Back to Journals » OncoTargets and Therapy » Volume 12
Tanshinone IIA Reverses Oxaliplatin Resistance In Human Colorectal Cancer Via Inhibition Of ERK/Akt Signaling Pathway
Authors Zhang Y, Ge T, Xiang P, Zhou J, Tang S, Mao H, Tang Q
Received 31 May 2019
Accepted for publication 22 October 2019
Published 14 November 2019 Volume 2019:12 Pages 9725—9734
DOI https://doi.org/10.2147/OTT.S217914
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Yonggang Zhang,1 Tingrui Ge,1 Ping Xiang,1 Jingyi Zhou,1 Shumin Tang,1 Haibing Mao,1 Qiang Tang2
1Department of Anus and Intestine Surgery, The First People’s Hospital of Lianyungang, Xuzhou Medical University Affiliated Hospital of Lianyungang, Lianyungang, Jiangsu, 222061, People’s Republic of China; 2Department of Gastrointestinal Surgery, The First People’s Hospital of Lianyungang, Xuzhou Medical University Affiliated Hospital of Lianyungang, Lianyungang, Jiangsu 222061, People’s Republic of China
Correspondence: Qiang Tang
Department of Gastrointestinal Surgery, The First People’s Hospital of Lianyungang, Xuzhou Medical University Affiliated Hospital of Lianyungang, No. 18 Tongguan Road, Xinpu District, Lianyungang, Jiangsu 222061, People’s Republic of China
Email [email protected]
Background: Oxaliplatin (OXA)-based chemotherapy is generally used to treat human cancers, whereas OXA resistance is a main obstacle for the treatment of colorectal cancer (CRC). Evidence has shown that tanshinone IIA (Tan IIA) could induce apoptosis in CRC cells. However, the role of combination of OXA and Tan IIA on OXA-resistance CRC cells remains unknown. Thus, this study aimed to investigate the effects of Tan IIA in combination with OXA on OXA-resistance CRC cells.
Methods: MTT assay, Ki67 immunofluorescence staining and flow cytometry were used to detect viability, proliferation and apoptosis in OXA-resistant cell line SW480/OXA, respectively. The expressions of Bcl-2, Bax, active caspase 3, p-Akt and p-ERK in SW480/OXA cells were detected with Western blot. In vivo animal study was performed finally.
Results: In this study, the inhibitory effects of OXA on the proliferation and invasion of SW480/OXA cells were significantly enhanced by Tan IIA. In addition, Tan IIA obviously enhanced the anti-apoptosis effects of OXA on SW480/OXA cells via decreasing the levels of Bcl-2, p-Akt and p-ERK, and increasing the levels of Bax and active caspase 3. In vivo experiments confirmed that Tan IIA enhanced OXA sensitivity in SW480/OXA xenograft model.
Conclusion: We found that Tan IIA could reverse OXA resistance in OXA-resistance CRC cells. Therefore, OXA combined with Tan IIA might be considered as a therapeutic approach for the treatment of OXA-resistant CRC.
Keywords: colorectal cancer, tanshinone IIA, oxaliplatin, Akt/ERK signaling pathway
Introduction
Colorectal cancer (CRC) is one of the most malignant cancers worldwide.1 A family history of CRC, a diet with lower fiber, age and smoking are the possible risk factors for CRC.2 Most of the patients with CRC are diagnosed at advanced stage, whereas the 5-year survival rate is only 25–39% at advanced stage and the recurrence rate is extremely high.3 At present, chemotherapy is one of the main options for the treatment of CRC.4 Oxaliplatin (OXA) is a first-line chemotherapy drug for advanced-stage CRC, which exhibits strong activity against CRC.5,6 However, the resistance happened in nearly all patients after long-term treatment with oxaliplatin alone, and then the therapeutic efficacy was limited.7 Therefore, searching new agents responsible for tumor chemoresistance is a key method to enhance the efficacy of OXA for patients with CRC.
Tanshinone IIA (Tan IIA) is isolated and purified from the roots of Salvia miltiorrhiza Bunge.8 Recent studies have revealed that Tan IIA exhibited anti-tumor, antioxidant and anti-inflammatory effects.9–11 Tan IIA could inhibit proliferation, migration and invasion in CRC cells.12 In addition, Tan IIA could attenuate hypoxia-induced chemotherapy resistance in breast cancer.13 However, the effect of Tan IIA in OXA-resistance CRC cells remains poorly understood.
Akt/ERK signaling pathway has been considered as a key signaling cascade involved in regulating cell growth, proliferation and cell death in human cancers.14,15 Protein kinase B (Akt) and extracellular regulated protein kinases (ERK) are the essential anti-apoptotic proteins, which could mediate proliferation, metastasis and survival in tumor cells.16,17 Recent studies have reported that Tan IIA could inhibit apoptosis in human cancers via Akt and ERK pathways.18,19 However, the role of Tan IIA in OXA resistance of CRC cells remains unknown. In the present study, we aimed to investigate the anti-tumor effects of combination of OXA with Tan IIA in OXA-resistant human CRC cells, providing a promising therapeutic direction for patients with CRC.
Materials And Methods
Cell Culture
Human CRC cell lines SW480 and HT29 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Two OXA-resistant cell lines (SW480/OXA and HT29/OXA) were established by continuous exposure of CRC cells to a stepwise gradually concentration of OXA for more than 7 months. Briefly, the OXA-resistant CRC cells (SW480 or HT29) were obtained discontinuously by gradually increasing doses of OXA (range from 3 to 48 μM). Briefly, 3 μM OXA was added to the medium, and the medium was changed at a proper time. When the cells were harvested and then cultured in medium with an increased OXA concentration. After appropriately increasing the concentration of DDP over a period of 7 months, a stable drug-resistant cell line SW480/OXA or HT29/OXA was obtained. Cells were cultured in Roswell Park Memorial Institute 1640 medium (RPMI1640, Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (FBS, Thermo Fisher Scientific) and 1% penicillin/streptomycin (Corning New York, NY, USA) in an incubator with 5% CO2 at 37°C.
Cell Proliferation And Cell Death Assay
Cell proliferation was determined by using a CCK‐8 kit (Beyotime Biotechnology, Suzhou, China) according to the manufacturer’s instructions. SW480, SW480/OXA, HT29 or HT29/OXA cells (5 × 104 cells per well) were plated into 96-well plates and incubated at 37°C overnight, respectively. Cells were then treated with different concentrations of OXA (0, 3, 6, 9, 12, 24 or 48 μM) for 72 hrs at 37°C. After that, the cells were incubated with 10 μL CCK8 reagent at 37°C for another 2 hrs. The optical density (OD) of each well was measured at 450 nm using a microplate reader (Bio-Tek Instruments Inc., Winooski, VT, USA).
Colony Formation Assay
SW480/OXA cells (5000 cells per well) were plated into 6-well plates at 37°C overnight. Then, cells were treated with OXA or/and Tan IIA for 3 days at 37°C. After that, cells were fixed with 2.5% glutaraldehyde and then stained with methylene blue solution at room temperature for 60 mins. Plates were photographed (Fluorescence microscope, Olympus, Tokyo, Japan) after washing and air drying, and the number of cell colonies were manually counted.
Immunofluorescence Staining Assay
SW480/OXA cells (5 × 103 cells per well) were plated into 96-well plates at 37°C overnight. Then, cells were treated with OXA or/and Tan IIA for 48 hrs at 37°C. After that, the cells were permeabilized with 0.5% TritonX-10 for 20 mins and then fixed with 4% paraformaldehyde for 15 mins. After that, the cells were incubated with primary antibodies anti-Ki67 (1:1000, Abcam Cambridge, MA, USA) at 4°C overnight, followed by incubation with secondary antibodies (1:2000, Abcam) at 37°C for 1 hr. Nuclei were counterstained with DAPI for 30 mins. Finally, the cells were observed using a laser scanning confocal microscope (Olympus CX23 Tokyo, Japan).
Cell Apoptosis Assay
The apoptosis of SW480/OXA cells was assessed using an Annexin V-FITC apoptosis detection kit (KeyGen Biotech, Nanjing, China) according to the protocol. Cells were collected and washed twice with pre-cold PBS. After that, cells were stained with 5 μL annexin V-FITC (Thermo Fisher Scientific) and 5 μL propidium iodide (PI, Thermo Fisher Scientific) for 30 mins in the dark at room temperature. Cell apoptosis was assessed by flow cytometry (BD Biosciences, Mountain View, CA, USA).
Transwell Invasion Assay
Transwell assays were performed as described previously.20 SW480/OXA cells (5 × 104 cells per well) were seeded in Matrigel-coated upper chambers. RPMI1640 medium without 10% FBS was added into the upper chamber, and medium with 10% FBS was added in the lower chamber to induce cell invasion. Twenty-four hours later, the invaded cells on the lower membrane surface were stained with 0.1% crystal violet. Then, cell images were photographed using a laser scanning confocal microscope (Olympus).
Western Blotting Assay
SW480/OXA cells (4 × 105 cells per well) were plated into 6-well plates at 37°C overnight. Then, cells were treated with OXA or/and Tan IIA for 48 hrs at 37°C. The protein concentration was determined using a BCA Protein Assay Kit (Generay, Shanghai, China) as per manufacturer’s instruction. Proteins (30 μg per lane) were separated using 10% SDS-PAGE and then transferred onto the PVDF membrane (Sigma-Aldrich, St. Louis, MO, USA). Following this, the membranes were blocked with 5% non-fat milk for 1 hr at room temperature and then incubated with the primary antibodies at 4°C overnight. Anti-Bcl-2 (cat. no. ab32124, 1:1000), anti-Bax (cat. no. ab32503, 1:1000), anti-active caspase 3 (cat. no. ab2302, 1:1000), anti-p-Akt (cat. no. ab38449, 1:1000), anti-Akt (cat. no. ab8805, 1:1000), anti-p-ERK (cat. no. ab50011, 1:1000), anti-ERK (cat. no. ab17942, 1:1000), anti-β-actin (cat. no. ab8227, 1:1000). Antibodies were purchased from Abcam Cambridge (MA, USA). Later on, the membranes were incubated with goat anti-rabbit IgG antibody (1:5000, Abcam) at room temperature for 1 hr. Membranes were scanned by using an Odyssey Imaging System and analyzed with Odyssey v2.0 software (LICOR Biosciences, Lincoln, NE, USA).
Animal Study
Male BALB/c nude mice (n = 16, 6–8 weeks old, 20 ± 2 g weight) were obtained from Animal Center of Chinese Academy of Sciences (Shanghai, China). All mice were housed under standard animal room conditions according to the guidelines for laboratory animal care. SW480/OXA cells (5 × 106 cells) were transplanted subcutaneously into the right flank in each animal. When the tumor volume reached about 200 mm3, the mice were randomized into four groups: vehicle, Tan IIA_30 mg/kg, OXA_10 mg/kg and OXA (10 mg/kg) + Tan IIA (30 mg/kg) groups. Tan IIA was administered via intraperitoneal (i.p.) injection at doses of 30 mg/kg every day. OXA was administered via i.p. injection at doses of 10 mg/kg once. The vehicle group received PBS only. After 3 weeks, 16 mice were sacrificed under anesthesia by following the recommended procedures of National Institutes of Health guide for the care and use of laboratory animals. Tumor tissues were collected and tumor weight was calculated. These animal experiments were performed following a protocol approved by the Ethics Committees of the First People’s Hospital of Lianyungang, Xuzhou Medical University Affiliated Hospital of Lianyungang.
TUNEL Assay
Tumor tissues were fixed in 4% paraformaldehyde, embedded in paraffin and then cut into 5-μm sections. Cell apoptosis was evaluated by TUNEL assay with an apoptosis detection kit (Thermo Fisher Scientific) according to the protocol.
Statistical Analysis
GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA) was performed for statistical analysis. Data were represented as mean ± standard deviation (SD). All experiments were repeated at least three times. The comparisons among multiple groups were made with one-way analysis of variance (ANOVA) followed by Tukey’s test. P<0.05 was accepted as a statistically significant difference.
Results
Tan IIA Enhances The Cytotoxic Effect Of OXA In Human CRC Cells
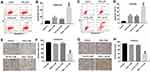
The chemical structure of Tan IIA was indicated in Figure 1A. To evaluate the effects of OXA at different doses on the viability of OXA-resistance CRC cells, CCK-8 assay was used. As shown in Figure 1B and C, SW480/OXA cells and HT29/OXA cells were resistant to OXA after gradient treatment compared with SW480 and HT29 parental cells. Furthermore, the cytotoxicity of Tan IIA in SW480/OXA cells and HT29/OXA cells were decreased compared with that in their parental SW480 and HT29 cells (Figure 1D and E). 9 μM OXA or 8 μM Tan IIA slightly inhibited the proliferation of SW480/OXA cells and HT29/OXA cells; therefore, 9 μM OXA and 8 μM Tan IIA were utilized in the following experiments. CCK-8 assay was further performed to analyze the effects of OXA and Tan IIA on viability of OXA-resistance CRC cells. 9 μM OXA induced about 14% growth inhibitions in SW480/OXA cells, which were further increased to about 73% in co-treatment group of OXA + Tan IIA (Figure 1F). Meanwhile, 9 μM OXA induced about 19% growth inhibitions in HT29/OXA cells, which were further increased to about 65% in co-treatment group (Figure 1G). The SW480/OXA cells were less sensitive to OXA-induced cell death than HT29/OXA cells. Therefore, SW480/OXA cells were utilized in the following studies.
Tan IIA Enhances The Anti-Proliferation Effect Of OXA In SW480/OXA Cells
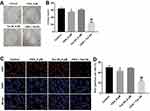
Next, the effects of Tan IIA and OXA on the proliferation of SW480/OXA cells were analyzed by colony formation and Ki67 immunofluorescence staining assay. As shown in Figure 2A and B, OXA significantly inhibited the proliferation of SW480/OXA cells and exhibited about 17% growth inhibition, which was further increased to 42% in co-treatment group. In addition, OXA significantly decreased the ki67-positive SW480/OXA cells, compared with control group. As expected, the ki67-positive cells were further decreased in the presence of Tan IIA, compared with OXA treatment group (Figure 2C and D). These data indicated that Tan IIA could enhance the anti-proliferation effect of OXA in SW480/OXA cells.
Tan IIA Enhances The Anti-Tumor Effects Of OXA In CRC Cells
Flow cytometric assay was applied to assess the apoptosis of SW480/OXA cells. As indicated in Figure 3A and B, the apoptosis rate in OXA-treated SW480/OXA cells was about 20%. As expected, the apoptosis rate in OXA-treated SW480/OXA cells was significantly increased to about 52% in the presence of Tan IIA. In addition, as shown in Figure 3C and D, OXA significantly induced apoptosis of HT29/OXA cells, which was markedly enhanced in the presence of Tan IIA. Moreover, Tan IIA enhanced the inhibitory effect of OXA on the invasion of SW480/OXA cells and HT29/OXA cells (Figure 3E–H). These results illustrated that Tan IIA could enhance the anti-tumor effects of OXA in CRC cells.
Tan IIA Affects OXA Resistance In CRC Cells By Regulation Of Akt/ERK Pathway
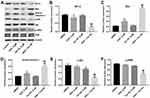
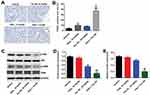
In order to explore the mechanisms by which Tan IIA regulates OXA resistance in CRC, the protein expressions of Bcl-2, Bax, active caspase 3, p-Akt and p-ERK were measured by Western blotting assay. The results showed that OXA decreased the levels of Bcl-2, p-Akt and p-ERK, and increased the levels of Bax and active caspase 3 in SW480/OXA cells and HT29/OXA cells, which were further enhanced in the presence of Tan IIA (Figures 4A–F and 5A–F). These data indicated that Tan IIA could enhance the sensitivity of CRC cells to OXA by regulating of Akt/ERK pathway.
Tan IIA Enhances OXA Sensitivity In SW480/OXA Xenograft In Vivo
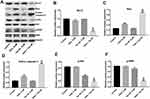
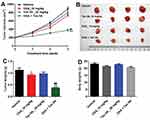
Given that Tan IIA could enhance the efficacy of OXA in vitro, we further assessed the effects of combination in the SW480/OXA xenograft mouse model. As shown in Figure 6A–C, OXA or Tan IIA treatment had limited effect on tumor volume and tumor weight, compared with control group. As expected, the combination of OXA with Tan IIA significantly decreased the tumor volume and tumor weight. In addition, combination treatment had very limited effect on body weight, which means combination treatment had no obvious toxicity in xenograft mouse model (Figure 6D). These results illustrated that Tan IIA could enhance OXA sensitivity in SW480/OXA xenograft in vivo.
Tan IIA Enhances Anti-Tumor Effects Of OXA In SW480/OXA Xenograft Via Regulation Of Akt/ERK Pathway
TUNEL analysis revealed that OXA significantly promoted tumor cell apoptosis compared with control group. Meanwhile, Tan IIA markedly increased the TUNEL positive cell rate induced by OXA, from 10% to 36% (Figure 7A and B). In addition, the data of Western blotting indicated that combination of Tan IIA with OXA treatment significantly downregulated the levels of p-Akt and p-ERK in tumor tissues, compared with OXA treatment group (Figure 7C–E). These data suggested that Tan IIA could enhance anti-tumor effects of OXA in SW480/OXA xenograft via inhibiting of Akt/ERK pathway.
Discussion
Colorectal cancer (CRC) is one of the most malignant tumors worldwide with poor prognosis.21 OXA is the first-line chemotherapy drug for the treatment of CRC.22 However, chemo-resistance is the main cause of death in patients with recurrent CRC and metastatic CRC.23 Recent study has shown that Chinese medicine could synergistically enhance anti-tumor effect of chemotherapeutics, such as OXA.24,25 In order to find an effective therapy to reverse OXA resistance in CRC, OXA-resistant human CRC cells were used.
Recent data have indicated that Tan IIA has anti-cancer activity on a large variety of cancer cells, including CRC cells and lung cancer cells.26,27 Guo et al indicated that Tan IIA enhanced anti-leukemia effect of imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia mouse models.18 In addition, combination of Tan IIA with cisplatin treatment significantly inhibited the proliferation of cisplatin-resistant ovarian cancer cells.28 In the present study, Tan IIA enhanced the anti-proliferation effect of OXA in SW480/OXA cells, which was consistent with previous studies. Moreover, we found that Tan IIA markedly enhanced the pro-apoptotic effect of OXA in SW480/OXA cells via downregulating the level of Bcl-2 and upregulating the expressions of Bax and active caspase 3. Meanwhile, the inhibitory effect of OXA on the invasion of SW480/OXA cells was enhanced by Tan IIA. Deng et al indicated that neferine could enhance OXA sensitivity in hepatocellular carcinoma cells via inducing apoptosis and inhibiting invasion.29 Tannic acid could reverse OXA resistance in CRC cells, which was consistent with our study.30 However, the mechanism underlying the chemo-preventive effect of Tan IIA has not been fully demonstrated.
Several studies have demonstrated that activation of Akt and ERK signaling pathways could directly induce drug resistance.31 The Akt/ERK pathway is implicated in tumor cell proliferation and apoptosis and is stimulated by various chemotherapeutic agents including OXA.32 Meanwhile, Tan IIA could induce apoptosis in CRC cells via inhibiting PI3K/Akt pathways.11 In this study, Western blotting assay showed that combination of Tan IIA with OXA obviously downregulated the expressions of phosphorylation of Akt/ERK in vitro and in vivo. Luo et al indicated that cetuximab enhanced the effect of OXA on gastric cancer cells via ERK/Akt pathways.33 In addition, capilliposide C reversed OXA resistance in esophageal squamous carcinoma cells via downregulating the PI3K/Akt signaling pathway.34 On the basis of the results, Tan IIA enhanced the sensitivity of SW480/OXA cells to OXA in vitro and in vivo by inhibiting Akt/ERK pathway.
Conclusion
Taken together, Tan IIA could reverse OXA resistance in SW480/OXA cells via inhibiting Akt/ERK pathway. Therefore, OXA combined with Tan IIA might be considered as a therapeutic approach for the treatment of OXA-resistant CRC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li X, Zhang X, Zhang Q, Lin R. miR-182 contributes to cell proliferation, invasion and tumor growth in colorectal cancer by targeting DAB2IP. Int J Biochem Cell Biol. 2019.
2. Shi C, Cai Y, Li Y, et al. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018;14:59–71. doi:10.1016/j.redox.2017.08.013
3. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745–761. doi:10.1053/j.gastro.2016.11.048
4. Li R, Xin T, Li D, et al. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018;18:229–243. doi:10.1016/j.redox.2018.07.011
5. Dumont F, Senellart H, Pein F, et al. Phase I/II study of oxaliplatin dose escalation via a laparoscopic approach using pressurized aerosol intraperitoneal chemotherapy (PIPOX trial) for nonresectable peritoneal metastases of digestive cancers (stomach, small bowel and colorectal): rationale and design. Pleura Peritoneum. 2018;3(3):20180120.
6. Huang H, Aladelokun O, Ideta T, et al. Inhibition of PGE2/EP4 receptor signaling enhances oxaliplatin efficacy in resistant colon cancer cells through modulation of oxidative stress. Sci Rep. 2019;9(1):4954. doi:10.1038/s41598-019-40848-4
7. Rothenberg ML. Efficacy of oxaliplatin in the treatment of colorectal cancer. Oncology (Williston Park). 2000;14(12 Suppl 11):9–14.
8. Yan SH, Zhao NW, Geng ZR, et al. Modulations of Keap1-Nrf2 signaling axis by TIIA ameliorated the oxidative stress-induced myocardial apoptosis. Free Radic Biol Med. 2018;115:191–201. doi:10.1016/j.freeradbiomed.2017.12.001
9. Fu J, Huang H, Liu J, et al. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568(1–3):213–221. doi:10.1016/j.ejphar.2007.04.031
10. Yen JH, Huang ST, Huang HS, et al. HGK-sestrin 2 signaling-mediated autophagy contributes to antitumor efficacy of Tanshinone IIA in human osteosarcoma cells. Cell Death Dis. 2018;9(10):1003. doi:10.1038/s41419-018-1016-9
11. Zhang X, Zhou Y, Gu YE. Tanshinone IIA induces apoptosis of ovarian cancer cells in vitro and in vivo through attenuation of PI3K/AKT/JNK signaling pathways. Oncol Lett. 2019;17(2):1896–1902. doi:10.3892/ol.2018.9744
12. Shan YF, Shen X, Xie YK, et al. Inhibitory effects of tanshinone II-A on invasion and metastasis of human colon carcinoma cells. Acta Pharmacol Sin. 2009;30(11):1537–1542. doi:10.1038/aps.2009.139
13. Fu P, Du F, Chen W, et al. Tanshinone IIA blocks epithelial-mesenchymal transition through HIF-1alpha downregulation, reversing hypoxia-induced chemotherapy resistance in breast cancer cell lines. Oncol Rep. 2014;31(6):2561–2568. doi:10.3892/or.2014.3140
14. Liu C, Mu X, Wang X, et al. Ponatinib inhibits proliferation and induces apoptosis of liver cancer cells, but its efficacy is compromised by its activation on PDK1/Akt/mTOR signaling. Molecules. 2019;24(7).
15. Zhang L, Mao Y, Mao Q, et al. FLOT1 promotes tumor development, induces epithelial-mesenchymal transition, and modulates the cell cycle by regulating the Erk/Akt signaling pathway in lung adenocarcinoma. Thorac Cancer. 2019;10(4):909–917. doi:10.1111/tca.2019.10.issue-4
16. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412–3419. doi:10.1016/j.ejca.2013.05.028
17. Li J, Guo Y, Duan L, et al. AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signaling. Oncotarget. 2017;8(20):33694–33703. doi:10.18632/oncotarget.16624
18. Guo Y, Li Y, Xiang B, et al. Nutlin-3 plus tanshinone IIA exhibits synergetic anti-leukemia effect with imatinib by reactivating p53 and inhibiting the AKT/mTOR pathway in Ph+ ALL. Biochem J. 2017;474(24):4153–4170. doi:10.1042/BCJ20170386
19. Wang H, Su X, Fang J, et al. Tanshinone IIA attenuates insulin like growth factor 1 -induced cell proliferation in PC12 cells through the PI3K/Akt and MEK/ERK pathways. Int J Mol Sci. 2018;19(9).
20. Wang Y, Zhang Y, Yang T, et al. Long non-coding RNA MALAT1 for promoting metastasis and proliferation by acting as a ceRNA of miR-144-3p in osteosarcoma cells. Oncotarget. 2017;8(35):59417–59434. doi:10.18632/oncotarget.19727
21. Harada K, Okamoto W, Mimaki S, et al. Comparative sequence analysis of patient-matched primary colorectal cancer, metastatic, and recurrent metastatic tumors after adjuvant FOLFOX chemotherapy. BMC Cancer. 2019;19(1):255. doi:10.1186/s12885-019-5479-6
22. Cai J, Wang H, Jiao X, et al. The RNA-binding protein HuR confers oxaliplatin resistance of colorectal cancer by upregulating CDC6. Mol Cancer Ther. 2019;18:1243–1254. doi:10.1158/1535-7163.MCT-18-0945
23. Liu Z, Yu M, Fei B, Sun J, Wang D. Nonhomologous end joining key factor XLF enhances both 5-florouracil and oxaliplatin resistance in colorectal cancer. Onco Targets Ther. 2019;12:2095–2104. doi:10.2147/OTT.S192923
24. Zhang Y, Zhang Q, Fan Z, et al. A Chinese herbal formula, Chang-Wei-Qin, synergistically enhances antitumor effect of oxaliplatin. Pathol Oncol Res. 2015;21(2):389–397. doi:10.1007/s12253-014-9831-5
25. Fan R, Li X, Deng J, et al. Dual drug loaded biodegradable nanofibrous microsphere for improving anti-colon cancer activity. Sci Rep. 2016;6:28373. doi:10.1038/srep28373
26. Sui H, Zhao J, Zhou L, et al. Tanshinone IIA inhibits beta-catenin/VEGF-mediated angiogenesis by targeting TGF-beta1 in normoxic and HIF-1alpha in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017;403:86–97. doi:10.1016/j.canlet.2017.05.013
27. Kim EO, Kang SE, Im CR, et al. Tanshinone IIA induces TRAIL sensitization of human lung cancer cells through selective ER stress induction. Int J Oncol. 2016;48(5):2205–2212. doi:10.3892/ijo.2016.3441
28. Jiao JW, Wen F. Tanshinone IIA acts via p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and lung-resistance protein in cisplatin-resistant ovarian cancer cells. Oncol Rep. 2011;25(3):781–788. doi:10.3892/or.2010.1107
29. Deng G, Zeng S, Ma J, et al. The anti-tumor activities of Neferine on cell invasion and oxaliplatin sensitivity regulated by EMT via Snail signaling in hepatocellular carcinoma. Sci Rep. 2017;7:41616. doi:10.1038/srep41616
30. Ren Y, Li X, Han B, et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur J Pharm Sci. 2019;128:279–289. doi:10.1016/j.ejps.2018.12.007
31. Matsuoka H, Tsubaki M, Yamazoe Y, et al. Tamoxifen inhibits tumor cell invasion and metastasis in mouse melanoma through suppression of PKC/MEK/ERK and PKC/PI3K/Akt pathways. Exp Cell Res. 2009;315(12):2022–2032. doi:10.1016/j.yexcr.2009.04.009
32. Huang WS, Hsieh MC, Huang CY, et al. The association of CXC receptor 4 mediated signaling pathway with oxaliplatin-resistant human colorectal cancer cells. PLoS One. 2016;11(9):e0159927. doi:10.1371/journal.pone.0159927
33. Luo HY, Wei W, Shi YX, et al. Cetuximab enhances the effect of oxaliplatin on hypoxic gastric cancer cell lines. Oncol Rep. 2010;23(6):1735–1745. doi:10.3892/or_00000819
34. Shen Z, Xu L, Li J, Zhang N. Capilliposide C sensitizes esophageal squamous carcinoma cells to oxaliplatin by inducing apoptosis through the PI3K/Akt/mTOR pathway. Med Sci Monit. 2017;23:2096–2103. doi:10.12659/MSM.901183
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.