Back to Journals » Journal of Pain Research » Volume 8
Tapentadol prolonged release for patients with multiple myeloma suffering from moderate-to-severe cancer pain due to bone disease
Authors Coluzzi F , Raffa RB , Pergolizzi J, Rocco A, Locarini P, Cenfra N, Cimino G, Mattia C
Received 25 February 2015
Accepted for publication 23 March 2015
Published 8 May 2015 Volume 2015:8 Pages 229—238
DOI https://doi.org/10.2147/JPR.S83490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Flaminia Coluzzi,1,2 Robert B Raffa,3 Joseph Pergolizzi,4 Alessandra Rocco,1 Pamela Locarini,1 Natalia Cenfra,5 Giuseppe Cimino,5 Consalvo Mattia1,2
1Department of Medical and Surgical Sciences and Biotechnologies, Faculty of Pharmacy and Medicine, Unit of Anaesthesiology, Intensive Care Medicine and Pain Therapy, Polo Pontino, Sapienza University of Rome, Latina, Italy; 2SIAARTI Study Group on Acute and Chronic Pain, Rome, Italy; 3Department of Pharmaceutical Sciences, Temple University School of Pharmacy, Philadelphia, PA, USA; 4Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 5Department of Cellular Biotechnology and Hematology, Sapienza University of Rome, Rome, Italy
Context: Myeloma bone disease (MBD) is a devastating complication of multiple myeloma that leads to severe pain.
Objectives: The aim of this study was to evaluate the efficacy and tolerability of tapentadol prolonged release (PR) in the management of patients with MBD suffering from moderate-to-severe cancer pain.
Methods: A 12-week prospective study was carried out in 25 opioid-naïve MBD patients. Patients initially received twice-daily doses of tapentadol PR 50 mg. Doses were then managed to maintain adequate relief or dose-limiting toxicity. The following parameters were recorded at weekly intervals for 4 weeks, and then at weeks 8 and 12: pain, opioid-related adverse effects, use of other analgesics, DN4 (Douleur Neuropathique 4) score. Quality of life (SF-36 [36-item short-form health survey]) was measured at baseline and at final evaluation.
Results: Of 25 patients, 22 completed the study. Pain intensity significantly decreased from baseline to all the week intervals (P<0.01). Quality of life significantly improved with respect to all SF-36 subscale parameters (P<0.01), and so did both the physical and mental status (P<0.01). Tapentadol PR significantly reduced DN4 mean value (P<0.01) and the number of patients with neuropathic component (DN4 ≥4) (P<0.01). After 8 weeks of treatment, all patients were negative for the DN4 score. Tapentadol PR was well tolerated, and the use of other analgesics was reduced during the study period.
Conclusion: Tapentadol PR started in doses of 100 mg/day was effective and well tolerated in opioid-naïve MBD patients with moderate-to-severe pain. Tapentadol PR can be considered a first-choice opioid in cancer patients suffering from mixed pain with a neuropathic component.
Keywords: tapentadol, multiple myeloma, bone, neuropathic pain, opioids
Introduction
Osteolytic bone disease is the most common complication of multiple myeloma (MM). It results in skeletal-related events that cause significant pain, morbidity, and mortality.1 Myeloma bone disease (MBD) is a devastating complication of MM that leads to pain, fractures, mobility issues, and neurological deficits.2 In addition, bone pain is the most common presenting complaint, concomitant with anaemia, raised serum creatinine levels, and high serum lactate dehydrogenase and C-reactive protein levels.3 In one-third of patients, MM is diagnosed after a pathological fracture occurs; such fractures commonly involve the axial skeleton. Most case series report that 70% of patients complain of bone pain emanating from the back, long bones, skull, and/or pelvis.
In recent years, a number of promising therapeutic targets have been identified for the management of MBD, eg, receptor activator of nuclear factor kappa-B ligand (RANKL), osteoprotegerin (OPG) system (RANKL/OPG), wingless (Wnt), dickkopf-1 (Wnt/DKK1) pathway.4 However, conventional approaches for metastatic bone pain, including analgesics, bisphosphonates, radiotherapy, and surgical interventions, still have a significant role in the treatment of MBD.5 Among analgesics, opioids still represent the cornerstone of management in cancer patients with moderate-to-severe chronic pain. Most opioids produce analgesia by binding to mu opioid receptors (MOR) in the central nervous system. The activation of these receptors inhibits neurone transmitter release in the dorsal horn of the spinal cord and interrupts the transmission of pain signals from primary afferent fibers by a presynaptic inhibitory action. Moreover, they reduce spinal neuronal activity through hyperpolarization of postsynaptic neurons. Unfortunately, the MOR agonistic interactions responsible for opioid activity are not limited to the neurons of the pain pathway. Opioid receptors present throughout the nervous system and periphery, plus the interactions of opioids with nonanalgesic receptors, contribute to many of the side effects associated with opioid treatment. In clinical practice, the onset of intolerable side effects often leads to the decision to reduce drug dosage, leading to inadequate analgesic relief. This vicious circle is a current drawback of traditional MOR agonists, resulting in more than 30% of patients discontinuing opioid therapy, because of adverse events, particularly involving the gastrointestinal tract.6
Tapentadol is an innovative centrally acting analgesic agent that has two mechanisms of action: MOR agonism and norepinephrine reuptake inhibition (MOR/NRI). The moderate affinity to MOR and the opioid-sparing effect of inhibition of norepinephrine reuptake would be consistent with tapentadol producing fewer opioid-related adverse effects than typical MOR agonists7 and being effective in a variety of pain models.8,9 The efficacy and favorable tolerability of tapentadol prolonged release (PR) have been reported in the management of moderate-to-severe chronic lower back pain,10 osteoarthritis,11 chronic painful diabetic peripheral neuropathy,12 and cancer pain.13–16 In particular, when compared with a traditional opioid, such as controlled-release (CR) oxycodone, tapentadol PR reduced the risk of discontinuation due to adverse events by 47%, the risk of nausea and vomiting by 47%, and the risk of constipation by 39%.17 In addition, the long-term tolerability of tapentadol PR has been demonstrated in patients with lower back pain during up to 1 year of treatment.18
Cancer pain is often multimechanistic, which is caused by multiple different natural and pathophysiologic mechanisms. In particular, bone lesions are often associated with mixed syndromes, where nociceptive and neuropathic components coexist.19,20 Unlike nociceptive pain, neuropathic pain is maladaptive and not proportional to the noxious stimulus, which makes chronic pain with a neuropathic pain component particularly challenging to manage.21 The pain may be perceived as shooting, lancinating, “electric shock-like”, or burning and may occur spontaneously. Negative signs, such as the loss of light touch, vibration, pinprick, or thermal sensation, may be accompanied by positive signs, such as allodynia, hyperalgesia, and hyperpathia. The prevalence of neuropathic pain has been estimated at 19%–39% among cancer patients when mixed pain is included.22
Recent literature underscores the relevance of the noradrenaline pathway in delaying the progression of chronic pain following nerve transection. This endogenous descending pain modulating system plays a key role in shaping the spatial and temporal expressions of the neuropathic pain phenotype following nerve injury.23 In general, the baseline pain sensitivity is only little influenced by the noradrenergic system, but in injured conditions, the noradrenergic system contributes to the feedback inhibition of pain. Following injury or inflammation, the central and peripheral noradrenergic systems are subject to plastic changes that mitigate against antinociceptive efficacy.24 Extensive findings suggest that an increase in noradrenaline in the spinal cord plays an important role in the antihyperalgesic effects of drugs for the treatment of neuropathic pain.25
Because mechanisms of descending noradrenergic modulation seem to be of particular importance in neuropathic pain components of chronic pain, multimechanistic tapentadol may be particularly well suited for the management of patients with bone lesions related to MM, in particular, back pain with a neuropathic pain component.
The aim of this open-label study was to assess the effectiveness and tolerability of tapentadol PR over up to 12 weeks in patients with MBD suffering from moderate-to-severe chronic cancer pain, with or without a neuropathic pain component.
Materials and methods
The study was carried out in a convenience sample of consecutive opioid-naïve MBD patients diagnosed at the hematologic unit and admitted to the Pain Therapy Unit of the Sapienza University, Polo Pontino, in Latina for a period of 6 months, from June to December 2013. For recruitment, medical staff asked patients to participate and informed consent was obtained.
Inclusion criteria were:
- at least 18 years of age,
- a diagnosis of MM with at least one bone lesion,
- moderate-to-severe cancer pain (more than 4 at movement on a numerical rating scale [NRS] from 0 to 10, see later text), unresponsive to step I analgesic ladder drugs (nonopioid drugs), or occasional use of step II opioids for moderate pain, and
- a Karnofsky status of 50 or more.
Exclusion criteria were:
- a history of, or laboratory values reflecting, severe renal or hepatic impairment,
- use of monoamine oxidase inhibitors within 14 days prior to screening,
- a history of drug abuse,
- cognitive failure,
- brain metastases or brain damage, and
- short expected survival.
During the titration phase, each patient initially received twice-daily doses of tapentadol PR 50 mg and oral morphine as rescue medication (5 mg). Doses were then titrated to adequate pain relief or dose-limiting toxicity, on the basis of the clinical response.
If patients were using nonopioid analgesics, such as acetaminophen (paracetamol), nonsteroidal anti-inflammatory drugs (NSAIDs), or adjuvant drugs (such as antidepressants or anticonvulsants), these were continued if tolerated by patients, according to medical decisions. Step II opioids were discontinued. Symptomatic drugs (antiemetics and laxatives), corticosteroids, bisphosphonates, radiotherapy, and chemotherapy were used according to clinical needs.
Patients were visited or contacted at least once weekly to monitor therapy and adjust it if needed, according to the clinical status. Data were recorded at baseline (W0 [week 0]) before starting the study, at weekly intervals for 4 weeks (W1, W2, W3, and W4), and at 8 weeks (W8) and 12 weeks (W12).
The following parameters were recorded:
- Pain intensity at rest using patients’ self-report on a 11-point NRS from 0 to 10.
- Pain intensity at movement using patients’ self-report on a 11-point NRS from 0 to 10.
- Neuropathic pain component using the DN4 score from 0 to 10.26 DN4 is a screening tool for neuropathic pain, consisting of interview questions and physical tests. If the score is 4 or higher, then the pain is likely to be neuropathic pain. DN4 can be used in cancer patients to detect neuropathic pain with higher sensitivity (87.5%) than other tools, such as the Leeds Assessment of Neuropathic Symptoms and Signs.27
- Adverse effects associated with opioid therapy (such as nausea and vomiting, drowsiness, confusion, constipation, dry mouth, myoclonus, and sweating) were rated using a verbal scale from 0 to 3 (not at all, slight, a lot, severe).
- Quality of life (QoL) was measured with the SF-36 at W0 and W12. The SF-3628 is a 36-item survey that evaluates eight dimensions of functional health and well-being (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health), each scored from 0 (“lowest level of health”) to 100 (“highest level of health”). The individual SF-36 item scores were summarized as physical and mental health composite scores.
Statistical analyses
Sample size
We set as a clinically relevant primary end point a significant (P<0.05) decrease in the pain intensity at movement (NRS score) with an effect size of at least 0.6 (standardized mean difference: basal versus first week – medium to large effect size). Then with α=0.05 (two-sided) and β=0.20 (power 80%), 25 patients were needed.
Statistical analyses
Demographic data were reported as descriptive statistics. The NRS scores of pain intensity at movement and at rest and the scores of DN4 collected at each visit were analyzed using the Friedman test for multiple paired data. The variation of the frequency of patients with neuropathic pain component versus basal was analyzed using the McNemar test.
The eight scales of SF-36 that aggregate 2–10 items each, the two summary measures that aggregate scales, and the total SF-36 score collected at the beginning of the trial and after 12 weeks of therapy were analyzed using the Wilcoxon test for paired data.
The frequency of opioid-related symptoms and the frequency of use of other drugs at each visit are reported as absolute and relative frequencies. The doses of tapentadol PR prescribed at each visit are reported as the mean and SD (standard deviation).
Analyses were performed by the BioMeDical Package statistical software.
Results
Of the 25 patients recruited for the study, 12 (48%) were female, and the mean (SD) age was 68.6 (9.8) years. The mean Karnofsky status was 73.6 (9.5). Demographic and baseline characteristics are summarized in Table 1. Bone lesions were in a rank order: back (n=23/25), ribs (n=7/25), skull (n=5/25), neck (n=4/25), long bones (n=3/25), pelvis (n=3/25), others (n=5/25). Three patients did not conclude the study; one died during the study period, one had adverse effects (severe headache) requiring a switch to other opioid analgesics, and one was lost to follow-up.
  | Table 1 Demographic data |
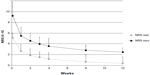
The results regarding pain intensity at the different time intervals are presented in Table 2. Mean (SD) pain intensity at movement (observed-case analysis) at (W0) was 9.2 (0.87); mean (SD) pain intensity scores decreased significantly from W0 to the end of the study (W12) (mean [SD] change from W0, −6.73 [1.32]; P<0.01). A statistically significant reduction of pain score at rest (P<0.05) and at movement (P<0.05) was recorded after the first week of treatment with tapentadol at the daily dose of 100 mg. Mean (and SD) pain intensity at rest and at movement for overall pain over time are shown in Figure 1.
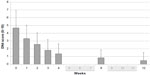
Tapentadol PR significantly reduced DN4 mean value from 4.68 (2.43) at W0 to 0.41 (0.91) at W12 (final evaluation) (P<0.01) (Figure 2). At W0, the values were negative (DN4 <4) and positive (DN4 ≥4) in 7 (28%) and 18 (72%) patients, respectively. Patients with neuropathic component (DN4 ≥4) showed a significant reduction from W0 to W12 (P<0.01) (Table 2): 18 at W0, 14 at W1, 9 at W2, 3 at W3, 3 at W4, 0 at W8, 0 at W12 (P<0.01) (Figure 3). After 8 weeks of treatment, no patients presented a positive neuropathic component.
All opioid-related symptoms were mild and are presented in Table 3. Some symptoms varied in prevalence during the study period. The incidence of constipation decreased from 32% at W0 to 9.1% at W12. Drowsiness increased at W1 (16%), and reduced progressively, disappearing at W4 in all patients. Dry mouth was observed throughout the study period in a minority of patients: 4% of patients reported dry mouth at W0; then, it increased at W1 (16%) and decreased from W1 to W12, at 4.6%. No patients reported nausea, vomiting, myoclonus, or sweating. Only one patient reported mild confusion at W1.
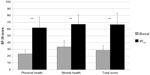
Significant improvements were observed in all SF-36 subscale scores, from W0 to W12 (P<0.01 for all eight scale scores; Figure 4). Mean changes in SF-36 subscale scores were respectively: +45.2 for physical functioning, +50.1 for role-physical, +60.8 for bodily pain, +23.3 for general health, +36.3 for vitality, +43.6 for social functioning, +42.7 for role-emotional, and +42.7 for mental health. Similarly, a statistically significant improvement basal-final (P<0.01) was recorded for physical, mental, and total SF-36 scores, as shown in Figure 5.
The initial mean dose of tapentadol PR was 100 mg daily for all patients, by inclusion criteria. Doses of tapentadol PR at the different time intervals are presented in Table 4. Tapentadol PR daily doses significantly increased up to a mean (SD) dosage of 243.5 mg (71.2) at W4 and subsequently reduced to a mean (SD) dosage of 213.6 mg (94.1) at W12. Ten patients used daily doses of tapentadol ≥300 mg without significant side effects.
The use of other analgesics was relatively low at W0: 0 patients were using NSAIDs, 7 (28%) used acetaminophen, 6 (24%) used anticonvulsants, and 5 (20%) antidepressants. Most patients reduced their use during the study period. Acetaminophen, anticonvulsants, and antidepressants were discontinued respectively by 5 (71.4%), 5 (83.3%), and 4 (80%) patients during the observed period (Table 4). The percentage of patients taking laxatives reduced from 16% at W0 to 9.1% at W12. No patients required antiemetics.
No difference in the use of other drugs, specifically prescribed by hematologists for MM (such as corticosteroids, bisphosphonates, radiotherapy, and chemotherapy), was observed during the study period.
Discussion
The present study evaluated tapentadol PR with starting doses of 100 mg/day in opioid-naïve patients with MBD, suffering from moderate-to-severe cancer pain. Pain intensity significantly decreased after the first week of treatment, and adequate pain relief was maintained during the entire study period. The dose of tapentadol PR, given according to the patients’ clinical response, slowly increased over the first 4 weeks to a stable daily dose of over 200 mg in the following 2 months.
Although data on tapentadol PR in cancer patients are not extensive, the doses used in this study were similar to those in previous studies with a similar design in cancer patients.13,29
The reduced tendency for dose escalation during the study is possibly due to the pharmacological characteristics of the tapentadol molecule, which has a dual mechanism of analgesic action. For this reason, tapentadol PR seems to be particularly appropriate for patients with mixed pain including a neuropathic component.30
In our study, the DN4 score significantly reduced from W0 to the 12-week evaluation, and after 8 weeks of treatment, no patients presented a positive neuropathic component.
The identification of a neuropathic pain component in chronic cancer pain may be a particularly important indicator regarding optimal choice of treatment strategy for patients who may experience neuropathic pain in addition to nociceptive pain.27
Cancer patients are currently treated mainly with traditional strong opioids according to the World Health Organization analgesic ladder. However, it is well known that neuropathic pain is relatively poorly responsive to MOR agonists. Osteolytic lesions are the most common feature of MM and give rise to multiple sources and mechanisms of pain. Because sensory and sympathetic neurons are present within the bone marrow, mineralized bone, and periosteum, and all these compartments are ultimately impacted by fractures or by the presence of tumor cells, sensory fibers in any of these tissues may play a role in the generation and maintenance of neuropathic bone cancer pain.31,32 In particular, sensory nerve fibers that innervate the tumor-induced bone lesions undergo a pathological sprouting and reorganization, which likely generate and maintain chronic pain. Preventive treatments, if they can be taken, may block this ectopic sprouting and attenuate cancer pain.33
In light of prior findings, we can suggest that bone cancer pain is driven by a neuropathic pain component. In our study, indeed, over 70% of patients had a neuropathic pain component at W0. At W8, all patients were negative, suggesting the strong efficacy of tapentadol PR in the management of this neuropathic pain component. Tapentadol PR was equally effective when assessed by clinical judgment or by tools commonly used for assessing predominantly neuropathic pain, such as the DN4 score. In other forms of neuropathic pain, such as in patients with lower back pain34 and painful diabetic peripheral neuropathy,12,35 tapentadol PR was effective in reducing neuropathic pain symptoms. In chronic pain patients with diabetic polyneuropathy, the analgesic effect of tapentadol PR was shown to be dependent on the activation of descending inhibitory pain pathways, as observed by conditioned pain modulation (an experimental measure of endogenous pain inhibition that gates incoming pain signals as a consequence of a preceding tonic painful stimulus) responses.36
Tapentadol PR was well tolerated in this study, as only one patient discontinued due to adverse events. The occurrence of expected opioid-related symptoms was extremely low and, when present, their intensity was mild. Most of these effects resolved over time.
Symptoms observed at W0, such as constipation and dry mouth, were not opioid-related symptoms, as patients were opioid-naïve, but they were part of patients’ medical history. The reason for the significant drop in constipation observed from W0 (32%) to W12 (9.1%) is unclear. However, it is reasonable to hypothesize a key role of patients’ education (adequate fiber and fluid intake, healthy toileting habits), lifestyle modification (increased mobility, moderate physical daily activity), and the choice of tapentadol PR, well known for its better gastrointestinal tolerability compared with traditional opioids. Notably, patients did not need antiemetics during tapentadol PR therapy. The use of corticosteroids could have been a confounding factor on the absence of nausea and vomiting, but they were used in repeated cycles as part of the hematological protocols for MM. The low dropout rate suggests that tapentadol PR may be of particular benefit to opioid-naïve patients or in frailty patients, for example, in the elderly.
Recently, in a multicenter study conducted on about 500 patients suffering from chronic cancer pain, tapentadol PR has been shown to provide comparable efficacy to that of CR morphine sulphate, with a better tolerability profile.15 Similarly, pain relief from tapentadol PR was noninferior to that achieved with CR oxycodone, with fewer gastrointestinal side effects.16
A recent study showed that the QoL of symptomatic patients with MM may be significantly affected by the side effects of analgesics, particularly by opioids.37 The good tolerability of tapentadol PR, probably related to its lower affinity for the MOR, would be clinically relevant in patients who have to be treated for lengthy periods.38 The low incidence of side effects, including in the first phase of treatment (during titration), is critical for improving patients’ adherence and acceptance of the proposed treatment. That tapentadol PR was well tolerated in our study is consistent with the results of other trials conducted in patients with neuropathic pain.12,34,35 Moreover, other studies suggest a better gastrointestinal tolerability, in terms of nausea and constipation, compared with the combination oxycodone/naloxone, which is specifically designed to limit opioid-induced constipation.39–41
Finally, an important finding was that the QoL score was significantly improved after the initiation of tapentadol PR therapy, and the use of adjuvant drugs administered prior to entering the study was reduced during the study period, confirming the efficacy of tapentadol PR.
To our knowledge, this study was the first to be performed in patients with MM. Therefore, although the relatively low number of patients participating in this study and the uncontrolled design do not allow definitive conclusions, and the findings require confirmation in controlled studies with a larger number of patients, our quest to identify subjects who could best benefit from tapentadol PR, nevertheless, demonstrated that tapentadol PR can provide effective and safe analgesia in patients with MBD suffering from moderate-to-severe chronic cancer pain.
Conclusion
Tapentadol PR started in doses of 100 mg/day was effective and well tolerated in opioid-naïve patients with MBD suffering from moderate-to-severe chronic cancer pain. Most patients were responsive at a mean stable dose of 200 mg/day, suggesting only a slow development of analgesic tolerance. Eight weeks after starting tapentadol PR, the neuropathic component of pain was eliminated in all the treated patients. QoL was significantly improved in both physical and mental status after 12 weeks of treatment.
Disclosure
FC, RBR, JP, and CM are speakers, consultants, and/or basic science investigators for several pharmaceutical companies involved in analgesic research, but they receive no royalty (cash or otherwise) from the sale of any product. FC serves as a speaker, consultant, and researcher for Grunenthal, Angelini, Mundipharma, and Prostrakan. RBR was previously employed by Johnson & Johnson, was on the scientific advisory board of Pain Therapeutics, and was a scientific consultant for Adolor, Alteon, Ampio, Asta Medica, Depomed, Discovery Research Consultants, Endo, Galleon, Grünenthal, Inspirion, Iroko, Johnson & Johnson, Kirax, LaboPharm, LAPID, Mallinckrodt/Covidien, Novartis, Onconova, Pfizer, Purdue Pharma, Trevena, and Vyrix. JP serves as a speaker, consultant, and researcher for Grunethal, Mundi Pharma, Purdue, Baxter, Depomed, Jansen, Inspirion, and Astra Zeneca. CM serves as a speaker, consultant, and researcher for Grunenthal, Angelini and Cephalon. AR, PL, NC, and GC disclose they have no financial relationships that could have influenced the manuscript. The authors report no other conflicts of interest in this work.
References
Terpos E, Berenson J, Raje N, Roodman GD. Management of bone disease in multiple myeloma. Expert Rev Hematol. 2014;7:113–125. | |
Hameed A, Brady JJ, Dowling P, Clynes M, O’Gorman P. Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastasis. 2014;10:33–42. | |
Kaur P, Shah BS, Baja P. Multiple myeloma: a clinical and pathological profile. Gulf J Oncolog. 2014;1:14–20. | |
Coluzzi F, Mandatori I, Mattia C. Emerging therapies in metastatic bone pain. Expert Opin Emerg Drugs. 2011;16:441–458. | |
Coluzzi F, Di Bussolo E, Mandatori I, Mattia C. Bone metastatic disease: taking aim at new therapeutic targets. Curr Med Chem. 2011;18:3093–3115. | |
Coluzzi F, Berti M. Change pain: changing the approach to chronic pain. Minerva Med. 2011;102:289–307. | |
Wade WE, Spruill WJ. Tapentadol hydrochloride: a centrally acting oral analgesic. Clin Ther. 2009;31:2804–2818. | |
Schröder W, Vry JD, Tzschentke TM, Jahnel U, Christoph T. Differential contribution of opioid and noradrenergic mechanisms of tapentadol in rat models of nociceptive and neuropathic pain. Eur J Pain. 2010;14:814–821. | |
Christoph T, De Vry J, Tzschentke TM. Tapentadol, but not morphine, selectively inhibits disease-related thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Neurosci Lett. 2010;470:91–94. | |
Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled phase III study. Expert Opin Pharmacother. 2010;11:1787–1804. | |
Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30:489–505. | |
Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin. 2011;27:151–162. | |
Mercadante S, Porzio G, Ferrera P, et al. Tapentadol in cancer pain management: a prospective open-label study. Curr Med Res Opin. 2012;28:1775–1779. | |
Mercadante S, Porzio G, Adile C, et al. Tapentadol at medium to high doses in patients previously receiving strong opioids for the management of cancer pain. Curr Med Res Opin. 2014;30:2063–2068. | |
Kress HG, Koch ED, Kosturski H, Steup A, Karcher K. Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain Physician. 2014;17(4):329–343. | |
Imanaka K, Tominaga Y, Etropolski M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr Med Res Opin. 2013;29(10):1399–1409. | |
Coluzzi F, Ruggeri M. Clinical and economic evaluation of tapentadol extended release and oxycodone/naloxone extended release in comparison with controlled release oxycodone in musculoskeletal pain. Curr Med Res Opin. 2014;30:1139–1151. | |
Wild JE, Grond S, Kuperwasser B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010;10:416–427. | |
Muller-Schwefe G, Ahlbeck K, Aldington D, et al. Pain in the cancer patient: different pain characteristics CHANGE pharmacological treatment requirements. Curr Med Res Opin. 2014;30:1895–1908. | |
Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol. 2014;32:1647–1654. | |
Brunelli C, Bennett MI, Kaasa S, et al. Classification of neuropathic pain in cancer patients: a Delphi expert survey report and EAPC/IASP proposal of an algorithm for diagnostic criteria. Pain. 2014;155:2707–2713. | |
Bennett MI, Rayment C, Hjermstad M, et al. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain. 2012;153:359–365. | |
Hughes SW, Hickey L, Hulse RP, Lumb BM, Pickering AE. Endogenous analgesic action of the pontospinal noradrenergic system spatially restricts and temporally delays the progression of neuropathic pain following tibial nerve injury. Pain. 2013;154:1680–1690. | |
Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 2013;716:2–7. | |
Nakajima K, Obata H, Iriuchijima N, Saito S. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain. 2012;153:990–997. | |
Van Seventer R, Vos C, Meerding W, et al. Linguistic validation of the DN4 for use in international studies. Eur J Pain. 2010;14:58–63. | |
Pérez C, Sànchez-Martinez N, Ballesteros A, et al. Prevalence of pain and relative diagnostic performance of screening tools for neuropathic pain in cancer patients: a cross-sectional study. Eur J Pain. Epub September 30, 2014. | |
Apolone G, Mosconi P. The Italian SF-36 health survey translation, validation and norming. J Clin Epidemiol. 1998;51:1025–1036. | |
Mercadante S, Porzio G, Aielli F, et al. Opioid switching from and to tapentadol extended release in cancer patients: conversion ratio with other opioids. Curr Med Res Opin. 2013;6:661–666. | |
Bee LA, Bannister K, Rahman W, Dickenson AH. Mu-opioid and noradrenergic a2-adrenoreceptor contributions to the effects of tapentadol on spinal electrophysiological measures of nociception in nerve-injured rats. Pain. 2011;152:131–139. | |
Jimenez-Andrade JM, Mantyh WG, Bloom AP, et al. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–181. | |
Peters CM, Ghilardi JR, Keyser CP, et al. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. 2005;193:85–100. | |
Jimenez-Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30:14649–14656. | |
Baron R, Kern U, Muller M, et al. Effectiveness and tolerability of a moderate dose of tapentadol prolonged release for managing severe, chronic low back pain with a neuropathic component: an open-label continuation arm of a randomized phase 3b study. Pain Pract. Epub April 18, 2014. | |
Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37:2302–2309. | |
Niesters M, Proto PL, Aarts L, et al. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth. 2014;113:148–156. | |
Sloot S, Boland J, Snowden JA, et al. Side effects of analgesia may significantly reduce quality of life in symptomatic multiple myeloma: a cross-sectional prevalence study. Support Care Cancer 2015;23:671–678. | |
Coluzzi F, Pergolizzi J, Raffa RB, Mattia C. The unsolved case of “bone-impairing analgesics”: the endocrine effects of opioids on bone metabolism. Ther Clin Risk Manag. 2015;2015:515–523. | |
Baron R, Schwittay A, Binder A, et al. Effectiveness of tapentadol prolonged release (PR) versus oxycodone/naloxone PR for severe chronic low back pain with a neuropathic pain component. Poster presented at: Pain Week 2014, September 2–6, 2014, Las Vegas, NV. | |
Binder A, Baron R, Schwittay A, et al. Safety and tolerability of tapentadol prolonged release (PR) versus oxycodone/naloxone PR for severe chronic low back pain with a neuropathic pain component. Poster presented at: Pain Week 2014, September 2–6, 2014, Las Vegas, NV. | |
Schwittay A, Baron R, Binder A, et al. Effects of tapentadol prolonged release (PR) versus oxycodone/naloxone PR on quality of life and function measures in patients with severe chronic low back pain with a neuropathic pain component. Poster presented at: Pain Week 2014, September 2–6, 2014, Las Vegas, NV. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.