Back to Journals » Infection and Drug Resistance » Volume 15
Tandem Repeat of blaNDM-1 and Clonal Dissemination of a fosA3 and blaKPC-2 Co-Carrying IncR-F33: A–: B– Plasmid in Klebsiella pneumoniae Isolates Collected in a Southwest Hospital in China, 2010–2013
Authors Hu Y , Zhang W , Shen X , Qu Q , Li X, Chen R , Wang Z , Ma R , Xiong Z , Wang Y , Wang P
Received 29 September 2022
Accepted for publication 6 December 2022
Published 15 December 2022 Volume 2022:15 Pages 7431—7447
DOI https://doi.org/10.2147/IDR.S391144
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Ying Hu,1,* Wei Zhang,1,* Xiufen Shen,1,* Qiaoli Qu,1 Xiao Li,2 Rucai Chen,3 Zhuo Wang,1 Run Ma,1 Zaikun Xiong,3 Yuming Wang,1 Pengfei Wang3
1Department of Clinical Laboratory, The Second Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 2State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, and Key Laboratory for Southwest Microbial Diversity of the Ministry of Education, Yunnan University, Kunming, People’s Republic of China; 3Department of Key Laboratory, The Second Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuming Wang, Department of Clinical Laboratory, The Second Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China, Tel +86 13708406058, Fax +86-0871-65334416, Email [email protected] Pengfei Wang, Department of Key Laboratory, The Second Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China, Tel +86 15288453604, Email [email protected]
Introduction: Carbapenem-resistant Klebsiella pneumoniae (CRKP) has been widespread in coastal cities of eastern China since 2009. However, how CRKP spreads and evolves in southwest China is unclear.
Aim: We investigated the genetic characteristics and dissemination mechanisms of carbapenemase genes in forty-one non-repetitive CRKP isolates collected from a southwest hospital, Kunming, Yunnan, during 2010– 2013.
Methodology: Drug susceptibilities were analyzed by using VITEK 2 compact system. Genetic relationships were ascertained based on multilocus sequence typing (MLST) and Pulsed-field gel electrophoresis (PFGE) analysis. Genetic backgrounds of blaKPC-2 and blaNDM-1 were revealed by DNA walking and high-throughput sequencing.
Results: All isolates were highly resistant to common antibiotics except for tigecycline. In total, 34 blaKPC-2, 3 blaNDM-1, 1 blaIMP-4 and 3 blaIMP-26 genes were identified and KP67 plasmid 1 co-harbored blaNDM-1 and blaIMP-26. Five sequence types, namely ST11, ST290, ST340, ST395 and ST437, were recognized by MLST. Surprisingly, blaKPC-2 was only detected in ST11 strains. We described a clonal dissemination of fosA3-positive IncR-IncF33:A-:B- multireplicon plasmid carrying the gene cassettes IS 26-ΔTn 3-ISKpn27-blaKPC-2-ΔISKpn6-korC-klcA-ΔrepB-Tn 1721 in all ST11 isolates. Three blaNDM-1 positive isolates belonged to three different ST types and their blaNDM-1 genetic backgrounds were also distinct. Interestingly, the flanking regions of blaNDM-1 in KP67 and KP72 were duplicated into one to five copies in a form of tandem repeat by the transposition of IS 91 like element. The blaNDM-1 of KP82 was carried on a common IncX3 plasmid.
Conclusion: This study described the early epidemiological characteristics of blaKPC-2/blaNDM-1-carrying CRKP, and reported a new tandem repeat pattern of blaNDM-1 cluster in Yunnan. These findings extend our knowledge on the carbapenemase gene evolutions.
Keywords: carbapenem-resistant Klebsiella pneumoniae, clonal dissemination, genetic backgrounds, IS 91 transposons, blaNDM-1 duplication
Introduction
Over the last fifty years, the development and the widespread use of antibacterial drugs has greatly helped people against bacterial infections. Clinically, the most commonly used antibacterial drugs are β-lactam antibiotics, such as cephalosporin and carbapenems, due to their broad-spectrum antimicrobial properties and strong activity against several extended-spectrum beta-lactamases.1 Recently, many gram-negative Enterobacteriaceae strains have been reported resistant to carbapenems by producing carbapenemases, termed Carbapenems resistant Enterobacteriaceae (CRE).2,3 Among CRE, the carbapenem-resistant Klebsiella pneumoniae (CRKP) is a taxon of considerable interest.4,5 Three highly reactive carbapenemases have been described in CRKP, known as class A KPC-type, class B NDM-type, and class D OXA-48-like.6,7 These carbapenemase-encoding genes are usually located on drug-resistant plasmids with the ability to transfer between bacterial cells through the concerted activities of mobile genetic elements.8,9 Therefore, CRKP infections are recognized as a major challenge in health-care settings and are receiving growing concerns worldwide.10,11
The first blaKPC-2 producing CRKP was identified in the United States in 1996, and subsequently spread worldwide,12 whereas it was first reported in Zhejiang, a coastal province in southeast China, in 2004.13 Although blaKPC had been disseminated in diverse K. pneumoniae sequence types (STs), the vast majority of them were found to be restricted in a genetic related group, namely clone complex 258 (CC258).14,15 This clone complex includes more than a thousand STs, of which the ST11 is the predominant clone in China.4,16,17 With the continuous spread and evolution of blaKPC-2 harboring plasmid in CRKP, the mobilization of different resistance gene cassettes facilitated the co-carriage of them on a single mosaic plasmid. Recently, a fosfomycin resistance gene, namely fosA3, encoded the FosA3 which inactivate fosfomycin by exerting glutathione-S-transferase activity, has been characterized on blaKPC-2-carrying plasmids in China.18–20 In those cases, researchers raised the possibility that fosA3 may be transmitted to humans from animal sources and fosfomycin may not be recommended as an alternative option for treating CRKP infections. The more global concerned β-lactamase were blaNDM-1 which first emerged in the Indian sub-continent in 2009.21 Most previous studies have reported a single copy of the blaNDM-1 gene encoded by diverse plasmids.22–24 However, recent studies have demonstrated the existence of multiple copies of plasmid encoded blaNDM-1.25–27 These findings suggest a potential relationship between mobile elements and blaNDM gene duplication, as well as future research trends on the interaction between gene replication and host phenotypic.
Occurrences of CRKP have been rising gradually in recent years in China, and have caused dissemination of nosocomial infections. The outbreaks of blaKPC-2/NDM-1-carrying K. pneumoniae have been reported in some hospitals in eastern coastal cities of China, such as Shandong,23 Shanghai,28 Jiangsu17,29 and Zhejiang.30,31 Meanwhile, several surveys exhibited an increase in the prevalence of K. pneumoniae with decreasing susceptibility to Carbapenems in the southwest China since 2009.32–34 However, few studies have focused on the molecular epidemiology of CRKP in Yunnan, a province of southwest China bordering the Indian sub-continent. In the present study, we aimed to describe the genetic characteristics and dissemination mechanisms of carbapenemase-encoding genes in CRKP isolates collected from hospitalized patients at the second affiliated hospital of Kunming medical university, Kunming, Yunnan, during 2010–2013.
Methods
Strains and Drug Susceptibilities
From 2010 to 2013, a total of 41 non-repetitive CRKP clinical isolates were collected from patients who had poor prognosis after carbapenem treatment during hospitalization (Table 1). Most of the samples were collected consecutively during February to August in 2013, while some isolates were collected intermittently before 2013. CRKP was defined as any K. pneumoniae isolate showing ertapenem and/or imipenem minimum inhibitory concentrations (MICs) of ≥2 mg/L or 4 mg/L, respectively. The minimal inhibitory concentrations (MICs) of several frequently used antibiotics (augmentin, piperacillin/tazobactam, cefuroxime axetil, cefuroxime sodium, cefoxitin, ceftazidime, ceftriaxone, cefoperazone sodium, cefepime, ertapenem, imipenem, amikacin, levofloxacin, tigecycline) were evaluated on the VITEK 2 compact system (bioMérieux, Craponne, France) and interpreted according to the 2021 Clinical and Laboratory Standards Institute (CLSI) guideline M100-S31 or Food and Drug Administration (U.S.).
 |
Table 1 Clinical Features, STs, Gene Identifications and Flanking Fragment Identifications Among 41 CRKP Isolates |
MLST and PFGE Molecular Typing
Isolates were recovered on LB medium. Cells were harvested at the period of logarithmic growth. Genomic DNA was prepared using the DNA extraction kit (TIANamp Bacteria DNA Kit, Tiangen) following the manufacturer’s protocol. Forty-one isolates were initially genotyped by multilocus sequence typing (MLST) as described previously.35 The Nucleotide sequence of each locus was submitted to the K. pneumoniae online MLST database to obtain allele number. Allelic profile of each isolate was matched to the existing sequence types (STs) numbers (http://www.pasteur.fr). Additionally, pulsed-field gel electrophoresis (PFGE) genotyping was also performed.36 Briefly, plugs were prepared in agarose blocks (1%) and digested with restriction enzyme XbaI. DNA fragments were electrophoresed for 24 h at 14 °C under the following conditions: 6V/cm voltage gradient, switch time from 4 to 40s, and with pulse angle of 120°. After separation, gels were stained with GelRed (Biotium, USA). The Cluster analysis was performed from the PFGE data using the BioNumerics software v7.6 (Applied Maths Inc., Austin, Texas, USA). The similarity of PFGE patterns was calculated based upon Dice coefficients with a tolerance setting of 1.4% band tolerance and a 1.5% optimization setting for the whole profile, and the Dice similarity coefficient required to be >80% for the pattern to be considered as belonging to the same PFGE type. Dendrogram was constructed using the unweighted-pair group method with mathematical averaging (UPGMA).
Molecular Identification of Carbapenemase Genes
Five carbapenemase encoding genes (KPC, NDM, IMP, VIM and OXA-48) were detected by PCR.37–41 The relevant primers are listed in Table S1. PCR amplification was performed in a 25 µL reaction containing 1 µL genomic DNA (about 200 ng), 22 µL Gold Mix TSE101 (TSINGKE, Beijing) and 1 µL of each primer (10 µM) under thermocycle conditions of 98 °C for 2 minutes, 35 × (98 °C for 10 sec, 15 sec at specific annealing temperature for each set of primers and 72 °C for 15 sec), and 72 °C for 5 minutes on a T100 Thermal Cycle (BIO-RAD, USA). Positive bands were cut out and purified, and then sequenced at TSINGKE Company (Kunming, China), bi-directionally. The sequencing results were checked and assembled using the ContigExpress (independent module from Vector NTI Advance V 11, Invitrogen, USA). Sequence comparison was performed using BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi).42
BlaKPC-2 Genetic Background Analysis and Southern Blot
MLST analysis showed that most isolates belonged to ST11 type. Therefore, we randomly selected KP21 as a pioneer to identify flanking genes of the blaKPC-2 gene using genome walking kit (TaKaRa, following the manufacturer’s protocol). The flow diagram and walking primers were present in the Figure S1 and Table S2, respectively. After sequencing the flanking fragments, gene contents of the blaKPC-2 genetic background were displayed. Specific scanning primers were designed based on the franking sequences (Table S3), and the corresponding flanking fragments of blaKPC-2 positive isolates were amplified by Takara LA Taq Polymerase (TaKaRa) under the conditions of 94 °C for 2 minutes, 35 × (94 °C for 30 sec, 60 °C for 30 sec and 72 °C for 3 or 7 min according to the flanking fragment size), and 72 °C for 5 minutes. Positive amplicons were further analyzed based on restriction fragment length polymorphism (PCR-RFLP). Briefly, PCR products were loaded on an agarose gel (1%) and separated by electrophoresis. Bands were excised from gels and purified using TIANgel Midi Purification Kit (Tiangen). The upstream and downstream fragments were digested by HaeII (TaKaRa) and EcoT14 (styI, TaKaRa), respectively. All digestions were performed at 37°C overnight and then separated by agarose gel (2%) electrophoresis at 120 V for 70 min. The representative amplicons with different types of PCR-RFLP fingerprints were further sequenced bi-directionally. S1 nuclease-pulsed-field gel electrophoresis (S1-PFGE) and southern blotting hybridization were performed to determine the location of blaKPC-2 gene. Briefly, the plugs were digested with S1 nuclease (TaKaRa) at 37°C for 30 min and then separated by electrophoresis. Southern blot hybridizations were performed by following the manufacturer’s instructions of the DIG-High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics) using the blaKPC-2 digoxigenin-labeled specific probe. Salmonella serotype Braenderup strain H9812 was digested by XbaI and used for the molecular marker.43 The gel bands of blaKPC-2-carrying plasmids were cut out and purified. IncR replicon, IncFII replicon and fosA3 genes were detected in these blaKPC-2-carrying plasmids by PCR amplification as previously described.44–46
High-Throughput Sequencing
Three blaNDM-1 positive isolates, two blaKPC-2 positive isolates with different RFLP fingerprints together with KP21 were used for high-throughput sequencing. Briefly, genomic DNA was extracted as described above. Libraries for whole-genome sequencing were constructed and then sequenced on an Oxford Nanopore ONT platform (Shanghai Personal Biotechnology Co., Ltd., China). To correct sequencing errors, libraries (400 bp) for second-generation sequencing were also constructed and sequenced in 2×150 bp paired-end mode with a minimal coverage of 100 × on an Illumina NovaSeq sequencer. After quality control, the Oxford Nanopore data were assembled with HGAP447 and CANU (Version 1.6)48 and then corrected by the second-generation sequencing data using pilon (Version 1.22)49 under default parameter. These whole genome shotgun projects have been deposited at DDBJ/ENA/GenBank under the accession PRJNA858206, PRJNA858218, PRJNA742185 and the annotations were added by the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) (release 2021).50
Bioinformatics Analysis
To count the copy number of vital resistance genes in a certain plasmid, the full-length nucleotide sequences of blaKPC-2 and blaNDM-1 were BLAST searched against the Oxford Nanopore sequencing raw data using CLC Genomics Workbench v21.0.2. In brief, multi-BLAST tables were filtered with the following parameters: Number of HSPs≥1, Lowest E-value=0.00 and Greatest HSP length≥300 bp. The repeat level of each gene was ascertained by checking the high-scoring segment pairs (HSP) number in each multi-BLAST table. The entire nucleotide sequences of two blaNDM-1 repeat structures were imported and searched again by setting the Greatest HSP length≥7000 bp (for KP67) or 15,000 bp (for KP72). Genetic characteristic of each HSPs were checked manually. The representative blaKPC-2 and blaNDM-1-carrying plasmids were compared with those available in NCBI nucleotide databases (https://ftp.ncbi.nlm.nih.gov/blast/db/) and NCBI plasmid reference database (https://ftp.ncbi.nlm.nih.gov/genomes/refseq/plasmid/). The plasmid sequences of related hits were downloaded via NCBI accession number (Table S4). These plasmids were annotated with RAST 2.0 (https://rast.nmpdr.org/). ISFinder (https://www-is.biotoul.fr/) was used to determine the insertion sequence elements. Easyfig (v2.2.5) was used to map whole plasmid comparisons between closely related plasmids.51
Results
Antibiotic Susceptibilities and Carbapenemase Gene Detections
The minimum inhibitory concentrations (MICs) of 14 antimicrobial agents against 41 CRKP isolates showed multidrug resistance profiles, particularly to the tested β-lactamase inhibitor combinations, third-generation cephems and ertapenem (Table S5). Of the tested antimicrobial agents, levofloxacin (4.87%) and amikacin (31.70%) exhibited low sensitivity. In contrast, all isolates were susceptible to tigecycline (100%). In total, four types of carbapenemases were identified in these samples, including blaKPC-2 (n=34), blaNDM-1 (n=3), blaIMP-26 (n=3) and blaIMP-4 (n=1), as shown in Table 1. The blaVIM and blaOXA-48 genes were not detected in any of the isolates, and the blaKPC-2/blaNDM-1 co-harboring isolate was also not detected. Remarkably, KP67 was found to co-harbor blaNDM-1 and blaIMP-26.
Distribution of MLST Sequence Types and PFGE Patterns
MLST analysis revealed 5 different sequence types (Table 1): ST11 (n=34), ST290 (n=3), ST340 (n=1), ST395 (n=2), and ST437 (n=1). All blaKPC-2 positive isolates belonged to ST11. Three blaNDM-1 positive isolates were ST290, ST395 and ST437, respectively. Three blaIMP-26 positive isolates were confined to ST290 type, whereas blaIMP-4 was only found in ST340 type. PFGE released five clades, consistent with the results of MLST (Figure 1). The genetic similarity of 34 isolates in the main clade of PFGE was 96.5%, and they all belonged to MLST genotype ST11, as shown by the red arrow in Figure 1. However, the genetic similarity of three isolates belonging to ST290 in PFGE was 82.7%, suggesting that their plasmids were quite different. In addition, only ST11 CRKP isolates can be detected in four wards (EICU, urology, emergency and hematology wards) and mostly from the sputum (n=13) and urine samples (n=5), suggestive of the likelihood of a clonal dissemination of ST11 CRKP among hospitalized patients after surgery or invasive therapy. We also noticed that two ST11 CRKP isolates from the urology wards could be dated back to early collecting years, one from May 2012 and the other from September 2011, indicating a long period spread of this clone.
BlaKPC-2 Genetic Background Analysis and Southern Blot
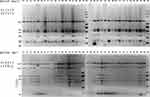
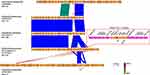
After three-rounds of thermal asymmetric interlaced PCR (TAIL-PCR) on isolate KP21, three bands were sequenced, as shown in Figure S1. Sequence analysis showed that the assembled fragment was 11,275 bp length with a genetic background composed of IS26-ΔtnpR(Tn3)-ISKpn27-blaKPC-2-ΔISKpn6-korC-klcA-ΔrepB-Tn1721 (Figure 2). This genetic background exhibited a high degree of similarity to the previously published plasmids with 100% query coverage and 100% nucleotide identity.19,52,53 Specific scanning primers (KPC-IS26F/U-SP2, KPC-D-SP2/Tn1771R) worked well and generated a 2880 bp PCR product for the upstream and an 8005 bp product for the downstream, as expected. As shown in Table 1, all 34 blaKPC-2 positive isolates successfully produced amplicons similar to that of KP21. PCR-RFLP showed a highly similar RFLP profiles among them, except for the downstream amplicon of KP29 (Figure 3). Further sequencing for this variant revealed an IS26 element inserted into the tnpA (Tn1721) gene (Figure 2).
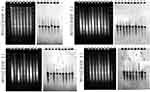
Based on PCR-RFLP results, we speculated that these blaKPC-2-carrying plasmids shared a homologous backbone. S1-PFGE and southern blot results revealed that these blaKPC-2 positive CRKP isolates contained one or two large plasmids, and the blaKPC-2 was located on the smaller one of ~120 kb (Figure 4). Since the PCR-RFLP results of the blaKPC-2 downstream sequences of KP29 were different from those of other samples, KP21, KP29 and KP65 were selected for high-throughput sequencing to further compare the homologies among the three blaKPC-2 bearing plasmids. The annotation showed that blaKPC-2 was located on a fosA3-harboring plasmid with IncR-IncF33:A-:B- multireplicon. Then IncR FW/RV, IncFII FW/RV and fosA3 specific primers were used to characterize replicons and fosfomycin resistance gene among blaKPC-2-carrying plasmids. Results showed that all test samples produced positive bands for IncR and IncFII replicons. However, two isolates were negative for fosA3 gene detection (data not show). The nucleotide sequence identity of two blaKPC-2-carrying plasmids (KP29 plasmid 2 and KP21 plasmid 1) was 99.99%. The two plasmids differed in their DNA sequences by one insertion region and two inversion regions (Figure 2). High sequence similarities were also observed in the comparison of KP21 plasmid 1 and KP65 plasmid 1 (Figure 2). The KP21 plasmid 1 matched with several multireplicon plasmids with high gene arrangement similarity and gene nucleotide identity. By reviewing the information of their host bacteria (scientific names, collection locations and dates), it noticed that almost of them were K. pneumoniae collected in China and several strains were isolated within the same year or earlier than this study, such as pXHKP6-1 (CP066888.1, 2010.09), pXHKP53-1 (CP066892.1, 2012.06), p1068-KPC (MF168402.1, 2012),54 pKP1034 (KP893385.1, 2010.05).19 These results suggest that this fosA3 and blaKPC-2 co-carrying IncR-F33: A–: B– plasmid may have been circulating in China since 2010.
Copy Number Analyses of blaNDM-1 and blaKPC-2
The coding sequences of blaNDM-1 and blaKPC-2 were respectively used as genetic markers to analysis their gene distributions in each Nanopore sequencing dataset. Multiple insertion events were discovered in KP67 plasmid 1 (replicon: IncHI2A) and KP72 plasmid 4 (replicon: IncFII(Yp)), resulting in the blaNDM-1 gene duplicated into one to five copies in the form of tandem repeats (Table 2). Results showed that the maximum number of blaNDM-1 HSPs found in a query read was up to 5 for KP72, while that was 4 for KP67. However, only one read with 2 HSPs of blaNDM-1 was detected in KP82. Similar result was noticed for blaKPC-2 gene in KP29. For KP21 and KP65, no more than one blaKPC-2 HSPs was found among their sequencing reads. Genetic analysis of the repeat unit of KP67 revealed a 10,844 bp length fragment composed of four elements, i e IS91-like insertion element (IS91-sul1-qacE), rifampicin and chloramphenicol resistance element (arr-3-catB3), carbapenem resistance core (ΔISaba125-blaNDM-1-ble-prai-ΔdsbD) and another IS91-like insertion element repeat, as shown in Figure 5. For KP72, the repeat unit was 23,853 bp length composed of IS91-like insertion element (ΔTn3-ΔintI1-aadA1-qacE-sul1-IS91), blaNDM-1 resistance core (aphA-6-ISaba125-blaNDM-1-prai-dsbD-cutA-groES-ΔgroEL) and another IS91-like insertion element repeat, as shown in Figure 6. The nucleic acid sequences of two IS91-like genes were identical and all belonged to ISCR1 family transposase. Considering, some multiple HSPs may reflect the gene arrangement in a part of the corresponding plasmid due to the limitation of read length; we further inspected the integrity of gene cassette in each repeat, manually. Results showed that KP67 contained one to three intact repeat units (Table 3). A variety of incomplete structures were also found and some of them contained more than three repeats. For KP72, only one intact repeat unit was detected and the remnant reads revealed one to six repeats (Table 3). Notably, we also detected blaNDM-1 repeat units of other genetic patterns, such as repeats with mobile elements inserted occasionally, or repeats translocating into chromosome or other plasmids. Together, these findings supported that the highly active IS91-like transposons mediated the tandem repeat of blaNDM-1 in this study.
 |
Table 2 Copy Number Analyses of blaNDM-1 and blaKPC-2 Genes |
 |
Table 3 Polymorphism Analyses of blaNDM-1 Genetic Background in KP67 and KP72 |
High-Throughput Sequencing and Comparative Analysis
Overall, blaNDM-1 gene located on the resistance plasmids of 318,781 bp, 113,479 bp and 54,035 bp for KP67, KP72 and KP82, respectively. The gene organization of the repeat cassette identified from P67 plasmid 1 had been reported in two published plasmids, pTMTA97342 (Phytobacter diazotrophicus, AP025336.1) and pEC8-NDM-1 (Escherichia coli, CP060954.1, 2016.07), with 100% coverage and 99% identity (pEC8-NDM-1 plasmid was showed in Figure 5). Interestingly, it is unusual to found a resistance element (Arr-3-CatB3) adjacent to the blaNDM-1. To the best of our knowledge, we reported the earliest collection of strain with this trait. The three elements of the repeat unit in KP67 plasmid 1 were found to be scattered in some other plasmids, such as pNDM-2262 (Citrobacter freundii, MH892479.1, 2016.09) and pICEPvuBC22 (Proteus vulgaris, MH160822.1, 2018). The backbone of KP67 plasmid 1 shared a large region with high similarity to a plasmid (CP091493.1) in a strain of Enterobacter cloacae collected in 2021 (Figure 5). However, the blaNDM-1 coding sequence of the Enterobacter cloacae plasmid was absent. Interestingly, the blaIMP-26 coding sequence was found at the same locus in the two plasmids, both of which were identified as IncHI2A replicons, indicating a common origin.
Two plasmids were highly similar to KP72 plasmid 4 in gene content and nucleotide sequence, including pKP46_2_KPC (Klebsiella pneumoniae, CP090128.1, 2017.12) and pEA49-KPC (Enterbacter aerogenes, KU318419.1, 2013.08). The major differences between the above two plasmids, referring to the adjacent genes in the upstream of blaKPC-2, are ISKpn19 and Tn3 insertion element, respectively (Figure 6). However, blaKPC-2 and relevant upstream and downstream contents were absent in KP72 plasmid 4 which was introduced by a blaNDM-1 gene cluster instead. The blaNDM-1 gene in KP72 plasmid 4 formed a combination with a neighbored intact ISaba125, which was considered to originate from Acinetobacter baumannii according to previous studies.55 This blaNDM-1 resistance core was usually found in many blaNDM-1-carrying plasmids, such as pdm186b (CP095599.1, 2016.11), but the entire repeat unit has not been reported yet.
For KP82 plasmid 4 (replicon: IncX3), we identified an ISAba125 inserted by an IS5 in the upstream of the blaNDM-1 gene (Figure 7). Besides, another IS91 family transposase, distinct from the former identified, was also found at the downstream of the blaNDM-1 gene cluster. The IS91 family transposase was adjacent to an IS26 element, an active transposon implicating resistance gene transposition and intermolecular replication.56 This blaNDM-1 genetic background were generally found in many carbapenem-resistant Enterobacteriaceae plasmids, such as pRor-30818cz (Raoultella ornithinolytica, MG252893.1, 2016.09)57 and pNDM-1_IncX3 (Escherichia coli, CP050161.1, 2012.08)2 and always carried on an IncX3 plasmid which spread carbapenemase genes worldwide.58
Discussion
Carbapenem-resistant K. pneumoniae (CRKP) has emerged as a major source of antibiotic-resistant bacteria of global concern. Recently, the plasmid-mediated mobile tigecycline resistance gene tet(A) and its variants have been reported to decrease the tigecycline susceptibility in Enterobacteriaceae strains.59,60 In our study, antimicrobial susceptibility test demonstrated highly resistant to common antibiotics other than tigecycline and the tet(A) gene was not found in our whole genome sequencing data. Therefore, tigecycline remains an important treatment option for CRKP infection. However, it is worth noting that under selective pressure, the coexistence of plasmid carrying blaKPC-2 and tet(A) gene in CRKP strains can develop a high-level tigecycline resistance.61 We should continuously highlight the importance of monitoring the emergence of tigecycline-resistant in CRKP.
KPC was first reported in 2001 in the USA from a K. pneumoniae strain isolated in 1996.12 In China, the first KPC-producing K. pneumoniae was isolated in Zhejiang Province in 2004.13 Subsequently, some institutional outbreaks of this taxon were reported in central and eastern China with the ST11 type as the dominant clone.16,28,33,62 In the present study, 34 out of 41 CRKP isolates were identified as ST11 type suggesting a rapid spread of blaKPC-2-carrying K. pneumoniae in Yunnan since 2011. Moreover, the 34 carriers are genetically closely related based on the results of blaKPC-2 genetic backgrounds analysis, southern blotting, plasmid replicon detection and fosA3 gene detection. In general, these data suggested that the prevalence of CRKP in Yunnan may be caused by the clonal spread of ST11 strains associated with fosA3 positive IncR-IncF33:A-:B- like drug-resistant plasmids. Our results were consistent with the prevalence of IncR-IncF33:A-:B- plasmid among CRKP in China.18,19 However, sight but obvious genetic divergences were detectable in the PFGE pattern among these ST11 strains and in the schematic representation of the three representative IncR-IncF33:A-:B- plasmids. More than that, the majority of these strains were collected within a six-month period (February to July in 2013), with the earliest samples dating back to December 2011. The above findings suggested that these ST11 isolates may be prevalent in our hospital earlier than our primary sampling period and could have developed genetic mutations after prolonged dissemination.
The blaKPC-2 genetic background described here was structurally identical to that in many previously reported strains which dated back to 2010 and sustained transmission ever since. Interestingly, those strains were mostly originated in China and seemed to be associated with K. pneumoniae ST11 type.4,19,54,63–69 These data implied that plasmids carrying this genetic background have been confined to K. pneumoniae ST11 so far and circulated since 2010 in mainland China. This resistant genetic background usually integrated into a fosA3-positive IncR-IncFII plasmid which was composed of three genetically and physically distinct modules: a pHN7A8-derived module which contains fosfomycin resistance gene fosA3 and IncFII replicon, a pKPC-LK30-derived core module which harbors blaKPC-2 gene and IncR replicon, and another 10 kb module.19 Comparative analysis showed that the core module tested in this study was significantly different from that of pKP1034, but more similar to those of p5-1 (isolated from Kunming) and pWCHKP020039 (collected from Chengdu, which is close to Kunming). These results indicated that the origin of plasmids with this core module in Kunming has a different evolutionary history from that of pKPC-LK30 and may have been widely spread in southwest China.
Interestingly, investigators reported that the fosA3-positive IncR-IncFII like plasmid was unsuccessfully transferred to E. coli J53Azi by conjugation experiments.19,54,63 Despite their limitation in conjugation transfer, nontransmissible IncR plasmids can serve as a reservoir by fusion with other types of resistance plasmids or directly cotransfer with the helper self-transferable IncN3 plasmid, thereby enhancing its ability to transmit to other K. pneumoniae lineage or Enterobacteriaceae species.70,71 More importantly, recent studies reminded the public that strains with this plasmid became high virulence and multidrug resistances.61,66,72–74 In this study, the tra regions in the three sequenced plasmids were incomplete compared with that in pHN7A8 and the blaKPC-2 gene was only detected in ST11, suggesting a limited horizontal transmission of this plasmid.
NDM was first reported in K. pneumoniae and E. coli in 2009 and subsequently spread worldwide via vertical and horizontal transmission.21,75 In China, the earliest NDM positive strains can be traced to Acinetobacter baumannii isolated in 2009 and then successively identified in other species, including E. coli and K. pneumoniae.22,76–78 Acinetobacter baumannii has been suggested as an intermediate in the transmission of the blaNDM-1 gene from environmental pathogens to Enterobacteriaceae species.55 Fu et al found that blaNDM-1 positive Acinetobacter spp. (represented by strain ABC7926, accession number JQ080305) shared a similar genetic structure in which blaNDM-1 genetic background made up of ISAba125-blaNDM-1-ble-ΔtrpF-ΔgroES-groEL-InsE-ISAba125. In our study, fragment deletions were found in the region downstream of the blaNDM-1 in the three tested isolates resulting in the missing of the downstream ISAba125. The ISAba125 upstream of the blaNDM-1 in KP82 was also truncated by IS5, while only a small residue of the ISAba125 was retained in KP67. The three blaNDM positive isolates belonged to three different ST types, and each had its unique blaNDM-1 genetic backgrounds, providing evidences on the complexity and diversity of genetic features associated with the blaNDM-1 gene. The comparative analyses showed that the sequence of KP82 plasmid 4 can be found in many other species in China with more than 99% identity and 99% coverage, such as Escherichia coli (KX094555.1), Enterobacter cloacae (KF976405.1),79 Citrobacter freundii (JX254913.2), Enterobacter cloacae (KY296103.1),80 Enterobacter hormaechei (MF344560.1) and Enterobacter kobei (CP088232.1). This transmissibility and plasticity implies an alarming potential of KP82-like blaNDM-1-carrying plasmid to spread and diversify among bacterial populations. It is also worth noting that two IS91-like elements with identical nucleotide sequence mediated blaNDM-1 flanking region duplicated into one to five copies in KP67 and KP72. This rather unusual IS element IS91 differs from the insertion sequence paradigm in that they lack conventional terminal inverted repeats (IRs) and can mobilize adjacent DNA sequences via a process called rolling circle replication.8,81 Recently, multiple copies of blaNDM-1-encoding plasmids have been reported with increasing frequency.25–27,82–84 In those cases, diverse mechanisms mediated the generation of tandem repeat of blaNDM-1 gene by different IS elements, such as Tn3-like,27 ISaba125 83 and ISCR1.25,82 Importantly, there are different conclusions on whether repeat sequences affect the resistance phenotype in those strains.25,27,82 Our findings emphasized the necessity for monitoring the prevalence of multicopy blaNDM-1 gene mediated by IS91-like IS element. Further research is warranted to clarify its potential role in the impact of host phenotype.
Conclusions
In summary, the clonal dissemination of CRKP ST11 clone harbors a fosA3 and blaKPC-2 co-carrying IncR-F33: A–: B– plasmid was identified in multiple departments in our hospital, indicating an extensive cross-transmission of CRKP isolates among high-risk departments. In addition, we reported tandem repeat of blaNDM-1 gene mediated by the transposition of IS91 element on two plasmids with different replicons, providing data on the complexity and diversity of genetic features associated with the blaNDM-1 gene. These findings help to better understand the spread history of CRKP in southwest China, and will provide reference for relevant studies in other regions or different periods in China.
Abbreviations
CRE, Carbapenems resistant Enterobacteriaceae; CRKP, Carbapenem-Resistant Klebsiella pneumoniae; NDM, New Delhi metallo-β-lactamase; UP, upstream; DN, downstream; ESBL, extended-spectrum β-lactamase; MIC, Minimal inhibitory concentration; CLSI, Clinical and Laboratory Standards Institute; MLST, multilocus sequence typing; STs, sequence types; PFGE, pulsed-field gel electrophoresis; PCR, Polymerase chain reaction; TAIL-PCR, thermal asymmetric interlaced PCR; NCBI, National Center for Biotechnology Information; BLAST, Basic Local Alignment Search Tool; RFLP, Restriction fragment length polymorphism; ICU, intensive care unit; SICU, surgical intensive care unit; EICU, Emergency Intensive Care Unit; AMC, amoxicillin-clavulanate; PIP/T, piperacillin/tazobactam; CXM, cefuroxime axetil; CXM-S, cefuroxime sodium; FOX, cefoxitin; CAZ, ceftazidime; CRO, ceftriaxone; SCF, cefoperazone/sulbactam; FEP, cefepime; ETP, ertapenem; IMP, imipenem; AMK, amikacin; LVX, levofloxacin; TGC, tigecycline.
Ethical Approval
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University. Patients participating in the study were anonymous, as a result of the retrospective study, so informed consent was not obtained.
Authors and Contributors
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 31960029), Yunnan Health Training Project of High Level Talents (grant number H-2019062 and D-2018041) and Yunnan Provincial Science and Technology Department (grant number 2017FE468 (−207) and 2019FE001 (−229)).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Lima LM, da Silva BNM, Barbosa G, Barreiro EJ. β-lactam antibiotics: an overview from a medicinal chemistry perspective. Eur J Med Chem. 2020;208:112829. doi:10.1016/j.ejmech.2020.112829
2. Zhu C, Li C, Lai CK, et al. Longitudinal genomic characterization of carbapenemase-producing Enterobacteriaceae (CPE) reveals changing pattern of CPE Isolated in Hong Kong Hospitals. Int J Antimicrob Agents. 2021;58(5):106430. doi:10.1016/j.ijantimicag.2021.106430
3. Yan WJ, Jing N, Wang SM, et al. Molecular characterization of carbapenem-resistant Enterobacteriaceae and emergence of tigecycline non-susceptible strains in the Henan province in China: a multicentre study. J Med Microbiol. 2021;70(3):001325. doi:10.1099/jmm.0.001325
4. Zhou Y, Ai W, Guo Y, et al. Co-occurrence of rare ArmA-, RmtB-, and KPC-2–encoding multidrug-resistant plasmids and hypervirulence iuc operon in ST11-KL47 Klebsiella pneumoniae. Microbiol Spectr;2022. e02371–e02321. doi:10.1128/spectrum.02371-21
5. Xu M, Zhao J, Xu L, et al. Emergence of transferable ceftazidime–avibactam resistance in KPC-producing Klebsiella pneumoniae due to a novel CMY AmpC β-lactamase in China. Clin Microbiol Infect. 2022;28(1):136.e131–136. e136. doi:10.1016/j.cmi.2021.05.026
6. Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem Rev. 2005;105(2):395–424. doi:10.1021/cr030102i
7. Queenan AM, Bush K. Carbapenemases: the Versatile β-Lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi:10.1128/CMR.00001-07
8. Partridge SR, Kwong SM, Firth N, Jensen SO. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31(4):e00088–e00017. doi:10.1128/CMR.00088-17
9. Schultsz C, Geerlings S. Plasmid-Mediated Resistance in. Enterobacteriaceae Drugs. 2012;72(1):1–16. doi:10.2165/11597960-000000000-00000
10. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–1106. doi:10.1086/592412
11. Zhu W-M, Yuan Z, Zhou H-Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: a systematic review and meta-analysis antimicrob. Resist Infect Control. 2020;9(1):1–13.
12. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151–1161. doi:10.1128/AAC.45.4.1151-1161.2001
13. Wei Z-Q, Du -X-X, Yu Y-S, Shen P, Chen Y-G, Li L-J. Plasmid-Mediated KPC-2 in a Klebsiella pneumoniae Isolate from China. Antimicrob Agents Chemother. 2007;51(2):763–765. doi:10.1128/AAC.01053-06
14. Kitchel B, Rasheed JK, Patel JB, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother. 2009;53(8):3365–3370. doi:10.1128/AAC.00126-09
15. Peirano G, Bradford PA, Kazmierczak KM, Chen L, Kreiswirth BN, Pitout JD. Importance of clonal complex 258 and IncFK2-like plasmids among a global collection of Klebsiella pneumoniae with bla KPC. Antimicrob Agents Chemother. 2017;61(4):e02610–e02616. doi:10.1128/AAC.02610-16
16. Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother. 2011;66(2):307–312. doi:10.1093/jac/dkq431
17. Cheng L, Cao XL, Zhang ZF, et al. Clonal dissemination of KPC-2 producing Klebsiella pneumoniae ST11 clone with high prevalence of oqxAB and rmtB in a tertiary hospital in China: results from a 3-year period. Ann Clin Microbiol Antimicrob. 2016;15:1. doi:10.1186/s12941-015-0109-x
18. Jiang Y, Shen P, Wei Z, et al. Dissemination of a clone carrying a fosA3-harbouring plasmid mediates high fosfomycin resistance rate of KPC-producing Klebsiella pneumoniae in China. Int J Antimicrob Agents. 2015;45(1):66–70. doi:10.1016/j.ijantimicag.2014.08.010
19. Xiang DR, Li JJ, Sheng ZK, et al. Complete sequence of a novel IncR-F33: a-:B-plasmid, pKP1034, Harboring fosA3, blaKPC-2, blaCTX-M-65, blaSHV-12, and rmtB from an Epidemic Klebsiella pneumoniae Sequence Type 11 Strain in China. Antimicrob Agents Chemother. 2015;60(3):1343–1348. doi:10.1128/AAC.01488-15
20. Hao Y, Zhao X, Zhang C, et al. Clonal dissemination of clinical carbapenem-resistant Klebsiella pneumoniae isolates carrying fosa3 and blakpc–2 coharboring plasmids in Shandong, China. Front Microbiol. 2021;12. doi:10.3389/fmicb.2021.771170
21. Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-β-Lactamase Gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53(12):5046–5054. doi:10.1128/AAC.00774-09
22. Wu H-S, Chen T-L, Chen IC-J, et al. First Identification of a Patient Colonized With Klebsiella pneumoniae Carrying blaNDM-1 in Taiwan. J Chin Med Assoc. 2010;73(11):596–598. doi:10.1016/S1726-4901(10)70129-5
23. Jin Y, Shao C, Li J, Fan H, Bai Y, Wang Y. Outbreak of multidrug resistant NDM-1-producing Klebsiella pneumoniae from a neonatal unit in Shandong Province, China. PLoS One. 2015;10(3):e0119571. doi:10.1371/journal.pone.0119571
24. Dong F, Lu J, Wang Y, et al. A five-year surveillance of carbapenemase-producing Klebsiella pneumoniae in a pediatric hospital in China reveals increased predominance of NDM-1. Biomed Environ Sci. 2017;30(8):562–569. doi:10.3967/bes2017.075
25. Jiang B-W, Ji X, Lyu Z-Q, et al. Detection of Two Copies of a blaNDM-1-Encoding Plasmid in Escherichia coli Isolates from a Pediatric Patient with Diarrhea. Infect Drug Resist. 2022;15:223. doi:10.2147/IDR.S346111
26. Yang L, He H, Chen Q, et al. Nosocomial outbreak of carbapenemase-producing proteus mirabilis with two novel salmonella genomic island 1 variants carrying different blaNDM–1 gene copies in China. Front Microbiol. 2021;12:800938.
27. Huang T-W, Chen T-L, Chen Y-T, et al. Copy number change of the NDM-1 sequence in a multidrug-resistant Klebsiella pneumoniae clinical isolate. PLoS One. 2013;8(4):e62774. doi:10.1371/journal.pone.0062774
28. Sun K, Chen X, Li C, Yu Z, Zhou Q, Yan Y. Clonal dissemination of multilocus sequence type 11 Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in a Chinese teaching hospital. APMIS. 2015;123(2):123–127. doi:10.1111/apm.12313
29. Gu B, Bi R, Cao X, Qian H, Hu R, Ma P. Clonal dissemination of KPC-2-producing Klebsiella pneumoniae ST11 and ST48 clone among multiple departments in a tertiary teaching hospital in Jiangsu Province, China. Ann Transl Med. 2019;7(23):716. doi:10.21037/atm.2019.12.01
30. Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46. doi:10.1016/S1473-3099(17)30489-9
31. Zhao Y, Zhang X, Torres VVL, et al. An outbreak of carbapenem-resistant and hypervirulent Klebsiella pneumoniae in an intensive care unit of a major teaching hospital in Wenzhou, China. Front Public Health. 2019;7:229. doi:10.3389/fpubh.2019.00229
32. Xia Y, Liang Z, Su X, Xiong Y. Characterization of carbapenemase genes in Enterobacteriaceae species exhibiting decreased susceptibility to carbapenems in a University Hospital in Chongqing, China. Ann Lab Med. 2012;32(4):270–275. doi:10.3343/alm.2012.32.4.270
33. Chen S, Feng W, Chen J, et al. Spread of carbapenemase-producing Enterobacteria in a southwest hospital in China. Ann Clin Microbiol Antimicrob. 2014;13:42. doi:10.1186/s12941-014-0042-4
34. Rui Z, Dehua L, Hua N, et al. Carbapenemase-producing Enterobacteriaceae in Yunnan Province, China. Jpn J Infect Dis. 2016;69(6):528–530. doi:10.7883/yoken.JJID.2015.471
35. Diancourt L, Passet V, Verhoef J, Grimont PAD, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43(8):4178–4182. doi:10.1128/JCM.43.8.4178-4182.2005
36. Hunter SB, Vauterin P, Lambert-Fair MA, et al. Establishment of a universal size standard strain for use with the pulsenet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the New Size Standard. J Clin Microbiol. 2005;43(3):1045–1050. doi:10.1128/JCM.43.3.1045-1050.2005
37. Castanheira M, Farrell SE, Deshpande LM, Mendes RE, Jones RN. Prevalence of β-lactamase-encoding Genes among Enterobacteriaceae bacteremia isolates collected in 26 U.S. Hospitals: report from the SENTRY antimicrobial surveillance program (2010). Antimicrob Agents Chemother. 2013;57(7):3012–3020. doi:10.1128/AAC.02252-12
38. Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi:10.1016/j.diagmicrobio.2010.12.002
39. Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JDD. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol. 2012;50(12):3877–3880. doi:10.1128/JCM.02117-12
40. Pitout JDD, Gregson DB, Poirel L, McClure J-A, Le P, Church DL. Detection of pseudomonas aeruginosa producing metallo-β-lactamases in a large centralized laboratory. J Clin Microbiol. 2005;43(7):3129–3135. doi:10.1128/JCM.43.7.3129-3135.2005
41. Marchiaro P, Mussi MA, Ballerini V, et al. Sensitive EDTA-based microbiological assays for detection of metallo-β-lactamases in nonfermentative gram-negative bacteria. J Clin Microbiol. 2005;43(11):5648–5652. doi:10.1128/JCM.43.11.5648-5652.2005
42. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi:10.1016/S0022-2836(05)80360-2
43. Xu M, Fu Y, Fang Y, et al. High prevalence of KPC-2-producing hypervirulent Klebsiella pneumoniae causing meningitis in Eastern China. Infect Drug Resist. 2019;12:641–653. doi:10.2147/IDR.S191892
44. Villa L, García-Fernández A, Fortini D, Carattoli A. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother. 2010;65(12):2518–2529. doi:10.1093/jac/dkq347
45. García-Fernández A, Fortini D, Veldman K, Mevius D, Carattoli A. Characterization of plasmids harbouring qnrS1, qnrB2 and qnrB19 genes in Salmonella. J Antimicrob Chemother. 2009;63(2):274–281. doi:10.1093/jac/dkn470
46. Ho P, Chan J, Lo W, et al. Dissemination of plasmid-mediated fosfomycin resistance fosA3 among multidrug-resistant E scherichia coli from livestock and other animals. J Appl Microbiol. 2013;114(3):695–702. doi:10.1111/jam.12099
47. Chin C-S, Peluso P, Sedlazeck FJ, et al. Phased diploid genome assembly with single-molecule real-time sequencing. Nat Methods. 2016;13(12):1050–1054. doi:10.1038/nmeth.4035
48. Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27(5):722–736. doi:10.1101/gr.215087.116
49. Walker BJ, Abeel T, Shea T, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9(11):e112963. doi:10.1371/journal.pone.0112963
50. Tatusova T, DiCuccio M, Badretdin A, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44(14):6614–6624. doi:10.1093/nar/gkw569
51. Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–1010. doi:10.1093/bioinformatics/btr039
52. Tang Y, Shen P, Liang W, Jin J, Jiang X. A putative multi-replicon plasmid co-harboring beta-lactamase genes bla KPC-2, bla CTX-M-14 and bla TEM-1 and trimethoprim resistance gene dfrA25 from a Klebsiella pneumoniae sequence type (ST) 11 strain in China. PLoS One. 2017;12(2):e0171339. doi:10.1371/journal.pone.0171339
53. Wang J, Yao X, Luo J, Lv L, Zeng Z, Liu J-H. Emergence of Escherichia coli co-producing NDM-1 and KPC-2 carbapenemases from a retail vegetable, China. J Antimicrob Chemother. 2017;73(1):252–254. doi:10.1093/jac/dkx335
54. Shi L, Feng J, Zhan Z, et al. Comparative analysis of bla KPC-2-and rmtB-carrying IncFII-family pKPC-LK30/pHN7A8 hybrid plasmids from Klebsiella pneumoniae CG258 strains disseminated among multiple Chinese hospitals. Infect Drug Resist. 2018;11:1783. doi:10.2147/IDR.S171953
55. Fu Y, Liu L, Li X, et al. Spread of a common blaNDM-1-carrying plasmid among diverse Acinetobacter species. Infect Genet Evol. 2015;32:30–33. doi:10.1016/j.meegid.2015.02.020
56. Dong N, Lin D, Zhang R, Chan EW, Chen S. Carriage of blaKPC-2 by a virulence plasmid in hypervirulent Klebsiella pneumoniae. J Antimicrob Chemother. 2018;73(12):3317–3321. doi:10.1093/jac/dky358
57. Paskova V, Medvecky M, Skalova A, et al. Characterization of NDM-encoding plasmids from Enterobacteriaceae recovered from Czech hospitals. Front Microbiol. 2018;9:1549. doi:10.3389/fmicb.2018.01549
58. Mouftah SF, Pál T, Darwish D, et al. Epidemic IncX3 plasmids spreading carbapenemase genes in the United Arab Emirates and worldwide. Infect Drug Resist. 2019;12:1729. doi:10.2147/IDR.S210554
59. Akiyama T, Presedo J, Khan AA. The tetA gene decreases tigecycline sensitivity of Salmonella enterica isolates. Int J Antimicrob Agents. 2013;42(2):133–140. doi:10.1016/j.ijantimicag.2013.04.017
60. Du X, He F, Shi Q, et al. The rapid emergence of tigecycline resistance in bla KPC–2 Harboring Klebsiella pneumoniae, as mediated in vivo by mutation in tetA during tigecycline treatment. Front Microbiol. 2018;9:648. doi:10.3389/fmicb.2018.00648
61. Xu J, Zhu Z, Chen Y, Wang W, He F. The plasmid-borne tet (A) gene is an important factor causing tigecycline resistance in ST11 carbapenem-resistant Klebsiella pneumoniae under selective pressure. Front Microbiol. 2021;12:328.
62. Hu F, Chen S, Xu X, et al. Emergence of carbapenem-resistant clinical Enterobacteriaceae isolates from a teaching hospital in Shanghai, China. J Med Microbiol. 2012;61(1):132–136. doi:10.1099/jmm.0.036483-0
63. Zhang W, Zhu Y, Wang C, et al. Characterization of a multidrug-resistant porcine Klebsiella pneumoniae sequence type 11 strain coharboring bla KPC-2 and fosA3 on two novel hybrid plasmids. Msphere. 2019;4(5):e00590–e00519. doi:10.1128/mSphere.00590-19
64. Chen Y-T, Lin J-C, Fung C-P, et al. KPC-2-encoding plasmids from Escherichia coli and Klebsiella pneumoniae in Taiwan. J Antimicrob Chemother. 2014;69(3):628–631. doi:10.1093/jac/dkt409
65. Chen J-Y, Liou M-L, Kuo H-Y, et al. Dissemination of carbapenem-resistant Klebsiella pneumoniae harboring KPC-carrying plasmid pKPC_P16, a pKPC_LK30 variant, in northern Taiwan. Diagn Microbiol Infect Dis. 2018;91(3):291–293. doi:10.1016/j.diagmicrobio.2018.02.014
66. Zhang Y, Jin L, Ouyang P, et al. Evolution of hypervirulence in carbapenem-resistant Klebsiella pneumoniae in China: a multicentre, molecular epidemiological analysis. J Antimicrob Chemother. 2020;75(2):327–336. doi:10.1093/jac/dkz446
67. Wang L, Guo L, Ye K, Yang J. Genetic characteristics of OXA-48-producing Enterobacterales from China. J Glob Antimicrob Resist. 2021;26:285–291. doi:10.1016/j.jgar.2021.07.006
68. Chen X, Li P, Sun Z, Xu X, Jiang J, Su J. Insertion sequence mediating mrgB disruption is the major mechanism of polymyxin resistance in carbapenem-resistant Klebsiella pneumoniae isolates from China. J Glob Antimicrob Resist. 2022;30:357–362. doi:10.1016/j.jgar.2022.07.002
69. Zhang X, Ouyang J, He W, et al. Co-occurrence of rapid gene gain and loss in an interhospital outbreak of carbapenem-resistant hypervirulent ST11-K64 Klebsiella pneumoniae. Front Microbiol. 2020;11:579618.
70. Kocsis E, Gužvinec M, Butić I, et al. bla NDM-1 carriage on IncR plasmid in Enterobacteriaceae strains. Microb Drug Resist. 2016;22(2):123–128. doi:10.1089/mdr.2015.0083
71. Wang X, Tang B, Liu G, et al. Transmission of nonconjugative virulence or resistance plasmids mediated by a self-transferable IncN3 Plasmid from carbapenem-resistant Klebsiella pneumoniae. Microbiol Spectr. 2022;10:e01364–e01322. doi:10.1128/spectrum.01364-22
72. Huang Q-S, Liao W, Xiong Z, et al. Prevalence of the NTEKPC-I on IncF plasmids among Hypervirulent Klebsiella pneumoniae isolates in Jiangxi Province, South China. Front Microbiol. 2021;12. doi:10.3389/fmicb.2021.622280
73. Tan D, Zhang Y, Qin J, et al. A frameshift mutation in wcaJ associated with phage resistance in Klebsiella pneumoniae. Microorganisms. 2020;8(3):378. doi:10.3390/microorganisms8030378
74. Zhu X, Sun C, Chen H, et al. Co-occurrence of three different plasmids in an extensively drug-resistant hypervirulent Klebsiella pneumoniae isolate causing urinary tract infection. J Glob Antimicrob Resist. 2020;23:203–210. doi:10.1016/j.jgar.2020.09.002
75. Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11(5):355–362. doi:10.1016/S1473-3099(11)70059-7
76. Chen Y, Zhou Z, Jiang Y, Yu Y. Emergence of NDM-1-producing Acinetobacter baumannii in China. J Antimicrob Chemother. 2011;66(6):1255–1259. doi:10.1093/jac/dkr082
77. Ho PL, Lo WU, Yeung MK, et al. Complete sequencing of pNDM-HK encoding NDM-1 Carbapenemase from a multidrug-resistant Escherichia coli strain isolated in Hong Kong. PLoS One. 2011;6(3):e17989. doi:10.1371/journal.pone.0017989
78. Fu Y, Du X, Ji J, Chen Y, Jiang Y, Yu Y. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J Antimicrob Chemother. 2012;67(9):2114–2122. doi:10.1093/jac/dks192
79. Ho P-L, Li Z, Lo W-U, et al. Identification and characterization of a novel incompatibility group X3 plasmid carrying bla NDM-1 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. Emerg Microbes Infect. 2012;1(1):1–6. doi:10.1038/emi.2012.37
80. Lin D. Examination of factors promoting progressive emergence of antibiotic resistance among microbiota strains of gastrointestinal tract; 2017.
81. Toleman MA, Bennett PM, Walsh TR. IS CR elements: novel gene-capturing systems of the 21st century? Microbiol Mol Biol Rev. 2006;70(2):296–316. doi:10.1128/MMBR.00048-05
82. Xiang R, Li M. Identification of Tn 6835 and a novel genomic island, MMGI-1, in a pan-resistant Morganella morganii strain. Antimicrob Agents Chemother. 2021;65(4):e02524–e02520. doi:10.1128/AAC.02524-20
83. Rojas LJ, Wright MS, De La Cadena E, et al. Initial assessment of the molecular epidemiology of bla NDM-1 in Colombia. Antimicrob Agents Chemother. 2016;60(7):4346–4350. doi:10.1128/AAC.03072-15
84. Stoesser N, Giess A, Batty E, et al. Genome sequencing of an extended series of NDM-producing Klebsiella pneumoniae isolates from neonatal infections in a Nepali hospital characterizes the extent of community-versus hospital-associated transmission in an endemic setting. Antimicrob Agents Chemother. 2014;58(12):7347–7357. doi:10.1128/AAC.03900-14
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.