Back to Journals » International Medical Case Reports Journal » Volume 13
Tamoxifen Induced Pachychoroid Pigment Epitheliopathy with Reversible Changes After Drug Discontinuation
Authors Ghassemi F , Masoomian B , Khodabandeh A , Khalili Pour E , Bazvand F , Riazi-Esfahani H
Received 2 April 2020
Accepted for publication 7 July 2020
Published 27 July 2020 Volume 2020:13 Pages 285—289
DOI https://doi.org/10.2147/IMCRJ.S256064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fariba Ghassemi,1,2 Babak Masoomian,1,2 Alireza Khodabandeh,1 Elias Khalili Pour,1 Fatemeh Bazvand,1 Hamid Riazi-Esfahani1,2
1Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran; 2Ocular Oncology Service, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran
Correspondence: Hamid Riazi-Esfahani
Ocular Oncology Service, Farabi Eye Hospital, Qazvin Square, South Kargar Street, Tehran, Iran
Tel +982155418113
Fax +982155421020
Email [email protected]
Abstract: We report a case of breast cancer with clinically significant retinal toxicity induced by 7 years’ Tamoxifen consumption in which patient’s visual acuity and paraclinical findings remarkably improved after drug discontinuation. A 49-year-old woman with a history of breast cancer and Tamoxifen consumption was referred to our clinic for evaluation and treatment of gradual and progressive decrease visual acuity of both eyes (more prominent in right eye). Funduscopy showed bilateral macular pigmentary changes, with diffused tiny yellow crystals and reduced macular tessellation. On spectral-domain optical coherence tomography (SD-OCT), there was retinal pigment epithelium (RPE) abnormality and ellipsoid zone discontinuity accompanied by retinal thinning and choroidal thickness that was more prominent in the right eye. One year after discontinuation of the drug, visual acuity was significantly improved and SD-OCT revealed some surprising recoveries in the photoreceptor layers especially in her right eye.
Keywords: retinal toxicity, pachychoroid, tamoxifen retinopathy, breast cancer
Introduction
Tamoxifen is one of the most commonly used oral nonsteroidal anti-estrogenic agents that has been used extensively for hormonal adjuvant therapy in breast cancer.1,2 Oncologists also currently prescribe it for patients with ductal carcinoma in situ of breast cancer, patients at risk for invasive form, and presence of a metastatic lesion of this tumor.1 Furthermore, there are other uses of Tamoxifen including management of malignant central nervous system (CNS) gliomas, treatment of infertility and precocious puberty.2
Some of the most important side effects of this drug are; major depression, thromboembolic events, and endometrial cancer.2 Ocular side effects also have been reported including vortex keratopathy, cataract, optic neuritis, and retinopathy.3 Crystalline retinopathy is the most common retinal side effect of this drug. Other side effects in the retina are pseudocysts foveal cavitation, cystoid macular edema, photoreceptor disruption and Pachychoroid pigment epitheliopathy (PPE).3–7
Based on previous reports, “Tamoxifen induced photoreceptor attenuation” is an irreversible event.3–6 Herein, we describe a case of breast cancer with pachychoroid pigment epitheliopathy and photoreceptor attenuation secondary to 7 years’ Tamoxifen consumption in which, after discontinuing of the drug partial photoreceptor recovery happened.
Case Report
A 49-year-old Iranian woman was referred to our clinic for evaluation and treatment of gradual and progressive visual acuity decrease of both eyes (more prominent in right eye) in the past 5 years. Best-corrected visual acuities (BCVA) was 20/100 in the right eye and 20/25 in the left eye (she stated that visual acuity was 20/20 in both eyes on examination recorded 5 years ago).
Due to breast cancer, the patient had a history of right breast mastectomy 7 years ago. She also underwent systemic adjuvant chemotherapy for 6 months after mastectomy and subsequently underwent Tamoxifen therapy (20 mg per day) during this period. The cumulative dose of Tamoxifen was 51.1 grams. There was no history of corticosteroid usage.
Anterior segment examination was unremarkable but, the fundus examination showed bilateral macular pigmentary changes with diffuse tiny yellow crystals and reduced macular tessellation (Figure 1).
On fundus autofluorescence (FAF), stippled hypo and hyper autofluorescence area were noticeable in the macular area of the right eye with patchy hyperautofluorescence area inferior to the fovea.
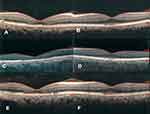
There was no significant change in autofluorescence pictures of the left eye (Figure 2). Infrared reflectance (IR) images revealed irregular hyperreflective and hyporeflective lesions bilaterally. On spectral-domain optical coherence tomography (SD-OCT) (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) there was retinal pigment epithelium (RPE) abnormality and ellipsoid zone discontinuity accompanied with retinal thinning that was more prominent in the right eye. Mild thickening of Haller’s layer was detectable exactly beneath the lesions by enhanced depth imaging OCT (EDI-OCT). Subfoveal choroidal thickness was 343 μm in the right eye and 321 μm in the left eye. Maximal choroidal thickness was 414 μm in the right eye and 365 μm in the left eye beneath the RPE lesions. Fluorescein angiography (FA) of the right eye revealed a punctuated hyperfluorescent patch in the central macular area without obvious leakage (Figure 2).
Based on these findings, our diagnosis was Tamoxifen induced Pachychoroid Pigment Epitheliopathy (PPE), therefore the patient was referred to an oncologist for changing the medication. Three months after the first visit, she still was under treatment with oral Tamoxifen surprisingly. Patient BCVA was considerably deteriorated in both eyes (right eye:20/200 and left eye:20/30). The EDI-OCT revealed the progression of the ellipsoid zone disruption in the right eye (Figure 3). With our strong recommendation, the patient finally stopped taking Tamoxifen by her oncologist.
One year later, visual acuity notably improved to 20/50 in the right eye and 20/25 in the left eye respectively. Evaluation with SD-OCT revealed some surprising recoveries in the photoreceptor layers, especially in her right eye (Figure 3).
Discussion
Tamoxifen is an effective drug for adjuvant treatment of breast cancer when used in low doses (Early Breast Cancer Trialists’ Collaborative Group 2005; US Food and Drug Administration, Centre for Drug Evaluation and Research 2006).1,8 In patients with hormone receptor-positive breast cancer, the five-year course of Tamoxifen therapy is standard of care. However, the global Adjuvant Tamoxifen, longer against shorter (ATLAS) trial has recently suggested that a course of at least 10 years treatment would reduce the mortality and recurrence rate of this cancer.8
Crystalline deposition in the inner retina is the most known side effect of this drug that could occur due to neuronal degeneration.5 Tamoxifen may also cause foveal schisis, cystic changes, or macular hole secondary to muller cell dysfunction.4 Although the incidence of retinal changes is less than 1% within the first 3 years of therapy,5 reports of subtle retinal changes like cystic changes or outer retinal disruption have been increased recently due to development of advanced retinal imaging technologies.5,6
The rates of retinopathy in patients who are being treated with 20mg tamoxifen ranged from 0% to 12.2%.1,2,9 Without using SD-OCT, retinopathy incidence probably has been underestimated.7 In a study by Chung and his colleagues, they found retinal outer retina cavitation in 12.2% of patients with SD-OCT. Also, crystalline retinopathy was observed in only one case due to prolonged Tamoxifen consumption (over 5 years).3 Recently Kim et al found that tamoxifen retinopathy may be more prevalent (12%) when using SD-OCT as an adjacent imaging modality than previously estimated by funduscopy only. Also, patients with high body mass index (BMI) and dyslipidemia were substantially more likely to suffer from retinopathy with tamoxifen.9
Pachychoroid pigment epitheliopathy (PPE) first time was introduced by Warrow et al in 2013.10,11 Pachychoroid is defined as choroidal thicknesses greater than 300 μm or focal extrafoveal choroidal thickness at least 50 μm more than the foveal center.10 It could be an isolated finding or could occur in fellow eyes of patients with unilateral Central Serous Chorioretinopathy (CSCR). The risk factors for these two entities are similar, including endogenous or exogenous hypercortisolism, pregnancy, Type A personality and exogenous testosterone therapy.12
There are many androgenic and estrogenic receptors in RPE and choroidal cells.13 Tamoxifen consumption, especially in postmenopausal women, can lead to an increase in serum total testosterone, cortisol, and cortisone levels, despite decreasing of estradiol level.13
The first report of PPE with a presumed association of Tamoxifen therapy has been reported by Ersoz et al.6 They revealed PPE in two cases that had been using Tamoxifen for about 4 and 6 months respectively. They contributed these phenomena to hormonal disturbance and elevation of the testosterone to estrogen level after Tamoxifen therapy. They did not report the patient’s outcome after drug cessation.
To the best of our knowledge, this is the first report of visual and anatomical recovery after Tamoxifen cessation in a patient with ellipsoid zone discontinuity and presence of PPE. This may be due to on-time drug cessation before irreparable effects happen.
Isolated crystal deposition without any other anatomical changes in EDI-OCT, may not be a strong reason for Tamoxifen discontinuing. Detection of retinal changes in the macular area like Ellipsoid zone disruption or intraretinal cavitations, CME, or macular hole formation could be potential signs of tamoxifen-induced retinal toxicity.3,5,8
However, these retinal changes in just the macular area may not be apparent in all patients, and as previous studies have shown, attention to retinal changes in the peripheral area away from the macula in wide-field imaging may indicate crystalline deposits in these patients.14 Besides the assessment of peripheral retina, it is important to appraise choroidal changes in these patients. With the introduction of this case, we also have tried to draw the attention of ophthalmologists to the valuation of the choroid especially the changes in choroidal thickness and the Haller layer in patients with a history of Tamoxiphen consumption in addition to retinal structural changes.
In conclusion, for patients treated with Tamoxifen, our recommendation is doing initially a baseline full ophthalmologic examination including visual acuity, fundus examination, and EDI-OCT. Then, these investigations should be repeated appropriately (maybe annually). Discontinuation of the drug at the beginning of toxicity could be a vision-saving attempt. More precise conclusions are precluded by just a single case report. A larger survey with more cases is necessary to clarify the situation.
Ethics
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional review board approval was not required. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
The abstract of this paper was presented at the “29th annual congress of the Iranian society of ophthalmology 2019” and “19th Euretina congress Paris” as a poster presentation with interim findings.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Murray DC, Gibson JM. Retinal changes associated with tamoxifen treatment for breast cancer. Eye (Lond). 1998;12(Pt 3a):485–486.
2. Tang R, Shields J, Schiffman J, et al. Retinal changes associated with tamoxifen treatment for breast cancer. Eye (Lond). 1997;11(Pt 3):295–297. doi:10.1038/eye.1997.64
3. Chung H, Kim D, Ahn SH, et al. Early detection of tamoxifen-induced maculopathy in patients with low cumulative doses of tamoxifen. Ophthalmic Surg Lasers Imaging. 2010;1–5.
4. Cronin BG, Lekich CK, Bourke RD. Tamoxifen therapy conveys increased risk of developing a macular hole. Int Ophthalmol. 2005;26(3):101–105. doi:10.1007/s10792-005-5424-3
5. Doshi RR, Fortun JA, Kim BT, Dubovy SR, Rosenfeld PJ. Pseudocystic foveal cavitation in tamoxifen retinopathy. Am J Ophthalmol. 2014;157(6):1291–1298 e1293. doi:10.1016/j.ajo.2014.02.046
6. Ersoz MG, Arf S, Karacorlu M, Hocaoglu M, Muslubas IS. Pachychoroid pigment epitheliopathy associated with tamoxifen. Ophthalmic Surg Lasers Imaging Retina. 2017;48(10):838–842. doi:10.3928/23258160-20170928-10
7. Nair SN, Anantharaman G, Gopalakrishnan M, Vyas J. Spectral domain optical coherence tomography findings in tamoxifen retinopathy–a case report. Retin Cases Brief Rep. 2013;7(2):128–130. doi:10.1097/ICB.0b013e31825956f1
8. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi:10.1016/S0140-6736(12)61963-1
9. Kim HA, Lee S, Eah KS, Yoon YH. Prevalence and risk factors of tamoxifen retinopathy. Ophthalmology. 2020;127(4):555–557. doi:10.1016/j.ophtha.2019.10.038
10. Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 2016;36(3):499–516. doi:10.1097/IAE.0000000000000742
11. Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33(8):1659–1672. doi:10.1097/IAE.0b013e3182953df4
12. Nudleman E, Witmer MT, Kiss S, Williams GA, Wolfe JD. Central serous chorioretinopathy in patients receiving exogenous testosterone therapy. Retina. 2014;34(10):2128–2132. doi:10.1097/IAE.0000000000000198
13. Baumgart J, Nilsson K, Stavreus Evers A, et al. Androgen levels during adjuvant endocrine therapy in postmenopausal breast cancer patients. Climacteric. 2014;17(1):48–54. doi:10.3109/13697137.2013.800039
14. Corradetti G, Violanti S, Au A, Sarraf D. Wide field retinal imaging and the detection of drug associated retinal toxicity. Int J Retina Vitreous. 2019;5(1):26. doi:10.1186/s40942-019-0172-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.