Back to Journals » Drug Design, Development and Therapy » Volume 13
Tanshinone I Inhibits IL-1β-Induced Apoptosis, Inflammation And Extracellular Matrix Degradation In Chondrocytes CHON-001 Cells And Attenuates Murine Osteoarthritis
Authors Wang X, Fan J, Ding X, Sun Y, Cui Z, Liu W
Received 21 May 2019
Accepted for publication 6 September 2019
Published 15 October 2019 Volume 2019:13 Pages 3559—3568
DOI https://doi.org/10.2147/DDDT.S216596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Xipeng Wang,1 Jianbo Fan,2 Xiaomin Ding,2 Yuyu Sun,2 Zhiming Cui,2 Wei Liu2
1Department of Orthopaedic Surgery, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430010, People’s Republic of China; 2Department of Orthopaedic Surgery, The Second Affiliated Hospital of Nantong University, Nantong, Jiangsu 226001, People’s Republic of China
Correspondence: Wei Liu
Department of Orthopaedic Surgery, The Second Affiliated Hospital of Nantong University, Nantong, Jiangsu 226001, People’s Republic of China
Email [email protected]
Background: Osteoarthritis (OA) is a prevalent degenerative joint disease, which was characterized by inflammation and cartilage degradation. Accumulating evidence has demonstrated that Tanshinone I has an anti-inflammatory effect in various diseases. However, the efficacy of Tanshinone I as an anti-inflammatory agent in OA remains unclear. This study aimed to explore the role of Tanshinone I on OA both in vitro and in vivo.
Methods: CHON-001 cells were treated with IL-1β (10 ng/mL) for 72 hrs to induce OA model in vitro. Meanwhile, CHON-001 cells were pre-treated with 20 μM Tanshinone I for 24 hrs and then stimulated with IL-1β (10 ng/mL) for 72 hrs. CCK-8, immunofluorescence and flow cytometry assays were used to detect the viability, proliferation and apoptosis in CHON-001 cells, respectively. Western blotting assay was used to detect the levels of collagen II, aggrecan, MMP-13, cleaved caspase 1, Gasdermin D, SOX11 and p-NF-κB in CHON-001 cells. In addition, the mouse model of OA was built by anterior cruciate ligament transection (ACLT) in the right knee. Meanwhile, the mice were administrated with 10 or 30 mg/kg Tanshinone I for 8 weeks. Safranin-O/Fast Green staining was used to assess cartilage destruction in a mouse model of OA.
Results: In this study, IL-1β significantly induced apoptosis, extracellular matrix degradation and inflammatory response in CHON-001 cells. Tanshinone I significantly inhibited IL-1β-induced apoptosis in CHON-001 cells. In addition, the IL-1β-induced collagen II, aggrecan degradation, SOX11 downregulation, and MMP-13 and p-NF-κB upregulation in CHON-001 cells were notably reversed by Tanshinone I treatment. Moreover, Tanshinone I alleviated cartilage destruction and synovitis and reduced OARSI scores and subchondral bone thickness in a mouse model of OA.
Conclusion: Our findings showed that Tanshinone I could alleviate the progression of OA in vitro and in vivo. These results demonstrated that Tanshinone I might be regarded as a promising therapeutic agent for the treatment of OA.
Keywords: osteoarthritis, IL-1β, Tanshinone I, chondrocytes
Introduction
Osteoarthritis (OA) is a prevalent degenerative joint disease, which seriously affects the health of the elderly people.1 The clinical manifestations of OA are chronic joint pain, activity limitation, bone hypertrophy and stiffness.2 In addition, inflammation, degradation of articular cartilage, chondrocyte apoptosis and synovitis are the main symptoms of OA.3,4 Articular cartilage injury is a vital pathological factor leading to OA.5 The structure of articular cartilage is difficult to renew when it was injured, eventually leading to pathological changes in OA.6 In addition, femoro-acetabular impingement is an important pathological factor leading to OA as well and is most common in young athletic men.7 An important manifestation of OA is the reduced production of collagen type II and aggrecan and the increased production of MMP-13 and SOX11.8,9 However, due to the pathogenesis of OA not fully elucidated, few effective methods of stopping the degradation of articular cartilage have been found.10 Therefore, novel therapies remain to be found to ameliorate the symptoms of OA.
Tanshinone I was extracted from a traditional Chinese medicine Danshen.11 Tanshinone I has been used as an anti-tumor, antioxidant and anti-inflammatory agent.11–13 It has been shown that Tanshinone I exerted a neuroprotection role via inhibiting pro-inflammatory gene expression in lipopolysaccharide (LPS)-stimulated microglia cells.14 Zhang et al indicated that Tanshinone I exhibited anti-inflammatory activity in rats with adjuvant-induced arthritis.15 In addition, in vitro evidence have found that Tanshinone IIA alleviated IL-1β-induced inflammatory injury in chondrocytes, suggesting the potential of Tanshinone IIA for use in the treatment of OA.16 However, the anti-inflammatory effect of Tanshinone I on OA is still unclear and needs to be illuminated.
Therefore, our main purpose of this study was to investigate the effect of Tanshinone I on chondrogenic CHON-001 cells. In this study, IL-1β‑induced chondrocyte injury model in CHON-001 cells and a mice model of OA were established firstly. The mechanism by which Tanshinone I regulates IL-1β-induced inflammatory injury in CHON-001 cells was evaluated as well.
Materials And Methods
Cell Culture
The chondrogenic cell line CHON-001 was obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% FBS and 100 units/mL of penicillin/streptomycin, and cultured under 5% CO2 at 37 °C. CHON-001 cells were stimulated with IL-1β (Sigma Aldrich, St. Louis, MO, USA) for OA model establishment.
Cell Viability Assay
The CHON-001 cells (5×103 cells/well) were plated onto a 96-well plate overnight. Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) was used to evaluate cell viability. Cells were treated with IL-1β, Tanshinone I or celecoxib for 24, 48 and 72 hrs. After that, 10 μL CCK-8 solution was added into each well, and the cells were incubated for another 2 hrs at 37 °C. Celecoxib was provided by Sigma Aldrich (St. Louis, MO, USA) and used as a positive drug. Then, the optical density (OD) of each well was measured by a microplate reader (Bio-Rad, Hercules, USA) at a wavelength of 450 nm. The experiment was repeated five times.
Flow Cytometry Assay
Apoptotic cells were detected by an Annexin V-FITC apoptosis detection kit (Thermo Fisher Scientific). The CHON-001 cells (5×104 cells/well) were plated onto 6-well plate overnight at 37 °C. When the cell confluence reached about 80%, cells were treated with IL-1β or Tanshinone I for 72 hrs. After that, cells were fixed with pre-cold 70% ethanol and then stained with 10 μL Annexin V-FITC and 5 μL propidium iodide (PI) for 30 mins. The apoptotic cells were discriminated using a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The experiment was performed in triplicate.
Quantitative Real‐Time PCR
To analyze the mRNA level expressions of collagen II, aggrecan and MMP-13, total RNA was extracted from CHON-001 cells by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) was applied to synthesize cDNA. Quantitative real‐time PCR was performed by using a SYBR Premix ExTaq kit (TaKaRa, Dalian, China) at ABI 7300. The qRT-PCR primers were as follows: GAPDH, Forward: 5’-CATCATCCCTGCCTCTACTGG-3’, Reversed: 5’-GTGGGTGTCGCTGTTGAAGTC-3’; Collagen II, Forward: 5’- ATGCCACACTCAAGTCCCTCA-3’, Reversed: 5’- GTCTCGCCAGTCTCCATGTTG-3’; Aggrecan, Forward: 5’- AAGGGCGAGTGGAATGATGT-3’, Reversed: 5’- CGCTTCTGTAGTCTGCGTTTGT-3’; MMP13, Forward: 5’- CAGAACTTCCCAACCGTATTGAT-3’, Reversed: 5’- TGTATTCAAACTGTATGGGTCCG-3’. GAPDH was used as an endogenous control, and the classic 2−ΔΔCt method was used to calculate the fold changes. The experiment was performed in triplicate.
Western Blot
The proteins were lysed by using radioimmunoprecipitation assay buffer (RIPA, Beyotime Biotechnology, Shanghai, China). Then, the proteins were quantified using a BCA™ Protein Assay Kit (Beyotime). Proteins (30 μg proteins per lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene difluoride (PVDF) membrane. After that, the membranes were blocked with 5% skim milk for 1 hr at room temperature and then incubated at 4°C overnight with primary antibodies against collagen II (1:1000, cat. no. ab34712), aggrecan (1:1000, cat. no. ab3778), MMP-13 (1:1000, cat. no. ab39012), GAPDH (1:1000, cat. no. ab181602), Cleaved caspase 1 (1:1000, cat. no. ab1872), Gasdermin D (1:1000, cat. no. ab239377), NF-κB (1:1000, cat. no. ab16502), p-NF-κB (1:1000, cat. no. ab86299), SOX-11 (1:1000, cat. no. ab170916, all from Abcam Cambridge, MA, USA). Later on, the membranes were incubated with HRP-goat anti-rabbit secondary antibody at room temperature for 1 hr. The bands were visualized using the Bio-Rad ChemiDoc Imaging system (Bio-Rad, Hercules, CA, USA) and the strength of the bands was quantified using Image Lab™ Software (Bio-Rad). GAPDH protein was used as the inner control of heterologous proteins. The experiment was performed in triplicate.
Immunofluorescence
The Ki67 protein is a cellular marker for proliferation, and Ki67 immunofluorescence assay was used to detect cell proliferation.17 CHON-001 cells were fixed in 4% formaldehyde for 10 mins and then permeated with 0.3% Triton X-100 for 15 mins at room temperature. After that, the cells were incubated with primary antibody against Ki67 (cat. no. ab197234, Abcam) overnight at 4 °C. After washing with PBS three times, the cells were incubated with goat anti-rabbit secondary antibody (cat. no. ab150077, Abcam) at 37 °C for 1 hr. Later on, cell nuclei were stained with DAPI (cat. no. ab104139, Abcam) for 5 mins. Images were captured using a laser scanning confocal microscope (Leica, Buffalo Grove, IL, USA). The experiment was performed in triplicate.
Elisa
The concentration of TNF-a in CHON-001 cells was analyzed by using an ELISA kit (cat. no. ab208348, Abcam) according to the manufacturer’s instruction. The experiment was performed in triplicate.
Animal Study
A total of 12 C57BL/6 mice (6–8 weeks old; 6 males and 6 females) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd., (Shanghai, China) and housed with a 12-hr light/dark cycle and fed a standard laboratory food and water. The animal experiments were approved by the Animal Care Committee of the Second Affiliated Hospital of Nantong University. The model of OA was built by anterior cruciate ligament transection (ACLT) in the right knees, as described previously.18,19 Briefly, the joint of mice were subjected a medial parapatellar arthrotomy. In order to expand the operation field, the patella was dislocated laterally with the leg. The anterior cruciate ligament was cut off using a #11 surgical blade. The mice were randomly divided into five groups: sham (control) group, OA group, OA + celecoxib (10 mg/kg) group OA + Tanshinone I (10 mg/kg) group and OA + Tanshinone I (30 mg/kg) group. Celecoxib was dissolved in saline with 0.1% DMSO and administered orally at a dosage of 10 mg/kg/day. In the OA + Tanshinone I group, the mice were treated with Tanshinone I via intraperitoneal injection once daily for 8 weeks after surgery. After 8 weeks, all mice were euthanized, and all animal cartilage tissues were collected for histological analysis. Joint samples were fixed in 4% paraformaldehyde at room temperature and then embedded in paraffin blocks. Each paraffin-embedded joint sample was sectioned at 5 mm, and each section was stained with Safranin-O/Fast Green (Sigma-Aldrich, St. Louis, MO, USA) to assess cartilage destruction. National Institutes of Health (NIH) guide for the care and use of laboratory animals was followed in the current study.
Histological Analysis
A histological scoring method issued by the Osteoarthritis Research Society International (OARSI) was used to determine the extent of cartilage deterioration, as described previously.20 OA cartilage histopathology grade assessment: grade 0, surface intact, cartilage intact; grade 1, surface intact; grade 2, surface discontinuity; grade 3, vertical fissures; grade 4, erosion; grade 5, denudation; and grade 6, deformation. We used a summed OARSI score (grade 0–12) from tibial plateau and femoral condyle to determine the degree of articular cartilage destruction. AxioVision software was used to measure the subchondral bone thickness according to Safranin-O-stained sections. The synovitis was assessed histologically using a scoring system as previously described:21 enlargement of the synovial lining cell layer: (0, thickness 1–2 cells, 1, thickness 2–4 cells, 2, thickness 4–9 cells, 3, thickness 10 or more cells); density of cells: (0, synovial stroma shows normal cellularity, 1, slightly increased cellularity, 2, moderately increased cellularity, 3, greatly increased cellularity and pannus formation).
Statistical Analysis
All data were repeated at least three times. Data are presented as mean ± standard error (SD). Graphs were generated using GraphPad Prism software (version 7.0, La Jolla, CA, USA). One-way analysis of variance (ANOVA) and Kruskal–Wallis tests were carried out for multiple group comparisons. A P-value < 0.05 was considered as statistically significant.
Results
IL-1β Induced Apoptosis In CHON-001 Cells
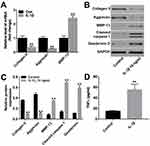
To assess the cytotoxic effect of IL-1β on chondrocytes, CCK-8 assay was applied. As shown in Figure 1A, CHON-001 cells were treated with different concentrations (2, 5, 10 or 20 ng/mL) of IL-1β for 0, 24, 48 and 72 hrs. IL-1β (10 ng/mL) induced about 50% growth inhibition (Figure 1A). In addition, 10 ng/mL IL-1β significantly induced apoptosis in CHON-001 cells (Figure 1B and C). Therefore, IL-1β at 10 ng/mL dose was utilized in the following in vitro experiments.
IL-1β Induced Extracellular Matrix Degradation In CHON-001 Cells
Previous evidence has demonstrated that degradation of extracellular matrix (ECM) underlies damage to articular cartilage in OA.22 To further investigate the role of IL-1β on chondrocytes, the levels of ECM-related protein collagen II, aggrecan and MMP-13 in CHON-001 cells were detected. QRT-PCR and Western blot assays indicated that IL-1β markedly downregulated the levels of collagen II and aggrecan, whereas notably upregulated the levels of MMP-13, cleaved caspase 1 and Gasfermin D in CHON-001 cells (Figure 2A–C). In addition, IL-1β obviously increased the production of TNF-α in CHON-001 cells (Figure 2D). These data indicated that IL-1β could induce ECM degradation and inhibited the expressions of inflammatory cytokines in CHON-001 cells.
Tanshinone I Inhibited IL-1β-Induced Apoptosis And Inflammation In CHON-001 Cells
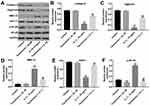
The effect of Tanshinone I on the viability of CHON-001 cells was examined using a CCK-8 assay. As indicated in Figure 3A, Tanshinone I at a concentration of 20 μM did not have an obvious cytotoxic effect on CHON-001 cells. Therefore, Tanshinone I at 20 μM dose was used in the subsequent experiments. As shown in Figure 3B, Tanshinone I or celecoxib markedly reversed IL-1β-induced cytotoxicity in CHON-001 cells. In addition, Tanshinone I or celecoxib significantly inhibited IL-1β-induced apoptosis in CHON-001 cells (Figure 3C and D). Meanwhile, Tanshinone I or celecoxib obviously increased the number of Ki67-positive CHON-001 cells, compared with IL-1β treatment group (Figures 3 and F). Moreover, ELISA assay indicated that Tanshinone I significantly reduced IL-1β-induced production of TNF-α in CHON-001 cells (Figure 3G). These results suggested that Tanshinone I could inhibit apoptosis and inflammation in IL-1β-stimulated CHON-001 cells.
Tanshinone I Prevented IL-1β-Induced Extracellular Matrix Degradation And Inflammation Response In CHON-001 Cells
Next, to evaluate the function of Tanshinone I in IL-1β-induced ECM degradation, Western blot was applied. As indicated in Figure 4A–E, the IL-1β-induced collagen II and aggrecan degradation, SOX11 downregulation and MMP-13 upregulation in CHON-001 cells were notably reversed by Tanshinone I treatment. In addition, the expression of NF-κB was significantly upregulated in IL-1β-stimulated CHON-001 cells, which was obviously reversed by Tanshinone I treatment (Figures 4A and F). These data suggested that Tanshinone I could attenuate IL-1β-induced degradation of ECM and inflammation response in CHON-001 cells.
Tanshinone I Alleviated OA Progression In A Murine OA Model
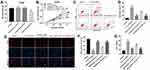
To assess the effect of Tanshinone I on the progression of OA in vivo, surgical OA models were established in mice. We first evaluated the effect of Tanshinone I on the body weight of mice. As indicated in Figure 5A,30 mg/kg Tanshinone I had very limited effect on the changes of body weight, while 50 mg/kg Tanshinone I notably decreased the body weight. Thus, 30 mg/kg Tanshinone was used in an animal study. As shown in Figure 5B, the Safranin O staining revealed the surface of the cartilage was undamaged in the control group. However, massive proteoglycan loss and reduction of cartilage thickness were observed in the OA group. In contrast, less cartilage degradation, cartilage erosion and reduction the amount of fibrous cartilage were observed by Tanshinone I treatment, compared to OA group (Figure 5B). In addition, the OARSI scores in the OA group were significantly higher than that in the control group. However, Tanshinone I markedly decreased OARSI scores, compared with the OA group (Figure 5C). Moreover, Tanshinone I treatment markedly decreased the subchondral bone thickness and synovitis scores, compared with the OA group (Figure 5D and E). Taken together, these data indicated that Tanshinone I could inhibit the development of OA in a mice model of OA.
Discussion
OA is one of the most common arthritis, which characterized by articular cartilage breakdown.23 Recently, compounds derived from traditional Chinese medicine have shown developing potentiality in the treatment of OA, due to their anti-inflammatory activities and few side effects.24,25 Tanshinone I, a lipophilic phenanthraquinone component, exhibited anti-inflammatory effect in acute kidney injury in mice.13 In the present study, we demonstrated that Tanshinone I obviously inhibited articular cartilage degradation in vitro and in vivo. These findings indicated that Tanshinone I might be a potential agent for the treatment of OA.
It has been indicated that IL-1β plays a vital role in regulating chondrocyte inflammation response in OA.26 In addition, IL-1β could induce apoptosis of chondrocyte via increasing the levels of Bax and caspase 3.27 IL-1β is positively associated with the severity of OA.28 In this study, CHON-001 cells were stimulated with IL-1β, in order to mimic an in vitro model of OA. Our data showed that IL-1β (10 ng/mL) markedly induced apoptosis and upregulated the level of pro-inflammatory cytokine TNF-α. It has been shown that pro-inflammatory cytokines, such as TNF-α and IL-1β, play vital roles in OA progression.29 Zhong et al indicated that the level of TNF-α was significantly upregulated in ACLT-injured cartilage degradation.30 In the present study, Tanshinone I inhibited IL-1β-induced TNF-α expression level in CHON-001 cells. Yin et al found that leonurine pretreatment could inhibit IL-1β-induced inflammation in chondrocytes, which was consistent with our results.25 These data indicated that Tanshinone I could protect CHON-001 cells from IL-1β-induced inflammatory injury.
Previous study indicated that IL-1β could induce transcription of matrix metalloproteinases (MMPs) in rabbit chondrocytes via upregulating pro-inflammatory protein serum amyloid A.31 Moreover, pro-inflammatory cytokines could induce cartilage degradation via activation of MMPs.32 Meanwhile, IL-1β could increase the expressions of proinflammatory genes, like MMP-9 and MMP-13, and inhibit aggrecan synthesis.33 MMP is a kind of proteolytic enzymes which could degrade the ECM components in OA.34 The destruction of ECM components of cartilage is the hallmark of OA.35 Type II collagen (Collagen II) and aggrecan mainly comprise of cartilage ECM structure, which plays an important role in maintaining movement of the joints.35,36 MMP-13 is considered as a central node in the cartilage degradation network because it could cleave collagen-II.37 In the present study, IL-1β markedly downregulated the levels of collagen II and aggrecan, whereas notably upregulated the level of MMP-13 in CHON-001 cells. However, the IL-1β-induced collagen II, aggrecan degradation and MMP-13 upregulation in CHON-001 cells were notably reversed by Tanshinone I treatment, which is in agreement with Jia et al, who found that Tanshinone IIA significantly alleviated articular cartilage degradation in OA.38 These results suggest that Tanshinone I prevented IL-1β-induced ECM degradation in CHON-001 cells.
SOX11, one of the SOXC transcription factors, plays an important role in skeletal formation and neurogenesis.39,40 Previous study found that SOX11 act as one of the molecules down-regulated in osteoarthritic cartilage.41 Kan et al indicated that SOX11 was downregulated in degraded articular cartilages.39 Consistent with these results, the expression of SOX11 was decreased in IL-1β-stimulated CHON-001 cells. However, the IL-1β-induced SOX11 downregulation in CHON-001 cells was markedly reversed by Tanshinone I treatment. These results suggest that Tanshinone I could alleviate the progression of OA via upregulation the level of SOX11.
NF-κB plays an important role in regulating the immune response, which could be stimulated by pro-inflammatory cytokines and extracellular matrix (ECM) degradation products.42 The IL-1β activated NF-κB pathway is associated with multiple inflammatory pathologies.43 The activated NF-κB molecules could induce destruction of the articular joint, leading to the progression of OA.42,44 Lin et al found that nobiletin could suppress IL-1β-induced inflammation in chondrocytes via inhibition of NF-κB.45 In the present study, we found that the expression of p-NF-κB was increased in IL-1β-stimulated CHON-001 cells, which was markedly reversed by Tanshinone I treatment. These data illustrated that Tanshinone I could alleviate the progression of OA via suppression the level of NF-κB. In addition, a murine OA model was established in this study to further investigate the protective effects of Tanshinone I. Morphological and pathological observations demonstrated that Tanshinone I obviously decreased cartilage degradation and reduced the OARSI scores in murine OA model. Taken together, the results suggested that Tanshinone I could attenuate the progression of OA in vitro and in vivo. However, further investigation is required to demonstrate the precise mechanism of action of Tanshinone I on the inflammatory process in OA.
Conclusion
In conclusion, this study demonstrated that Tanshinone I alleviated IL-1β-induced apoptosis, ECM degradation and inflammation in CHON-001 cells. In addition, Tanshinone I decreased the cartilage degradation as well as synovitis in a murine OA model. These results demonstrated that Tanshinone I might be regarded as a promising therapeutic agent for the treatment of OA.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wong M, Desai B, Bautista M, et al. YouTube is a poor source of patient information for knee arthroplasty and knee osteoarthritis. Arthroplast Today. 2019;5(1):78–82. doi:10.1016/j.artd.2018.09.010
2. Roman-Blas JA, Bizzi E, Largo R, Migliore A, Herrero-Beaumont G. An update on the up and coming therapies to treat osteoarthritis, a multifaceted disease. Expert Opin Pharmacother. 2016;17(13):1745–1756. doi:10.1080/14656566.2016.1201070
3. Chapman K, Valdes AM. Genetic factors in OA pathogenesis. Bone. 2012;51(2):258–264. doi:10.1016/j.bone.2011.11.026
4. Ma Y, Wu Y, Chen J, et al. miR-10a-5p promotes chondrocyte apoptosis in osteoarthritis by targeting HOXA1. Mol Ther Nucleic Acids. 2019;14:398–409. doi:10.1016/j.omtn.2018.12.012
5. Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18(1):24–33. doi:10.1016/j.joca.2009.08.010
6. Guermazi A, Niu J, Hayashi D, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham osteoarthritis study). Bmj. 2012;345:e5339.
7. Nardo L, Parimi N, Liu F, et al. Femoroacetabular impingement: prevalent and often asymptomatic in older men: the osteoporotic fractures in men study. Clin Orthop Relat Res. 2015;473(8):2578–2586. doi:10.1007/s11999-015-4222-0
8. Zhu B, Cui G, Zhang Q, Cheng X, Tang S. Desumoylation of aggrecan and collagen II facilitates degradation via aggrecanases in IL-1beta-mediated osteoarthritis. J Pain Res. 2019;12:2145–2153. doi:10.2147/JPR.S194306
9. Xu S, Yu J, Wang Z, et al. SOX11 promotes osteoarthritis through induction of TNF-alpha. Pathol Res Pract. 2019;215(7):152442. doi:10.1016/j.prp.2019.152442
10. Duan ZX, Huang P, Tu C, et al. MicroRNA-15a-5p regulates the development of osteoarthritis by targeting PTHrP in chondrocytes. Biomed Res Int. 2019;2019:3904923.
11. Jing X, Xu Y, Cheng W, et al. Tanshinone I induces apoptosis and pro-survival autophagy in gastric cancers. Cancer Chemother Pharmacol. 2016;77(6):1171–1181. doi:10.1007/s00280-016-3034-6
12. Dai C, Liu Y, Dong Z. Tanshinone I alleviates motor and cognitive impairments via suppressing oxidative stress in the neonatal rats after hypoxic-ischemic brain damage. Mol Brain. 2017;10(1):52.
13. Gao H, Huang L, Ding F, et al. Simultaneous purification of dihydrotanshinone, tanshinone I, cryptotanshinone, and tanshinone IIA from Salvia miltiorrhiza and their anti-inflammatory activities investigation. Sci Rep. 2018;8(1):8460.
14. Wang S, Jing H, Yang H, et al. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. J Ethnopharmacol. 2015;164:247–255. doi:10.1016/j.jep.2015.01.042
15. Kim SY, Moon TC, Chang HW, et al. Effects of tanshinone I isolated from Salvia miltiorrhiza bunge on arachidonic acid metabolism and in vivo inflammatory responses. Phytother Res. 2002;16(7):616–620. doi:10.1002/ptr.941
16. Luan L, Liang Z. Tanshinone IIA protects murine chondrogenic ATDC5 cells from lipopolysaccharide-induced inflammatory injury by down-regulating microRNA-203a. Biomed Pharmacother. 2018;103:628–636. doi:10.1016/j.biopha.2018.04.051
17. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi:10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9
18. Zhen G, Wen C, Jia X, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–712. doi:10.1038/nm.3143
19. Lu J, Ji ML, Zhang XJ, et al. MicroRNA-218-5p as a potential target for the treatment of human osteoarthritis. Mol Ther. 2017;25(12):2676–2688. doi:10.1016/j.ymthe.2017.08.009
20. Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi:10.1016/j.joca.2005.07.014
21. Lewis JS, Hembree WC, Furman BD, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage. 2011;19(7):864–873. doi:10.1016/j.joca.2011.04.011
22. Shi Y, Hu X, Cheng J, et al. A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development. Nat Commun. 2019;10(1):1914.
23. Zeng RM, Lu XH, Lin J, et al. Knockdown of FOXM1 attenuates inflammatory response in human osteoarthritis chondrocytes. Int Immunopharmacol. 2019;68:74–80. doi:10.1016/j.intimp.2018.12.057
24. Liu L, Gu H, Liu H, et al. Protective effect of resveratrol against IL-1beta-induced inflammatory response on human osteoarthritic chondrocytes partly via the TLR4/MyD88/NF-kappaB signaling pathway: an “in vitro study”. Int J Mol Sci. 2014;15(4):6925–6940. doi:10.3390/ijms15046925
25. Yin W, Lei Y. Leonurine inhibits IL-1beta induced inflammation in murine chondrocytes and ameliorates murine osteoarthritis. Int Immunopharmacol. 2018;65:50–59. doi:10.1016/j.intimp.2018.08.035
26. Yang X, Zhang Q, Gao Z, Yu C, Zhang L. Baicalin alleviates IL-1beta-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed Pharmacother. 2018;99:184–190. doi:10.1016/j.biopha.2018.01.041
27. Huang Y, Wu D, Fan W. Protection of ginsenoside Rg1 on chondrocyte from IL-1beta-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol Cell Biochem. 2014;392(1–2):249–257. doi:10.1007/s11010-014-2035-1
28. Cen X, Liu Y, Wang S, et al. Glucosamine oral administration as an adjunct to hyaluronic acid injection in treating temporomandibular joint osteoarthritis. Oral Dis. 2018;24(3):404–411. doi:10.1111/odi.12760
29. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459.
30. Zhong G, Liang R, Yao J, et al. Artemisinin ameliorates osteoarthritis by inhibiting the Wnt/beta-Catenin signaling pathway. Cell Physiol Biochem. 2018;51(6):2575–2590. doi:10.1159/000495926
31. Vallon R, Freuler F, Desta-Tsedu N, et al. Serum amyloid A (apoSAA) expression is up-regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. J Immunol. 2001;166(4):2801–2807. doi:10.4049/jimmunol.166.4.2801
32. Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35(12):2306–2312.
33. Madhavan S, Anghelina M, Rath-Deschner B, et al. Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthritis Cartilage. 2006;14(10):1023–1032. doi:10.1016/j.joca.2006.03.016
34. Nummenmaa E, Hamalainen M, Moilanen T, Vuolteenaho K, Moilanen E. Effects of FGF-2 and FGF receptor antagonists on MMP enzymes, aggrecan, and type II collagen in primary human OA chondrocytes. Scand J Rheumatol. 2015;44(4):321–330. doi:10.3109/03009742.2014.1000372
35. Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov. 2005;4(4):331–344. doi:10.1038/nrd1693
36. Gooljarsingh LT, Lakdawala A, Coppo F, et al. Characterization of an exosite binding inhibitor of matrix metalloproteinase 13. Protein Sci. 2008;17(1):66–71. doi:10.1110/ps.073130208
37. Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248.
38. Jia PT, Zhang XL, Zuo HN, Lu X, Li L. Articular cartilage degradation is prevented by tanshinone IIA through inhibiting apoptosis and the expression of inflammatory cytokines. Mol Med Rep. 2017;16(5):6285–6289. doi:10.3892/mmr.2017.7340
39. Kan A, Ikeda T, Fukai A, et al. SOX11 contributes to the regulation of GDF5 in joint maintenance. BMC Dev Biol. 2013;13:4.
40. Lefebvre V, Bhattaram P. SOXC genes and the control of skeletogenesis. Curr Osteoporos Rep. 2016;14(1):32–38. doi:10.1007/s11914-016-0296-1
41. Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3(11):e3740.
42. Rigoglou S, Papavassiliou AG. The NF-kappaB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45(11):2580–2584. doi:10.1016/j.biocel.2013.08.018
43. Scholz CC, Cavadas MA, Tambuwala MM, et al. Regulation of IL-1beta-induced NF-kappaB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci U S A. 2013;110(46):18490–18495. doi:10.1073/pnas.1309718110
44. Liu YX, Wang GD, Wang X, Zhang YL, Zhang TL. Effects of TLR-2/NF-kappaB signaling pathway on the occurrence of degenerative knee osteoarthritis: an in vivo and in vitro study. Oncotarget. 2017;8(24):38602–38617. doi:10.18632/oncotarget.16199
45. Lin Z, Wu D, Huang L, et al. Nobiletin inhibits IL-1beta-induced inflammation in chondrocytes via suppression of NF-kappaB signaling and attenuates osteoarthritis in mice. Front Pharmacol. 2019;10:570. doi:10.3389/fphar.2019.00848
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.