Back to Journals » OncoTargets and Therapy » Volume 12
Tanshinol inhibits the growth, migration and invasion of hepatocellular carcinoma cells via regulating the PI3K-AKT signaling pathway
Authors Zhu P, Liu Z, Zhou J, Chen Y
Received 31 August 2018
Accepted for publication 28 November 2018
Published 19 December 2018 Volume 2019:12 Pages 87—99
DOI https://doi.org/10.2147/OTT.S185997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Pingting Zhu,1,2,* Zhaoguo Liu,3,* JiaoJiao Zhou,1 Yuanyuan Chen1
1School of Nursing, Yangzhou University, Yangzhou, China; 2Jiangsu Key Laboratory of Zoonosis, Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, China; 3Department of Pharmacology, School of Pharmacy, Nantong University, Nantong, China
*These authors contributed equally to this work
Background: Tanshinol is an active constituent of Salvia miltiorrhiza and possess anti-inflammatory, antioxidant, and anti-bacterial activity. Herein, we explored the role of tanshinol on the growth and aggressiveness of hepatocellular carcinoma (HCC) cells in vitro and in vivo.
Materials and methods: The proliferation of a panel of HCC cell lines was measured using MTT assay. The expressions of phosphatidylinositol 3 kinase (PI3K) and protein kinase B (AKT) were detected by immunofluorescence staining and immunohistochemical assay. The levels of Bcl-2 and Bax were determined using immunoblotting assay. The secretions of matrix metalloproteinase-2 (MMP-2) and MMP-9 were detected by ELISA. The migration and invasion abilities of HepG2 cell were determined using wound healing and Transwell invasion assays. The apoptosis of HepG2 cell induced by tanshinol was analyzed by Annexin V/propidium iodide staining. A xenograft model was constructed to investigate the inhibitory effect of tanshinol on HepG2 cell growth in vivo. To further investigate the role of tanshinol on the metastasis of HepG2 cell in vivo, an experimental metastasis assay was performed.
Results: Tanshinol inhibited the growth and colony formation of HCC cell in vitro. Tanshinol also induced the apoptosis of HepG2 cell and inhibited the migration and invasion of HepG2 cell. In in vivo experiments, tanshinol suppressed the tumor growth and metastasis of HepG2 cell. Furthermore, the phosphorylation of PI3K and AKT was decreased by tanshinol in vitro and in vivo.
Conclusion: Tanshinol exerts its anti-cancer effects via regulating the PI3K-AKT signaling pathway in HCC.
Keywords: tanshinol, HCC, migration, invasion, apoptosis
Introduction
Hepatocellular carcinoma (HCC) is one of the common malignant tumors and remains the leading cause of cancer-related death worldwide.1,2 Multiple genes influence the tumorigenesis and progression of HCC and accumulation of multiple molecular changes are involved in the progression of HCC.3,4 Surgery, radiotherapy, and chemotherapy are the major therapeutic options available for patients with HCC.5 Unfortunately, the clinical outcomes of patients with HCC are still poor owing to the limited therapeutic strategies. Hence, it is urgent to find a novel therapeutic treatment for HCC.
With a history of thousands of years of clinical practice, traditional Chinese medicine plays important roles in maintaining the health of Asian people and is being increasingly applied all over the world.6–10 Salvia miltiorrhiza has a long history of use for medicinal purposes in China. Currently, it is frequently used in herbal medicine for its anti-inflammatory activity, anti-arthritic properties, wound and burn healing capabilities, and anti-bacterial/ anti-cancer properties.11–15 There are several biologically active constituents in S. miltiorrhiza, including tanshinol.16–18 Previous investigation has demonstrated that tanshinol regulates adhesion molecule expression of tumor necrosis factor alpha (TNF-α)-induced endothelial cells by blocking activation of nuclear factor kappa B (NF-κB).19 Additional, tanshinol inhibits the growth of vascular smooth muscle cell (VSMC) and protective effect on the VSMC damage induced by hydrogen peroxide by increasing the expression of nitric oxide (NO).20 Furthermore, combination of tanshinol and paeonol inhibits the isoproterenol-induced myocardial infarction in myocardial infarcted rats through inhibition of nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) and PI3K/AKT signaling pathway.21 Nevertheless, the potential roles of tanshinol in anti-HCC are not well investigated.
In the current study, we investigated the suppressive roles of tanshinol on the growth and metastasis of HCC cell. The results indicated that tanshinol remarkably inhibited the proliferation, migration, and invasion of HCC HepG2 cell in vitro. We further demonstrated that tanshinol induced the apoptosis of HepG2 cell and suppressed the activation of the PI3K-AKT signaling cascade. In vivo, tanshinol suppressed the growth and metastasis of HCC cell. All these results indicated the promising preclinical activity of tanshinol in the treatment of HCC.
Materials and methods
Cell culture
Tanshinol was purchased from Pure-one Biotechnology (P0907, Purity ≥98% Shanghai, China). The HCC cell lines, including SNU-423, PLC/PRF/5, SNU-449, and HepG2, were purchased from the GuangZhou Jennio Biotech Co., Ltd (GuangZhou, GuangDong, China) and were maintained in 1,1640 medium or DMEM (Invitrogen, Carlsbad, CA, USA) and supplemented with 10% FBS (Invitrogen), 100 U/mL penicillin/streptomycin (Sigma-Aldrich, St Louis, MO, USA).
MTT assay
Briefly, HepG2 cells (1×104) were seeded in 96-well plates and then treated with tanshinol (0, 1, 5, 10, 20, and 30 μM) for 48 hours. HepG2 cells were treated with 5 μM tanshinol for 24, 48, and 72 hours. A total of 10 μL of MTT (5 mg/mL; Sigma-Aldrich) was added into each well and then incubated for 4 hours at 37°C. Next, 200 μL of dimethyl sulfoxide (DMSO) was added to the 96-well plates. Finally, the absorbance was measured at 490 nm on a microplate reader. The overall experiments were repeated at least three times.
Trypan blue exclusion test
HepG2 cells (5×105 cells/mL) were incubated with various concentrations of tanshinol (1, 5, and 10 μM) for 48 hours in 96-well plates. HepG2 cells (5×105 cells/mL) were incubated with 5 μM tanshinol for 24, 48, and 72 hours. After culture, cell numbers were evaluated by staining with trypan blue and counting using light microscopy.
Colony formation assay
HepG2 cells (1×103) were cultured into 6-well plates and were allowed to grow for 2 weeks with added tanshinol (1, 5, and 10 μM). Then, colonies were stained using 1% crystal violet. The cell colonies were photographed and the number of colonies was counted.
Hochest 33342 staining
Cells (1×103) were treated with tanshinol (1, 5, and 10 μM) for 48 hours. Cells were washed three times with PBS and cell nucleus were stained using 5 μg/mL Hoechst 33342 (Beyotime, Nanjing, Jiangsu, China) for 10 minutes. Then, images were taken using the ZEN 2011 imaging software on a Zeiss invert microscope (CarlZeiss, Hallbergnoos, Germany).22,23
Apoptosis analysis
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Beyotime) was used to detect the apoptosis of tanshinol-treated HepG2 cells according to the manufacturer’s instructions. HepG2 cells were seeded into 6 well plates with a density of 1×105 cells/well for 24 hours and incubated with the tanshinol (1, 5, and 10 μM) for 48 hours. After incubating with tanshinol, HepG2 cells were harvested, washed three times with PBS, suspended in Annexin V binding buffer, and incubated with FITC-labeled Annexin V and PI for 5 minutes at room temperature in the dark. Then, the samples were immediately analyzed by Flow cytometry (BD Biosciences, San Jose, CA, USA).
Migration assay
Cells were cultured into 6-well plates overnight. When the degree of fusion reached 95% or more, a 100 μL sterile pipette tip was applied to create a wound in each well. After washing with PBS, cells were treated with medium containing tanshinol (1, 5, and 10 μM) for 24 hours. The wounds were observed and photographed at 0 hour or 24 hours under a Nikon microscope. The rate of scratch healing was calculated. Scratch healing rate = (0 hour width of scratch − 24 hours width of scratch)/0 hour width of scratch ×100%.24
Invasion assay
Transwell chambers (Corning Incorporated, Corning, NY, USA) with polycarbonate filters (8 μm pore size) were pre-coated with 50 μL Matrigel (BD Biosciences). HepG2 cells were pre-treated with tanshinol for 24 hours, and then cells were collected and re-suspended in FBS-free DMEM. A total of 100 μL cell suspensions (1×104) were plated into the upper chamber. After this, 600 μL DMEM medium containing 20% FBS was added into the lower chamber. After 12 hours, membranes were stained using 1% crystal violet and the number of the invasive cells was manually counted from five random fields by bright field microscopy.25 Each experiment was repeated thrice.
Immunocytochemistry staining
HepG2 cells (1×103) were grown on the glass slide and treated with tanshinol (1, 5, and 10 μM) for 24 hours. Then, cells permeabilized using 1% TritonX-100 for 10 minutes. Then, the cells were incubated with primary antibody Bcl-2 or Bax (1:100) (Cell Signaling Technology, Danvers, MA, USA) for overnight. After being washed with PBS for three times, cells were incubated with the biotin labeled secondary antibody for 30 minutes and stained with the chain mildew avidin peroxidase (Beyotime). Finally, 3,3′-diaminobenzidine was applied to color reaction.
ELISA
HepG2 cells (1×103) were cultured into 6-well plates for overnight and treated by tanshinol (1, 5, and 10 μM) for 24 hours. Then, the culture media were harvested and stored at −20°C. Commercially available human matrix metalloproteinase (MMP)-2/MMP-9 ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to measure the levels of MMP-2 and MMP-9, according to the manufacturer’s instructions.
Western blot assay
Proteins were extracted from HepG2 cells using RIPA lysis (Beyotime) according to manufacturer’s protocol and were quantified using a bicinchoninic acid kit (Beyotime). A total of 30 μg proteins were loaded to 10% SDS-PAGE gel and transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After blocking with 5% skim milk in Tris-buffered saline with Tween-20 (TBST), PVDF membrane was incubated with primary antibodies (phospho-PI3 Kinase p85Tyr458/p55Tyr199, phospho-AKTSer473, total-PI3K, total-AKT, Bcl-2, Bax, and glyceraldehyde-3-phosphate dehydrogenase) (Cell Signaling Technology) overnight. After that, the membrane was washed with TBST three times and incubated with a secondary antibody (1:5,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) for 2 hours at 37°C. Target proteins were detected using the enhanced chemiluminescence system (Millipore) and visualized with the ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA).
Mouse xenograft and metastasis model
A total of 100 μL HepG2 cells (2×106) were injected into 6-week-old athymic nude mice. Then, mice were randomly assigned to four groups: control group, vehicle-treated group, 10 mg/kg tanshinol-treated group, and 30 mg/kg tanshinol-treated group. Tanshinol was dissolved in DMSO. Mice in the experimental groups were administrated tanshinol intragastrically daily for 5 weeks. Control mice in vehicle-treated group received the same dose of vehicle. Tumor width (W) and length (L) were measured each week. The tumor volume was calculated using the following formula: L × W2/2. After 5 weeks, mice were sacrificed. In lung metastasis model, nude mice were injected with 100 μL HepG2 cells (1×106) via the lateral tail vein. Mice in the experimental groups were administrated tanshinol intragastrically daily. Tanshinol was dissolved in DMSO. Control mice in vehicle-treated group received the same dose of vehicle only. After 1 month, the mice were euthanized and the lungs were removed for H&E staining. Animal experiments were approved by the Ethics Committee of Yangzhou University. Our animal experiments were conducted in accordance with Institutional Guidelines and the Guide for the Care and Use of Laboratory Animals (NIH publication no 85-23, revised 1996).
Immunohistochemical (IHC) staining
Briefly, formalin-fixed paraffin-embedded tumor tissues were prepared in 4-μm sections. After performing dewaxing, rehydration, blocking endogenous peroxidase activity, and antigen retrieval steps, sections were blocked with 10% normal goat serum at room temperature for 15 minutes and incubated with primary antibodies against Bcl-2, Bax, p-PI3K, and p-AKT at 4°C overnight. The sections were subsequently treated with horseradish peroxidase-conjugated secondary antibody (Nichirei, Tokyo, Japan), and the reactions were visualized with diaminobenzidine. The number of immunopositive cells was counted using the BZ-9000 analysis software programmer (Keyence, Osaka, Japan).
Statistical analysis
Statistical analysis was conducted using SPSS (Statistical Package for the Social Sciences) 13.0 (SPSS Inc., Chicago, IL, USA). All data were expressed as mean ± SD. Differences were evaluated using either two-tailed Student’s t-test or one-way ANOVA followed by post hoc Dunnett’s test. P<0.05 was considered statistically significant.
Results
Tanshinol inhibits the growth of HCC cell in vitro
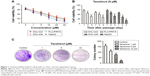
To evaluate the inhibitory effect of tanshinol on HCC cells, four human HCC cell lines were treated with different concentrations of tanshinol (0, 1, 5, 10, 20, and 30 μM) or 5 μM tanshinol for different times (24, 48, and 72 hours). As shown in Figure 1A and B, the MTT assay suggested that tanshinol suppressed the growth of HCC cells in a dose- dependent and time-responsive manner. We also found that the HepG2 cell was most sensitive to tanshinol. Consistent with the results of MTT test, the trypan blue exclusion assay demonstrated that tanshinol effectively inhibited the proliferation of HCC cell in a dose- dependent and time-responsive manner (Figure S1A and B). Hence, HepG2 cell was selected as the optimal cell model for the following examinations. We adopted the doses 0, 1, 5, and 10 μM of tanshinol and performed colony formation assay. Quantitative analysis revealed that the number of cell colonies was reduced by tanshinol (Figure 1C). All these data indicate that tanshinol suppresses the proliferation and colony formation of HCC cell in vitro.
Tanshinol induces the apoptosis of HepG2 cell
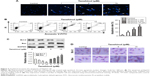
We further examined the mechanism behind impairment of HCC cell viability by tanshinol, the Hoechst 33342 nuclear staining assays were conducted to analyze the nuclear morphology of HepG2 cell after treating with tanshinol. As shown in Figure 2A, the nuclei of control cells were stained weakly and homogeneously blue, whereas in the cells that were treated with tanshinol, blue emission was much brighter than in the control cells. Then, the apoptosis of HepG2 cell induced by tanshinol was further confirmed using flow cytometry analysis. As shown in Figure 2B, the apoptosis of HepG2 cell treated with tanshinol was significantly increased compared with control group. Furthermore, the protein expression of pro- and anti-apoptotic mitochondria protein Bax and Bcl-2 in HepG2 cell were analyzed by immunoblotting assay. As shown in Figure 2C, tanshinol increased the level of Bax and conversely reduced the protein expression of Bcl-2 in HepG2 cell. The levels of Bax and Bcl-2 in HepG2 cell were also confirmed by IHC staining (Figure 2D). These findings suggest that the inhibitory impact of tanshinol on the growth of HCC cell is closely correlated with it inducing apoptosis.
Tanshinol inhibits the migration and invasion of HepG2 cell
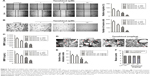
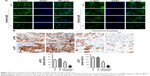
The effects of tanshinol on HepG2 cell metastasis were subsequently assessed. As shown in Figure 3A, tanshinol obviously inhibited the migration of HepG2 cell compared with control as shown in the wound healing assay. In Transwell assay, the number of invasive HepG2 cells was markedly decreased in tanshinol-treated group (Figure 3B). Besides, the secretions of MMP-2 and MMP-9 were significantly reduced by tanshinol (Figure 3C). In order to assess the impact of tanshinol on the metastasis of HepG2 cell in vivo, the animal model of experimental pulmonary metastasis was constructed. The number of metastatic colonies in the lung from the mice that were treated with tanshinol was significantly less than that in the control group (Figure 3D and E). Furthermore, there was a higher incidence of tumor nodules in the lung from mice without tanshinol treatment compared with those mice treated with tanshinol (Figure 3F). Our data suggest that tanshinol inhibits the metastasis of HCC cell in vivo and in vitro.
Tanshinol inhibits tumor growth of HepG2 cell in vivo
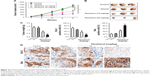
A xenograft model of HCC was constructed to further investigate the impact of tanshinol on the growth of HepG2 cell in vivo. Mice in experimental groups were treated with 10 mg/kg or 30 mg/kg of tanshinol daily for 5 weeks. The tumor volume of the control group was significantly larger than those of the experimental groups (Figure 4A and B). Consistently, the tumor weight was largest in control mice and was smaller in the tanshinol-treated mice (Figure 4C). Moreover, in tanshinol-treated xenograft tumors, the levels of Bax and Bcl-2 were detected using IHC staining assay (Figure 4D). Consistently, the protein expression of Bcl-2 was significantly inhibited by tanshinol compared with control group whereas the expression of Bax was remarkably increased by tanshinol treatment (Figure S2). These data suggest that tanshinol inhibits HepG2 cell growth in vivo.
Tanshinol inhibits the activities of the PI3K and AKT
After treatment of HepG2 cell with tanshinol, the phosphorylation of PI3K-AKT family member (PI3K and AKT), which regulate a wide variety of cellular functions was detected using Western blot assay. As shown in Figure 5A, the phosphorylation of PI3K and AKT in HepG2 cell was remarkably decreased by tanshinol. Furthermore, tanshinol significantly suppressed the phosphorylation of PI3K and AKT in vivo as demonstrated by IHC staining assay (Figure 5B). Consistently, the Western blotting assay indicated that the phosphorylation of PI3K and AKT in tumor tissue was significantly inhibited by tanshinol compared with control group (Figure S3). These data suggest that the PI3K/AKT signaling pathway is inactivated by tanshinol.
Discussion
HCC is still one of the leading causes of cancer-related death worldwide. During the past few years, the clinical diagnosis and treatment for patients with HCC have made great progress.26 However, a large proportion of patients with HCC are diagnosed at the late stage of disease, and recurrence or metastasis in HCC are closely associated to poor overall survival. Tanshinol is an active constituent of the Chinese medicinal herb S. miltiorrhiza and has been shown to exert anti-inflammatory and anti-fibrotic effects, and has anti-bacterial properties.27–32 Previous investigation indicates that tanshinol has anti-tumor activities in melanoma cell through inhibiting angiogenesis and cell metastasis.33 In addition, tanshinol promotes the radioresponse of Lewis lung carcinoma in mice model by alleviating tumor cell hypoxia.34 Recently, investigation has proved that tanshinol has protective effects on the carbon tetrachloride l4-induced liver fibrosis by suppressing oxidative stress and inflammation via regulating Nrf2/HO-1 signaling pathway.16 Nevertheless, the potential anti-cancer role of tanshinol in HCC should be further explored. In the present study, we mainly investigated the possible therapeutic values of tanshinol against HCC.
First, we found that tanshinol treatment inhibited the proliferation of HCC cell in both dose- and time-dependent manner. In colony formation assay, the clonogenic potential of HepG2 cell was remarkably inhibited by tanshinol, which further confirmed that tanshinol suppressed the growth of HepG2 cell in vitro. In addition, a marked increase of apoptosis in HepG2 cell was observed following tanshinol treatment. Importantly, tanshinol inhibited tumor growth of HepG2 cell in vivo. In all, our data suggest that tanshinol has a potential anti-cancer effect in HCC. Cancer metastasis requires several crucial interrelated events, such as cancer cell migration and invasion. In this study, we also investigated the migration and invasion of HepG2 cell that was treated with tanshinol. As expected, the migration and invasion abilities were remarkably reduced by tanshinol. Dissemination of cancer cells from the primary tumor is another crucial event in the process of cancer invasion and metastasis. This process involves degradation of the extracellular matrix by many proteases of which MMP-2 and MMP-9 appear to play a key role.35 Indeed, the up-regulation of the expression of MMPs correlates with the increased metastatic potential of cancer cells.36 Therefore, inhibiting the level of MMP-2/9 is an important approach in the fight against cancer metastasis. Here, we clearly demonstrated that the MMP-2 and MMP-9, which are promoters of distant metastasis, were also decreased in the tanshinol-treatment group. Collectively, our data show that tanshinol significantly inhibits the growth and metastasis of HCC cell.
The PI3K/AKT signaling pathway is elevated in a significant portion of primary and metastatic cancer.37,38 Recent studies have presented that PI3K/AKT pathway activation is a key character of the metastasis of several types of cancers.39,40 PI3K-AKT signaling pathway regulates various important cellular processes, including cancer cell proliferation, apoptosis survival, and adhesion.41,42 Hence, inhibition of PI3K-AKT signaling pathway offers a promising strategy in targeting malignant cancer. In our current study, the levels of PI3K and AKT phosphorylation were decreased in the tanshinol-treated HepG2 cell, indicating that tanshinol could inhibit the activation of PI3K/AKT pathway. Consistently, tanshinol reduced the activities of PI3K and AKT in the xenograft model of HCC that was established using HepG2 cell. Increased levels of MMP-2 and MMP-9 are known to promote migration, invasion, and metastasis of various types of cancers.43–45 PI3K-AKT is the most important positive regulator of the expressions of MMP-2 and MMP-9.46 As expected, tanshinol treatment significantly reduced the levels of MMP-2 and MMP-9 in HCC cell. Thus, we speculated that tanshinol inhibited the growth and metastasis of HCC via regulating the PI3K-AKT signaling pathway. Although, we found that tanshinol inhibited the growth, migration, invasion, and metastasis of HCC cell, the correlation between the anti-cancer effects of tanshinol and its suppression of the activity of PI3K-AKT has not been well investigated. In future studies, to confirm the anti-cancer effect of tanshinol on HCC, the specific PI3K-AKT inhibitor or siRNA interference could be applied.
Conclusion
In summary, the present study identified tanshinol as a novel therapeutic compound to inhibit the proliferation, migration, invasion, and metastasis of HCC cell. The anti-cancer activity of tanshinol might be through regulation of the PI3K-AKT signaling pathway.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81403260, No 81703884), China Postdoctoral Science Foundation (No 2014M551639), Natural Science Foundation of Jiangsu Province (No BK20140493), postdoctoral funding in Jiangsu province (No 1401138C), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No 17KJB360015, No 17KJB360012), technical project of Yangzhou city (No YZ2017090), and Young Backbone Teacher Foundation of Yangzhou University.
Disclosure
The authors report no conflicts of interest in this work.
References
Xue Y, Jia X, Li L, et al. DDX5 promotes hepatocellular carcinoma tumorigenesis via Akt signaling pathway. Biochem Biophys Res Commun. 2018;503(4):2885–2891. | ||
Mo Y, He L, Lai Z, et al. LINC01287 regulates tumorigenesis and invasion via miR-298/MYB in hepatocellular carcinoma. J Cell Mol Med. 2018;22(11):5477–5485. | ||
Xiao L, Wang Y, Liang W, et al. LRH-1 drives hepatocellular carcinoma partially through induction of c-myc and cyclin E1, and suppression of p21. Cancer Manag Res. 2018;10:2389–2400. | ||
Lapointe-Shaw L, Georgie F, Carlone D, et al. Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: a validation study. PLoS One. 2018;13(8):e0201120. | ||
Shao Z, Li Y, Dai W, et al. ETS-1 induces sorafenib-resistance in hepatocellular carcinoma cells via regulating transcription factor activity of PXR. Pharmacol Res. 2018;135:188–200. | ||
Zhong P, Yang H, Lin S, Peng J, Lin J. A traditional Chinese medicine herb mixture qingjie fuzheng granules inhibits hepatocellular carcinoma cells growth by inducing apoptosis. J Evid Based Integr Med. 2018;23:2515690X1878963. | ||
Pan B, Zang J, He J, Wang Z, Liu L. Add-on therapy with Chinese herb medicine Bo-Er-Ning capsule (BENC) improves outcomes of gastric cancer patients: a randomized clinical trial followed with bioinformatics-assisted mechanism study. Am J Cancer Res. 2018;8(6):1090–1105. | ||
Jiang JH, Pi J, Jin H, Yang F, Cai JY. Chinese herb medicine matrine induce apoptosis in human esophageal squamous cancer KYSE-150 cells through increasing reactive oxygen species and inhibiting mitochondrial function. Pathol Res Pract. 2018;214(5):691–699. | ||
Geng CA, Yang TH, Huang XY, et al. Anti-hepatitis B virus effects of the traditional Chinese herb Artemisia capillaris and its active enynes. J Ethnopharmacol. 2018;224:283–289. | ||
Liu JH, Cheng YY, Hsieh CH, Tsai TH. The herb-drug pharmacokinetic interaction of 5-Fluorouracil and its metabolite 5-Fluoro-5,6-Dihydrouracil with a traditional Chinese medicine in rats. Int J Mol Sci. 2017;19(1):25. | ||
Ma X, Yang J, Deng S, et al. Two new megastigmanes from Chinese traditional medicinal plant Sedum sarmentosum. Nat Prod Res. 2017;31(13):1473–1477. | ||
Bai Y, Wu C, Hong W, Zhang X, Liu L, Chen B. Anti-fibrotic effect of Sedum sarmentosum Bunge extract in kidneys via the hedgehog signaling pathway. Mol Med Rep. 2017;16(1):737–745. | ||
Bai Y, Lu H, Zhang G, et al. Sedum sarmentosum Bunge extract exerts renal anti-fibrotic effects in vivo and in vitro. Life Sci. 2014;105(1–2):22–30. | ||
Jung HJ, Kang HJ, Song YS, Park EH, Kim YM, Lim CJ. Anti-inflammatory, anti-angiogenic and anti-nociceptive activities of Sedum sarmentosum extract. J Ethnopharmacol. 2008;116(1):138–143. | ||
Kang TH, Pae HO, Yoo JC, et al. Antiproliferative effects of alkaloids from Sedum sarmentosum on murine and human hepatoma cell lines. J Ethnopharmacol. 2000;70(2):177–182. | ||
Wang R, Wang J, Song F, Li S, Yuan Y. Tanshinol ameliorates CCl4-induced liver fibrosis in rats through the regulation of Nrf2/HO-1 and NF-κB/IκBα signaling pathway. Drug Des Devel Ther. 2018;12:1281–1292. | ||
Peng R, Wang S, Wang R, Wang Y, Wu Y, Yuan Y. Antifibrotic effects of tanshinol in experimental hepatic fibrosis by targeting PI3K/AKT/mTOR/p70S6K1 signaling pathways. Discov Med. 2017;23(125):81–94. | ||
Lu C, Zeng YQ, Liu H, et al. Tanshinol suppresses cardiac allograft rejection in a murine model. J Heart Lung Transplant. 2017;36(2):227–236. | ||
Ding M, Zhao GR, Yuan YJ, Guo ZX. Aqueous extract of Salvia miltiorrhoza regulates adhesion molecule expression of tumor necrosis factor alpha-induced endothelial cells by blocking activation of nuclear factor kappaB. J Cardiovasc Pharmacol. 2005;45(6):516–524. | ||
Wu L, Li X, Li Y, Wang L, Tang Y, Xue M. Proliferative inhibition of danxiongfang and its active ingredients on rat vascular smooth muscle cell and protective effect on the VSMC damage induced by hydrogen peroxide. J Ethnopharmacol. 2009;126(2):197–206. | ||
Li H, Song F, Duan LR, et al. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci Rep. 2016;6:23693. | ||
Chen C, Wu J, Zhu P, Xu C, Yao L. Investigating isoquinoline derivatives for inhibition of inhibitor of apoptosis proteins for ovarian cancer treatment. Drug Des Devel Ther. 2017;11:2697–2707. | ||
Jin R, Xia Y, Chen Q, et al. Da0324, an inhibitor of nuclear factor-κB activation, demonstrates selective antitumor activity on human gastric cancer cells. Drug Des Devel Ther. 2016;10:979–995. | ||
Yin J, Ji Z, Hong Y, et al. Sh-MARCH8 inhibits tumorigenesis via PI3K pathway in gastric cancer. Cell Physiol Biochem. 2018;49(1):306–321. | ||
Cheng H, Dong H, Feng J, Tian H, Zhang H, Xu L. miR-497 inhibited proliferation, migration and invasion of thyroid papillary carcinoma cells by negatively regulating YAP1 expression. Onco Targets Ther. 2018;11:4711–4721. | ||
Ji K, Lin K, Wang Y, et al. TAZ inhibition promotes IL-2-induced apoptosis of hepatocellular carcinoma cells by activating the JNK/F-actin/mitochondrial fission pathway. Cancer Cell Int. 2018;18:117. | ||
Song W, Pu J, He B. Tanshinol protects human umbilical vein endothelial cells against hydrogen peroxide-induced apoptosis. Mol Med Rep. 2014;10(5):2764–2770. | ||
Tian DD, Jia WW, Liu XW, et al. Methylation and its role in the disposition of tanshinol, a cardiovascular carboxylic catechol from Salvia miltiorrhiza roots (Danshen). Acta Pharmacol Sin. 2015;36(5):627–643. | ||
Chen C, Cheng G, Yang X, Li C, Shi R, Zhao N. Tanshinol suppresses endothelial cells apoptosis in mice with atherosclerosis via lncRNA TUG1 up-regulating the expression of miR-26a. Am J Transl Res. 2016;8(7):2981–2991. | ||
Jia P, Wang S, Xiao C, et al. The anti-atherosclerotic effect of tanshinol borneol ester using fecal metabolomics based on liquid chromatography-mass spectrometry. Analyst. 2016;141(3):1112–1120. | ||
Luo S, Yang Y, Chen J, et al. Tanshinol stimulates bone formation and attenuates dexamethasone-induced inhibition of osteogenesis in larval zebrafish. J Orthop Translat. 2016;4:35–45. | ||
Yang Y, Wang L, Wu Y, et al. Tanshinol suppresses inflammatory factors in a rat model of vascular dementia and protects LPS-treated neurons via the MST1-FOXO3 signaling pathway. Brain Res. 2016;1646:304–314. | ||
Zhang LJ, Chen L, Lu Y, et al. Danshensu has anti-tumor activity in B16F10 melanoma by inhibiting angiogenesis and tumor cell invasion. Eur J Pharmacol. 2010;643(2–3):195–201. | ||
Cao HY, Ding RL, Li M, et al. Danshensu, a major water-soluble component of Salvia miltiorrhiza, enhances the radioresponse for Lewis lung carcinoma xenografts in mice. Oncol Lett. 2017;13(2):605–612. | ||
Littlepage LE, Sternlicht MD, Rougier N, et al. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010;70(6):2224–2234. | ||
Wilson MJ, Sellers RG, Wiehr C, Melamud O, Pei D, Peehl DM. Expression of matrix metalloproteinase-2 and -9 and their inhibitors, tissue inhibitor of metalloproteinase-1 and -2, in primary cultures of human prostatic stromal and epithelial cells. J Cell Physiol. 2002;191(2):208–216. | ||
Tan X, Chen S, Wu J, et al. PI3K/AKT-mediated upregulation of WDR5 promotes colorectal cancer metastasis by directly targeting ZNF407. Cell Death Dis. 2017;8(3):e2686. | ||
Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–156. | ||
Lim HJ, Crowe P, Yang JL. Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer. J Cancer Res Clin Oncol. 2015;141(4):671–689. | ||
Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. | ||
Danielsen SA, Eide PW, Nesbakken A, Guren T, Leithe E, Lothe RA. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim Biophys Acta. 2015;1855(1):104–121. | ||
Polivka J, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142(2):164–175. | ||
Li Z, Takino T, Endo Y, Sato H. Activation of MMP-9 by membrane type-1 MMP/MMP-2 axis stimulates tumor metastasis. Cancer Sci. 2017;108(3):347–353. | ||
Webb AH, Gao BT, Goldsmith ZK, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017;17(1):434. | ||
Liu J, Wen X, Liu B, et al. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP-2 and MMP-9. Mol Med Rep. 2016;13(3):2401–2408. | ||
Wu YJ, Neoh CA, Tsao CY, Su JH, Li HH. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci. 2015;16(7):16469–16482. |
Supplementary materials
  | Figure S2 The levels of Bcl-2 and Bax in tumor tissues that were formed by HepG2 cells were detected by Western blotting assay. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.