Back to Journals » Journal of Pain Research » Volume 12
Tapentadol in the treatment of osteoarthritis: pharmacological rationale and clinical evidence
Authors Rinonapoli G , Coaccioli S, Panella L
Received 8 October 2018
Accepted for publication 27 February 2019
Published 16 May 2019 Volume 2019:12 Pages 1529—1536
DOI https://doi.org/10.2147/JPR.S190161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Giuseppe Rinonapoli,1 Stefano Coaccioli,2 Lorenzo Panella3
1Dipartimento di Scienze Chirurgiche, s.c. Ortopedia e Traumatologia Università di Perugia, Ospedale S. Maria della Misericordia, 06100 Perugia, Italy; 2Department of Medicine, Sezione di Clinica Medica e Anatomia Patologia, Terni, Italy; 3Rehabilitation Department, ASST Pini-CTO, Milan, Italy
Abstract: Osteoarthritis (OA) is the most prevalent joint disease in older people worldwide. Pain owing to OA is considered one of the most frequent causes of chronic pain; however, current pharmacological approaches have some limitations in terms of efficacy and safety. Of note, descending inhibitory pain pathways are often disrupted in chronic OA pain, and pharmacotherapies targeting those pathways – eg, those that block norepinephrine reuptake may be more appropriate for managing chronic pain than pure μ-opioid receptor (MOR) agonists. Tapentadol is an analgesic molecule, which combines two synergistic mechanisms of action, MOR, and norepinephrine reuptake inhibition. This narrative review will briefly discuss the mechanisms contributing to the onset and maintenance of pain in OA patients; clinical data on the use of tapentadol in this setting will then be presented and commented.
Keywords: osteoarthritis, pain, tapentadol
Introduction
Osteoarthritis (OA) is the most prevalent joint disease in older people worldwide, with the knee and hips being the most affected joints, although hand OA is sometimes reported.1 It is characterized by the progressive destruction of articular cartilage, synovial inflammation, changes in subchondral bone and peri-articular muscle, and pain.2 In particular, pain due to OA is considered one of the most frequent causes of chronic pain:3,4 the prevalence of radiographic knee OA is estimated to be up to 28%.5
The management of OA may require three lines of pharmacological treatment in order to: 1) manage inflammation, only when it is present in its flares; 2) provide central analgesia, by modulating both ascending and descending pathways; and 3) prevent further joint destruction (which is a generator of peripheral pain), eg, by using condroprotectors.6,7
In particular, the treatment of pain is crucial to the management of the OA patient; however, current pharmacological approaches, including paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs), have some limitations in terms of efficacy and safety, limiting them to short-term use.8–12 Another option is represented by tramadol.13 Opioid analgesics may relieve OA pain, but are associated with well-known safety concerns.14,15 Remarkably, patients with OA are often submitted to total knee or hip arthroplasty if their pain is not well-controlled.16 However, patients still experience marked pain while in the waiting list for surgery and even after joint replacement.17
Of note, descending inhibitory pain pathways are disrupted in chronic OA pain, and pharmacotherapies targeting those pathways – eg, those that block norepinephrine reuptake may be more appropriate for managing chronic pain compared with pure μ-opioid receptor (MOR) agonists.18 Tapentadol is a dual-acting analgesic molecule, which combines two mechanisms of action, MOR agonism and norepinephrine reuptake inhibition (NRI).19,20 This narrative review will briefly discuss the mechanisms contributing to the onset and maintenance of pain in OA patients; clinical data on the use of tapentadol in this setting will then be presented and commented.
Mechanisms of pain onset in OA
OA pain can be considered a “mixed” pain state.21 Indeed, all structures of the joints, with the exception of the cartilage, are innervated by nociceptors, and hence structural alterations do stimulate pain.4 Although OA pain presents a wide heterogeneity,12,22 neuropathic mechanisms are also involved, since structural changes of joint innervation, such as local loss and/or sprouting of nerve fibers are common in OA. In addition, central sensitization, reduction of descending inhibition, descending excitation, and cortical atrophies were observed in OA;23 all these mechanisms contribute to transition to chronic pain and enhanced severity.12,21 Therefore, central analgesia becomes crucial to avoid the establishment of neuroplasticity phenomena leading to chronic pain.24,25 Owing to the chronic pain associated with functional limitation, patients may ultimately need to undergo knee or hip replacements. In most cases, the waiting period prior to surgery is characterized by moderate–severe pain, which limits the patients’ function and activity. Control of pain is also important postoperatively, since severe pain is associated with longer hospitalization, poor compliance with the rehabilitation program, a delay in starting to perform daily activities and an increase in postoperative complications.26,27
On these bases, a molecule able to act both on the nociceptive and the neuropathic components of pain can be highly effective in the treatment of OA pain. Tapentadol meets these requirements.28 Indeed, the pharmacological profile of tapentadol, combining synergistically MOR agonism and NRI in one molecule, appears to be unique and it seems reasonable to propose for tapentadol a new class of centrally acting analgesics, designated MOR-NRI,19 and can be considered an a priori choice for the treatment of chronic, neuropathic, and mixed pain.29
Tapentadol in the treatment of OA pain: clinical data
Solid clinical data support the efficacy and safety of tapentadol in the treatment of OA-associated pain, in line with its pharmacological rationale. Studies on tapentadol in this indication encompass both the non-surgical (Table 1) and the surgical setting; some pieces of evidence also support the use of tapentadol prolonged release (PR) in the rehabilitation setting.
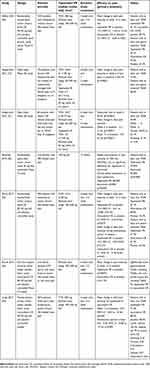 | Table 1 Key elements from clinical trials on tapentadol PR in the treatment of OA-related pain in the non-surgical setting |
Non-surgical setting
In a randomized, double-blind study, Afilalo et al evaluated the efficacy and safety of tapentadol PR compared with oxycodone controlled release (CR) in the management of moderate-to-severe chronic OA-related knee pain.30 In total, 1,030 patients receive tapentadol PR 100–250 mg twice daily, oxycodone HCl CR 20–50 mg twice daily, or placebo for a 3-week titration period followed by a 12-week maintenance period. Tapentadol PR significantly reduced pain intensity from baseline to week 12 of the maintenance period versus placebo and throughout the maintenance period. On the other hand, oxycodone CR significantly reduced average pain intensity from baseline throughout the maintenance period versus placebo but not at week 12. A higher percentage of patients achieved ≥50% improvement in pain intensity with tapentadol PR (32.0% [110/344]) compared with placebo (32.0 vs 24.3%; p=0.027), while a significantly lower percentage of patients achieved this goal in the oxycodone CR group (17.3%). Incidence of gastrointestinal events was 26.1% with placebo, 43.0% with tapentadol and 67.3% with oxycodone.
In a subsequent open-label, Phase IIIb study, Steigerwald et al evaluated the effectiveness and tolerability of tapentadol PR for severe, chronic OA knee pain inadequately managed or left untreated.14 In total, 195 patients received tapentadol PR (50–250 mg bid) for a 5-week titration period, followed by a 7-week maintenance period. The mean change from baseline to week 6 in pain intensity was −3.4±2.10 (p<0.0001). Significant decreases in pain intensity were also observed at weeks 6, 8, and 12.14 Improvements from baseline to weeks 6 and 12 were observed in the Western Ontario and McMaster Universities OA index,14 the EuroQol-5 Dimension health status questionnaire, the Short Form-36 health survey, and the Hospital Anxiety and Depression Scale. The same group evaluated the effectiveness and tolerability of tapentadol PR (50–250 mg twice daily) after rotation from WHO step III opioids in patients with severe OA knee pain who poorly tolerated this latter therapy.31 Patients received oral tapentadol PR (50–250 mg twice daily) over a 5-week titration and a 7-week maintenance period. In total, 63 patients received tapentadol PR. The responder rate (ie, patients with reduced pain intensity compared with baseline) at week 6 was 94.3%; mean pain intensity was 4.7±0.66 at baseline, 2.5±1.46 at week 6, and 1.8±1.4 at week 12 (p<0.0001 for all comparisons).31 Prevalence of adverse events (AEs) reported as associated with prior opioids and related to tapentadol treatment at week 12 decreased over time; the most frequent AEs were nausea (46.0 vs 24.1%) and constipation (31.7 vs 7.4%).
In a comparative, randomized, open labeled, controlled study by Banerjee et al, patients received either tapentadol (100 mg twice daily; n=108) or etoricoxib (30 mg twice daily; n=110) for 12 weeks.32 Steady improvement was seen in pain intensity VAS and WOMAC scores31 in both groups; moreover, a higher number of patients reported at least satisfactory response at the end of the study in the tapentadol group (p=0.036). AEs were less frequent with tapentadol PR (incidence: 37% vs 49%) with the most common events being nausea (22%) and dizziness (13%) with tapentadol and nausea (20%) and dyspepsia (15%) with etoricoxib.
Serrie et al also conducted a double-blind, placebo or oxycodone CR (20–50 mg bid)-controlled trial to assess the efficacy and safety of tapentadol PR (100–250 mg bid) administered for a 3-week titration and 12-week maintenance period in 990 patients with moderate-to-severe OA-related knee pain. The study did not meet its primary endpoints as both tapentadol and oxycodone did not significantly reduce pain intensity vs placebo after 12 weeks of treatment, nor over the maintenance period. However, the study did not demonstrate assay sensitivity and the finding that both primary end-points for tapentadol PR were not met therefore cannot be interpreted. The overall health status of patients treated with tapentadol was superior to that of patients treated with oxycodone; indeed, more patients in the tapentadol arm completed the study and the percentage of patients that rated, at least, “much improved” at the end of the study was higher (56% vs 42.5% for oxycodone). Tapentadol also showed a better tolerability profile with significantly reduced incidence of constipation (17.9% vs 35% for oxycodone) and of the composite of nausea and/or vomiting (23.8% vs 46.8%).33
Some retrospective studies have also investigated the efficacy and safety of tapentadol.
In a post-hoc analysis of pooled data, Biondi et al specifically evaluated the tolerability and analgesic efficacy of tapentadol PR compared with oxycodone CR in 210 elderly adult patients (≥75 years) with moderate-to-severe pain due to OA of knee or low back pain.34 Each study consisted of a 3-week titration and 12-week maintenance period, and patients received placebo, tapentadol PR (100–250 mg bid), or oxycodone CR (20–50 mg bid) for 15 weeks. Overall, the incidences of gastrointestinal treatment-emergent AEs overall and those of nausea/vomiting were significantly lower with tapentadol PR compared with oxycodone CR group (all p≤0.0206). Moreover, tapentadol extended release treatment determined a significant reduction in pain intensity from baseline to week 15 compared with placebo (p=0.0075), while the difference between the oxycodone CR and placebo group did not reach significance, likely due to a higher treatment discontinuation rate in the oxycodone CR group.
In addition, a pooled analysis of two randomized, double-blind, controlled studies conducted by Lange et al, suggested that tapentadol PR is superior to oxycodone in providing pain relief and improving overall health status in patients with moderate-to-severe chronic OA-related knee pain.35 Both studies consisted in a 3-week titration +12-week maintenance period, and patients were randomized to tapentadol PR (100–250 mg bid), oxycodone CR (20–50 mg bid), or placebo. Tapentadol treatment resulted in a more significant reduction in average pain intensity compared with oxycodone, both after 12 weeks of treatment (mean difference −0.41 [95% CI: −0.65 to −0.16], p=0.001) and over the maintenance period (−0.35 [95% CI: −0.58 to −0.12], p=0.003). Patients’ global impression of change measured by the Short Form-36 score and EuroQoL-5Dimensions health status index were also significantly higher for tapentadol vs oxycodone (p<0.001 for all). In terms of safety, treatment with tapentadol led to a reduced relative risk of vomiting, constipation, nausea, somnolence and pruritus, and to less cases of treatment discontinuation (42.2 vs 64% for oxycodone).
Surgical setting
Hartrick et al assessed the efficacy and tolerability of tapentadol immediate release (IR) in a 10-day randomized, double-blind, active (oxycodone IR) and placebo-controlled trial in patients candidate for joint replacement surgery.36 In total, 659 subjects were evaluated for efficacy. Tapentadol IR (50 and 75 mg) and oxycodone HCl IR (10 mg) were associated with significant reductions in pain intensity compared with placebo, at 2, 5, and 10 days after surgery; however, the incidence of gastrointestinal AEs was significantly lower for both doses of tapentadol IR compared with oxycodone HCl IR 10 mg. Rates of treatment discontinuation were 18% in the tapentadol IR 50-mg group, 26% in the tapentadol IR 75-mg group, 35% in the oxycodone HCl IR 10-mg group, and 10% in the placebo group. A similar 7-day study reached the same conclusions, and tapentadol was also associated with greater overall improvement as assessed by both patients and clinicians.37
The PR formulation of tapentadol in this setting has been evaluated by Haeseler et al, who conducted a randomized, observer-blinded, active-controlled (oxycodone/naloxone) trial in patients following orthopedic/trauma surgery.38 In total, 133 patients received tapentadol and an equal number oxycodone. Mean pain levels in the first 5 postoperative days were 2.8±1.3 in both groups. Overall, the two treatments showed comparable analgesic efficacy and a similar tolerability profile.
Rehabilitation setting
To date, attention has focused on analgesia in the preoperative period, while studies on the rehabilitation period are scant.39 However, pain control continues to be very important in the rehabilitation phase, since high-intensity pain during rehabilitation is associated with longer hospital stay and poor compliance with rehabilitation protocols, with marked consequences on QoL.
In a still-unpublished open-label study, Rinonapoli et al evaluated 49 patients waiting for knee replacement treated with tapentadol.40 In all these patients, the pain numerical rating scale (NRS) was ≥6. The initial dose of tapentadol was always 50 mg twice daily. The dose was increased to 100 mg twice daily after 4–5 days and could be further increased according to clinical needs. Most patients, 31 (63.2%) found sufficient benefit from the therapy at a dose of 300 mg/day. Mean NRS at baseline was 8.35, and it decreased to 5.33 at surgery, 4.96 15 days after surgery and 2.15 at 40 days. Sleep quality also improved increased. The average time to reach the best postoperative scores was 23.9 days.
In the pure rehabilitation setting, Panella et al conducted a 3-week, open study to assess the analgesia and tolerability of tapentadol PR (50–150 mg twice daily; n=91) compared with paracetamol 1000 mg bid (n=53), in patients in rehabilitation after knee replacement surgery and moderate-to-severe pain.39 During the study, more favorable progress was observed with tapentadol PR: in particular, pain, range of motion, and sleep quality showed a faster improvement in the patients treated with tapentadol PR (p<0.01 vs paracetamol). At the end of the study, the pain intensity reduced by 4.3 points with tapentadol PR versus 2.4 with paracetamol.
Conclusion
OA is a common disease of aged population and one of the leading causes of disability worldwide, associated with marked pain in most patients. Proper control of pain is crucial in OA, also in order to guarantee functional recovery and improve quality of life. In the surgical setting, control of pain allows improved surgical outcomes. Therefore, most guidelines on knee OA, drew up by the most authoritative international societies (AAOS, OARSI, ACR),11,41 dedicate a specific section to the pharmacological treatment of OA-associated pain, but point out that current therapies for this condition present a number of drawbacks including modest efficacy and poor safety, especially over the long term. Selection of treatment should take into consideration the mechanism of action of the analgesic therapy, which should be able to address both the nociceptive and the neuropathic components.
Remarkably, tapentadol was not considered in those guidelines, partly due to its more recent introduction in the pharmacological armamentarium for OA compared with other therapies. However, current data on the efficacy of tapentadol, especially in its PR formulation, are robust and were collected, in most cases, from well-designed studies in the non-surgical, surgical, and rehabilitation settings. Noteworthy, all studies pointed out the favorable safety profile of tapentadol, a finding of major importance in the long-term therapy. The efficacy and safety of tapentadol PR in this setting were consistent regardless of patients’ age.
Most of the published studies have included either oxycodone or paracetamol as comparators. To our knowledge, no study has directly compared tapentadol PR with tramadol; owing to the lack of head-to-head comparisons, we can speculate that the higher risk of AEs and pharmacological interactions with tramadol.42 Similarly, only one trial has compared tapentadol with a NSAIDs (etoricoxib),32 showing more pronounced efficacy and improved safety with tapentadol. Given the lack of other direct comparisons, it is not possible to know whether this improved efficacy and safety of tapentadol can be extended to other NSAIDs commonly used in the treatment of OA (eg , ibuprofen, diclofenac).
On these bases, we believe that tapentadol PR can be considered a first-line choice in the treatment of OA-associated pain and future guidelines should include this therapy among the recommended pharmacological therapies.
Key points
- OA is the leading causes of disability worldwide, associated with marked pain in most patients. Proper control of pain is crucial in OA, also in order to guarantee functional recovery and improve quality of life. In the surgical setting, control of pain allows improved surgical outcomes.
- Selection of pain treatment should take into consideration the mechanism of action of the analgesic therapy, which should be able to address both the nociceptive and the neuropathic components; moreover, it should avoid the phenomenon of central sensitization.
- Current data on the efficacy of tapentadol for the treatment of OA, especially in its PR formulation, are robust and were collected, in most cases, from well-designed studies in the non-surgical, surgical, and rehabilitation settings.
- All studies pointed out the favorable tolerability profile of tapentadol, a finding of major importance in the long-term therapy.
Acknowledgments
Editorial assistance was provided by Luca Giacomelli, PhD, Ambra Corti, and Aashni Shah. This assistance and fees for publications were supported by Grunenthal.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wenham CY, Conaghan PG. New horizons in osteoarthritis. Age Ageing. 2013;42(3):272–278. doi:10.1093/ageing/aft043
2. Kim JR, Yoo JJ, Kim HA. Therapeutics in osteoarthritis based on an understanding of its molecular pathogenesis. Int J Mol Sci. 2018;19(3):E674. doi:10.3390/ijms19030674
3. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi:10.1016/j.ejpain.2005.06.009
4. Eitner A, Hofmann GO, Schaible HG. Mechanisms of osteoarthritic pain. studies in humans and experimental models. Front Mol Neurosci. 2017;10:349. doi:10.3389/fnmol.2017.00349
5. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–1153. doi:10.1016/j.joca.2013.03.018
6. Cohen E, Lee Y. A mechanism-based approach to the management of osteoarthritis pain. Curr Osteoporos Rep. 2015;13:399–406. doi:10.1007/s11914-015-0291-y
7. Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014;10(6):374–380. doi:10.1038/nrrheum.2014.47
8. Saragiotto BT, Machado GC, Ferreira ML, Pinheiro MB, Abdel Shaheed C, Maher CG. Paracetamol for low back pain. Cochrane Database Syst Rev. 2016;(6):CD012230. doi:10.1002/14651858.CD012230
9. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi:10.1136/bmj.h1225
10. Mammucari M, Gigliotti S, Pucino A, Capezza M, Santé G,
11. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi:10.1016/j.joca.2014.01.003
12. Dell’Isola A, Steultjens M. Classification of patients with knee osteoarthritis in clinical phenotypes: data from the osteoarthritis initiative. PLoS One. 2018;13(1):e0191045. doi:10.1371/journal.pone.0191045
13. Raffa RB. Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin Pharmacother. 2012;13(10):1437. doi:10.1517/14656566.2012.696097
14. Steigerwald I, Müller M, Kujawa J, Balblanc JC, Calvo-Alén J. Effectiveness and safety of tapentadol prolonged release with tapentadol immediate release on-demand for the management of severe, chronic osteoarthritis-related knee pain: results of an open-label, phase 3b study. J Pain Res. 2012;5:121–138. doi:10.2147/JPR.S30540
15. Nuesch E, Rutjes AW, Husni E, Welch V, Juni P. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115. doi:10.1002/14651858.CD003115.pub3
16. Gademan MG, Hofstede SN, Vliet Vlieland TP, Nelissen RG. Marang-van de Mheen PJ. Indication criteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview. BMC Musculoskelet Disord. 2016;17(1):463. doi:10.1186/s12891-016-1134-4
17. Simões JL, Soares S, Sa-Couto P, et al. The influence of presurgical factors on the rehabilitation outcome of patients following hip arthroplasty. Rehabil Nurs. 2018:1. doi:10.1097/rnj.0000000000000126.
18. Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–581. doi:10.1016/j.pain.2010.04.003
19. Kress HG. Tapentadol and its two mechanisms of action: is there a new pharmacological class of centrally-acting analgesics on the horizon? Eur J Pain. 2010;14(8):781–783. doi:10.1016/j.ejpain.2010.06.017
20. Tzschentke TM, Jahnel U, Kogel B, et al. Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today (Barc). 2009;45:483–496.
21. Clauw DJ, Hassett AL. The role of centralised pain in osteoarthritis. Clin Exp Rheumatol. 2017;35 Suppl 107(5):79–84.
22. Dell’Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord. 2016;17(1):425. doi:10.1186/s12891-016-1134-4
23. Ghilardi JR, Freeman KT, Jimenez-Andrade JM, et al. Neuroplasticity of sensory and sympathetic nerve fibers in the painful arthritic joint. Arthritis Rheum. 2012;64:2223–2232. doi:10.1002/art.34385
24. Coluzzi F, Fornasari D, Pergolizzi J, Romualdi P. From acute to chronic pain: tapentadol in the progressive stages of this disease entity. Eur Rev Med Pharmacol Sci. 2017;21(7):1672–1683.
25. Morlion B, Coluzzi F, Aldington D, et al. Pain chronification: what should a non-pain medicine specialist know? Curr Med Res Opin. 2018;34(7):1169–1178. doi:10.1080/03007995.2018.1449738
26. Cherubino P. La gestione del dolore persistente in ortopedia [Management of persistent pain in orthopedics]. Minerva Ortop Traumatol. 2012;63(6):471–477. Italian.
27. Scardino M. La gestione del dolore muscoloscheletrico in riabilitazione ortopedica: focus su tapentadolo [Management of musculoskeletal pain in orthopedic rehabilitation: focus on tapentadol]. Cap. 2014;13. Italian.
28. Tzschentke TM, Folgering JH, Flik G, De Vry J. Tapentadol increases levels of noradrenaline in the rat spinal cord as measured by in vivo microdialysis. Neurosci Lett. 2012;507(2):151–155. doi:10.1016/j.neulet.2011.12.008
29. Langford RM, Knaggs R, Farquhar-Smith P, Dickenson AH. Is tapentadol different from classical opioids? A review of the evidence. Br J Pain. 2016;10(4):217–221. doi:10.1177/2049463716657363
30. Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30(8):489–505. doi:10.2165/11533440-000000000-00000
31. Steigerwald I, Schenk M, Lahne U, Gebuhr P, Falke D, Hoggart B. Effectiveness and tolerability of tapentadol prolonged release compared with prior opioid therapy for the management of severe, chronic osteoarthritis pain. Clin Drug Investig. 2013;33(9):607–619. doi:10.1007/s40261-013-0102-0
32. Banerjee M, Mondal S, Sarkar R, Mondal H, Bhattacharya K. Comparative study of efficacy and safety of tapentadol versus etoricoxib in mild to moderate grades of chronic osteorthritis of knee. Indian J Rheumatol. 2016;11(1):21–25. doi:10.1016/j.injr.2015.12.001
33. Serrie A, Lange B, Steup A. Tapentadol prolonged-release for moderate-to-severe chronic osteoarthritis knee pain: a double-blind, randomized, placebo- and oxycodone controlled release-controlled study. Curr Med Res Opin. 2017;33(8):1423–1432. doi:10.1080/03007995.2017.1335189
34. Biondi DM, Xiang J, Etropolski M, Moskovitz B. Tolerability and efficacy of tapentadol extended release in elderly patients ≥75 years of age with chronic osteoarthritis knee or low back pain. J Opioid Manag. 2015;11(5):393–403. doi:10.5055/jom.2015.0289
35. Lange B, von Zabern D, Elling C, Dubois C. Efficacy and safety of tapentadol prolonged release for moderate-to-severe chronic osteoarthritis knee pain: a pooled analysis of two double-blind, randomized, placebo- and oxycodone controlled release-controlled studies. Curr Med Res Opin. 2017;33(8):1413–1422. doi:10.1080/03007995.2017.1335188
36. Hartrick C, Van Hove I, Stegmann JU, Oh C, Upmalis D. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10-day, phase III, randomized, double-blind, active- and placebo-controlled study. Clin Ther. 2009;31(2):260–271. doi:10.1016/j.clinthera.2009.02.009
37. Vorsanger GJ, Klopfer AM, Xiang J, Benson CJ, Moskovitz BL, Rosenthal NR. Immediate-release tapentadol or oxycodone for treatment of acute postoperative pain after elective arthroscopic shoulder surgery: a randomized, phase IIIb study. J Opioid Manag. 2013;9(4):281–290. doi:10.5055/jom.2013.0170
38. Haeseler G, Schaefers D, Prison N, Ahrens J, Liu X, Karch A. Combatting pain after orthopedic/trauma surgery- perioperative oral extended-release tapentadol vs. extended-release oxycodone/naloxone. BMC Anesthesiol. 2017;17(1):91. doi:10.1186/s12871-017-0383-6
39. Panella L, Caserta AV, Ballarati R, Lopresti M, Parravicini L. Control of post-operative pain and rehabilitation compliance of patients undergoing knee replacement. Clinical Practice (Therapy). 2016;13:2.
40. Rinonapoli G, Caraffa A Tapentadolo PR in pazienti in attesa di protesizzazione di ginocchio: valutazione pre e post intervento. 101° Congresso SIOT Torino, Italy, October 28–31, 2016.
41. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:577–579. doi:10.5435/JAAOS-21-09-577
42. Faria J, Barbosa J, Moreira R, Queirós O, Carvalho F, Dinis-Oliveira RJ. Comparative pharmacology and toxicology of tramadol and tapentadol. Eur J Pain. 2018;22(5):827–844. doi:10.1002/ejp.1196
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
