant when they were transfected by pG-CX-bF (p < 0.0001) compared to pG-CM-bF (p < 0.01). The results of MTT assay were confirmed by trypan blue exclusion test (data not shown).
Discussion
We employed combined transcriptional and translational regulation strategy, using CXCR1 promoter and bFGF 5′UTR, to restrict PE38 toxin expression to BCSC and HER2+ populations of the breast cancer cell. BCSC and HER2+ cell lines are rational targets for breast cancer gene therapy, as they are known to increase aggressiveness and mortality.28–30
Collectively, the present study illustrates the following important points:
First, comparing MFE outcome with FACS data demonstrated that SK-BR-3 cell lines have a high ability to form mammosphere, despite low CD44high/CD24low subpopulation and MCF-10A cell lines have a low mammosphere-forming ability, although it contains a large subpopulation of CD44 high/CD24 low cells. Thus, as suggested by other researchers,31,32 there is no clear correlation between CD44high/CD24low phenotype and mammosphere-forming ability. Given that the percentage of CD44 high/CD24 low is not a unique and conclusive BCSC profile32,33 and considering the fact that each mammosphere represents a single stem-like cell of the parental population, mammosphere formation is believed to be more effective in measuring stem-like propagation.34 Our data agreed with this concept, as cell lines with higher MFE were more sensitive to our BCSC targeted therapy.
Second, our data indicated that gene expression rate under the control of CMV promoter was almost equally high in all the three cell lines tested. In contrast, under the control of CXCR1 promoter, SK-BR-3 and MDA-MB-231 had comparable gene expression but the expression was considerably lower in MCF-10A. This result provides a proof of the cancer specificity of the CXCR1 promoter compared to the commonly used strong, non-specific CMV promoter. It should be noted that IL-8 and CXCR1 are overexpressed in BSCSs and HER2+ breast cancer cells17 and the three cell lines chosen for this study differ in BSCS content and HER2 expression. HER2+ SK-BR-3 and triple-negative MDA-MB-231 demonstrated high MFE; thus, high CXCR1 activity in these tumorigenic cell lines can be explained by their high BCSC content and HER2 positivity in the case of SK-BR-3. However, moderate activity of CXCR1 in MCF-10A could be ascribed to the stem cell-like properties of this cell line.
Third, we observed that the specificity of the CXCR1 in combination with the bFGF 5ʹUTR was higher than CXCR1 alone. Furthermore, the specificity of the bFGF 5ʹUTR in combination with the CXCR1 was higher than when it was combined with the CMV, thus providing a proof of the effectiveness of our combined transcriptional and translational scheme. The cancer-specific translation of the Luciferase or PE38 protein when preceded by the bFGF 5′UTR can be explained by the high level of eIF4E in SK-BR-3 and MDA-MB-231, relative to non-malignant MCF-10A cells.35,36 Consistent with this, Mathis et al reported the successful usage of the rat bFGF 5′UTR to limit the expression of the HSV-1 thymidine kinase (HSV-Tk) suicide gene to breast cancer cells both in vitro and in vivo. Their results also demonstrated elevated HSV-Tk protein expression in the cells which had a higher level of eIF4E.37 Effectiveness of bFGF 5′UTR to target cancerous cells has been also demonstrated by some other researchers.20,21
And lastly, the PE38 gene product has been proved to successfully express and kill the transfected cells. We tested pGL4.14 empty vector in the cytotoxicity experiments and about 20% cell death was observed in all the cell lines. This level of cytotoxicity can be assigned to the transfection process, and so the excessive cell death observed in the cells transfected with the PE38 encoding constructs was mainly due to the cytotoxicity of PE38 protein. This result is in agreement with the previously published observations.13,14 Also, the levels of the PE38 protein correlated well with the activity of the CMV promoter, CXCR1 promoter and the bFGF 5ʹUTR in the three cell lines tested.
Importantly, despite the undesirable activity of the CXCR1 promoter in the MCF-10A, the addition of the 5′UTR of the bFGF to the CXCR1 promoter dramatically decreased the protein expression in this cell line. This result clearly shows the effectiveness of our binary transcriptional and translational regulation scheme in minimizing the toxicity of the killer gene toward normal cells with stem/progenitor phenotype in vitro. Nonetheless, considering the fact that there are normal cells in the body with high IL-8/CXCR1 expression other than cancer cells, in vivo effectiveness and safety of the proposed treatment remains to be assessed.
Conclusion
To direct PE38 killer gene expression to breast cancer cells, we utilized a combination of transcriptional and translational targeting. Our results confirmed that CXCR1 promoter and bFGF 5′UTR regulatory element can increase specific gene expression in BCSCs and HER2+ cell lines, with low toxicity for normal cells; thus, these three elements in combination can be considered as a promising tool for killer gene therapy of the breast cancer.
Acknowledgments
We would like to thank Professor Ira Pastan (NIH) for kindly providing the plasmid encoding PE38 (pBR391) used in this study. The work was supported in part by Tarbiat Modares University, Iranian Council for Stem Cell Sciences and Technologies [reference: Rep 170], the Biotechnology Development Council of the Islamic Republic of Iran (No: 950711) and Iran Nanotechnology Innovation Council (INIC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Phillips TM, McBride WH, Pajonk F. The response of CD24−/low/CD44+ breast cancer–initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. doi:10.1093/jnci/djj495
2. Yu F, Yao H, Zhu P, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–1123. doi:10.1016/j.cell.2007.10.054
3. Lodish HF. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974;251:385–388. doi:10.1038/251385a0
4. Siegall CB, Chaudhary VK, FitzGerald DJ, Pastan I. Functional analysis of domains II, Ib, and III of Pseudomonas exotoxin. J Biol Chem. 1989;264(24):14256–14261.
5. Wolf P, Elsässer-Beile U. Pseudomonas exotoxin A: from virulence factor to anti-cancer agent. Int J Med Microbiol. 2009;299(3):161–176. doi:10.1016/j.ijmm.2008.08.003
6. Jenkins CE, Swiatoniowski A, Issekutz AC, Lin T-J. Pseudomonas aeruginosa exotoxin A induces human mast cell apoptosis by a caspase-8 and-3-dependent mechanism. J Biol Chem. 2004;279(35):37201–37207. doi:10.1074/jbc.M405594200
7. Kreitman RJ, Siegall CB, Chaudhary VK, FitzGerald DJ, Pastan I. Properties of chimeric toxins with two recognition domains: interleukin 6 and transforming growth factor. alpha. at different locations in Pseudomonas exotoxin. Bioconjug Chem. 1992;3(1):63–68.
8. Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin anti-Tac (Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18(8):1622–1636. doi:10.1200/JCO.2000.18.8.1622
9. Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4 (dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23(27):6719–6729. doi:10.1200/JCO.2005.11.437
10. Kreitman RJ, Stetler-Stevenson M, Margulies I, et al. Phase II trial of recombinant immunotoxin RFB4 (dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27(18):2983. doi:10.1200/JCO.2008.20.2630
11. Wayne AS, Kreitman RJ, Findley HW, et al. Anti-CD22 immunotoxin RFB4 (dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin Cancer Res. 2010;16(6):1894–1903. doi:10.1158/1078-0432.CCR-09-2980
12. Hassan R, Lerner MR, Benbrook D, et al. Antitumor activity of SS (dsFv) PE38 and SS1 (dsFv) PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic culture in vitro. Clin Cancer Res. 2002;8(11):3520–3526.
13. Candolfi M, Xiong W, Yagiz K, et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc Natl Acad Sci. 2010;107(46):20021–20026. doi:10.1073/pnas.1008261107
14. Kimchi-Sarfaty C, Vieira WD, Dodds D, et al. SV40 Pseudovirion gene delivery of a toxin to treat human adenocarcinomas in mice. Cancer Gene Ther. 2006;13(7):648. doi:10.1038/sj.cgt.7700943
15. Robson T, Hirst DG. Transcriptional targeting in cancer gene therapy. Biomed Res Int. 2003;2003(2):110–137.
16. McDermott SP, Wicha MS. Targeting breast cancer stem cells. Mol Oncol. 2010;4(5):404–419. doi:10.1016/j.molonc.2010.06.005
17. Todorović-Raković N, Milovanović J. Interleukin-8 in breast cancer progression. J Interferon Cytokine Res. 2013;33(10):563–570. doi:10.1089/jir.2013.0023
18. Rosenwald IB. Upregulated expression of the genes encoding translation initiation factors eIF-4E and eIF-2α in transformed cells. Cancer Lett. 1996;102(1):113–123. doi:10.1016/0304-3835(96)04171-7
19. Kevil C, Carter P, Hu B, DeBenedetti A. Translational enhancement of FGF-2 by eIF-4 factors, and alternate utilization of CUG and AUG codons for translation initiation. Oncogene. 1995;11(11):2339–2348.
20. DeFatta RJ, Chervenak RP, De Benedetti A. A cancer gene therapy approach through translational control of a suicide gene. Cancer Gene Ther. 2002;9(6):505. doi:10.1038/sj.cgt.7700469
21. Fang YX, Zhang XB, Wei W, et al. Development of chimeric gene regulators for cancer-specific gene therapy with both transcriptional and translational targeting. Mol Biotechnol. 2010;45(1):71–81. doi:10.1007/s12033-010-9244-y
22. Hiremath L, Webb N, Rhoads R. Immunological detection of the messenger RNA cap-binding protein. J Biol Chem. 1985;260(13):7843–7849.
23. Duncan R, Milburn SC, Hershey J. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987;262(1):380–388.
24. Koromilas AE, Lazaris-Karatzas A, Sonenberg N. mRNAs containing extensive secondary structure in their 5ʹnon-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. Embo J. 1992;11(11):4153. doi:10.1002/j.1460-2075.1992.tb05508.x
25. Abedi-Gaballu F, Dehghan G, Ghaffari M, et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today. 2018;12:177–190. doi:10.1016/j.apmt.2018.05.002
26. Sonawane ND, Szoka FC, Verkman A. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278(45):44826–44831. doi:10.1074/jbc.M308643200
27. Pfaffl MW. Quantification strategies in real-time PCR. AZ Quant PCR. 2004;1:89–113.
28. Koutras AK, Evans TJ. The epidermal growth factor receptor family in breast cancer. Onco Targets Ther. 2008;1:5. doi:10.2147/OTT.S3842
29. Ménard S, Fortis S, Castiglioni F, Agresti R, Balsari A. HER2 as a prognostic factor in breast cancer. Oncology. 2001;61(Suppl. 2):67–72. doi:10.1159/000055404
30. Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi:10.1126/science.2470152
31. Bailey PC, Lee RM, Vitolo MI, et al. Single-cell tracking of breast cancer cells enables prediction of sphere formation from early cell divisions. iScience. 2018;8:29–39. doi:10.1016/j.isci.2018.08.015
32. Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11(4):254. doi:10.1038/nrc3023
33. Grimshaw MJ, Cooper L, Papazisis K, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10(3):R52. doi:10.1186/bcr2106
34. Barbieri F, Wurth R, Ratto A, et al. Isolation of stem-like cells from spontaneous feline mammary carcinomas: phenotypic characterization and tumorigenic potential. Exp Cell Res. 2012;318(7):847–860. doi:10.1016/j.yexcr.2012.02.008
35. Sorrells DL, Black DR, Meschonat C, et al. Detection of eIF4E gene amplification in breast cancer by competitive PCR. Ann Surg Oncol. 1998;5(3):232–237.
36. Pettersson F, Yau C, Dobocan MC, et al. Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clin Cancer Res. 2011;17(9):2874–2884. doi:10.1158/1078-0432.CCR-10-2334
37. Mathis JM, Williams BJ, Sibley DA, et al. Cancer‐specific targeting of an adenovirus‐delivered herpes simplex virus thymidine kinase suicide gene using translational control. J Gene Med. 2006;8(9):1105–1120. doi:10.1002/jgm.935
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.