Back to Journals » Drug Design, Development and Therapy » Volume 9
Tacrolimus in preventing transplant rejection in Chinese patients – optimizing use
Received 19 September 2014
Accepted for publication 13 November 2014
Published 13 January 2015 Volume 2015:9 Pages 473—485
DOI https://doi.org/10.2147/DDDT.S41349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Chuan-Jiang Li,1,* Liang Li2,*
1Department of Surgery, Nanfang Hospital, 2Department of Medical Genetics, School of Basic Medical Sciences, Southern Medical University, Guangzhou, People’s Republic of China
*The authors contributed equally to this work
Abstract: Tacrolimus is a product of fermentation of Streptomyces, and belongs to the family of calcineurin inhibitors. It is a widely used immunosuppressive drug for preventing solid-organ transplant rejection. Compared to cyclosporine, tacrolimus has greater immunosuppressive potency and a lower incidence of side effects. It has been accepted as first-line treatment after liver and kidney transplantation. Tacrolimus has specific features in Chinese transplant patients; its in vivo pharmacokinetics, treatment regimen, dose and administration, and adverse-effect profile are influenced by multiple factors, such as genetics and the spectrum of primary diseases in the Chinese population. We reviewed the clinical experience of tacrolimus use in Chinese liver- and kidney-transplant patients, including the pharmacology of tacrolimus, the immunosuppressive effects of tacrolimus versus cyclosporine, effects of different factors on tacrolimus metabolism on Chinese patients, personalized medicine, clinical safety profile, and patient satisfaction and adherence. This article provides guidance for the rational and efficient use of tacrolimus in Chinese organ-transplant patients.
Keywords: tacrolimus, liver transplantation, kidney transplant, Chinese, personalized medicine
Introduction
Organ transplantation is the treatment of choice for patients with end-stage organ failure. Kidney and liver transplantation accounts for greater than 90% of all large-organ transplants in the People’s Republic of China (PRC). According to data published by the Chinese Scientific Registry of Kidney Transplantation, 6,471 kidney transplantations were performed in the PRC in 2013. The China Liver Transplant Registry gathered data on 20,818 patients who underwent liver transplantation between February 2005 and December 2011. Although the PRC is the second-largest country after the United states (US) with regard to the annual number of organ transplants, only 10,000 transplants are actually performed per year in the PRC. However, approximately 300,000 patients require organ transplantation annually; many die due to shortage of donor organs. Increasing the success and long-term survival of grafts is an efficient way to offset donor organ shortage. Graft rejection is the primary cause of chronic loss of graft function in the PRC.1,2 The key to controlling graft rejection is appropriate immunosuppression. Immune-induction therapy, either intravenous antilymphocyte globulin or anti-IL-2 receptor monoclonal antibody and methylprednisolone pulse therapy, is frequently used in the perioperative period. Maintenance therapy includes dual-, triple-, or quadruple-therapy regimens, including cyclosporine A (CsA) or tacrolimus (FK506) as the main components (CsA or tacrolimus plus mycophenolate mofetil [MMF] plus prednisone) to prevent acute rejection.3 Tacrolimus was first used in organ transplantation in the PRC in the late 1990s.4 With greater immunosuppression and a more favorable safety profile over CsA, tacrolimus gradually became the preferred immunosuppressant after liver and kidney transplantation, in place of CsA. This article reviews the clinical utility of tacrolimus in the PRC.
Pharmacology, mode of action, and pharmacokinetics of tacrolimus
In 1984, tacrolimus, a macrolide lactone antibiotic, was isolated from the fermentation broth of Streptomyces tsukubaensis and was considered a novel immunosuppressant.5 In vitro animal experiments subsequently revealed that tacrolimus had a strong immunosuppressive effect.6,7 In 1989, the use of tacrolimus for the prevention of rejection in liver transplantation was published.8 In 1994, the US Food and Drug Administration approved the use of tacrolimus in liver transplantation.9,10 Its use has since expanded to other organ types, and today it is the most widely used posttransplant immunosuppressant medication.
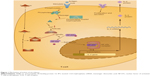
There are two main patterns of graft rejection following organ transplantation: acute and chronic rejection. During the process of graft rejection, T cells, B cells, and cytokines play important roles. T cells recognize the antigens of donors and are activated to proliferate, differentiate, and secrete cytokines. These cytokines stimulate B cells to produce antigraft antibodies. These cytokines also help cytotoxic T cells to develop cytotoxicity against the graft. The antigens of the graft are recognized by T-cell receptors; this stimulation induces generation of the second messengers of T cells. The activation of second messengers results in a sustained high level of intracellular calcium. Calcium activates calcineurin phosphatase, which changes the cytoplasmic component of nuclear factor and transports it into the nucleus. Then, transcription factor is formed, which can activate IL-2 gene transcription. IL-2 can induce T-cell proliferation and activation. Activated T cells secrete different types of cytokines. IL-2 also increases cytotoxic T-cell activity.11 Tacrolimus can form a complex with immunophilin FK-binding protein 12. This complex strongly inhibits calcineurin phosphatase activity and inhibits IL-2 expression. Subsequently, T-cell activation and cytokine secretion are inhibited. This is the mechanism by which tacrolimus prevents allograft rejection (Figure 1).
Tacrolimus is absorbed in the gastrointestinal tract, reaching peak blood concentrations in 1–3 hours, with a mean bioavailability of approximately 20%–25%.12 Tacrolimus mainly binds to erythrocytes. In the plasma, 99% of tacrolimus is bound to α1-acid glycoprotein. The blood/plasma tacrolimus-distribution ratio is about 20:1. Tacrolimus is the substrate of cytochrome P450 (CYP)-3A isoenzymes and P-glycoprotein. Tacrolimus is metabolized mainly by CYP3A4 and CYP3A5 in the liver and intestinal epithelium.13 P-glycoprotein, also known as multidrug-resistance protein (MDR)-1 or adenosine triphosphate-binding cassette subfamily B member 1, is an adenosine triphosphate-dependent efflux pump. It is expressed in intestinal epithelium cells, limiting the absorption of tacrolimus, and in the bile canalicular membrane of hepatocytes, mediating the biliary elimination of tacrolimus.14
Tacrolimus has wide interindividual variability in its pharmacokinetics. The maximum area under the curve (AUC) of tacrolimus was almost four times higher than the minimum AUC after the first oral dose in combination with MMF and prednisone among Chinese renal transplant recipients.15 In addition, it is characterized by a narrow therapeutic index. Underdosage of tacrolimus may result in graft rejection, while overdosage may result in toxicity.16,17 In order to avoid adverse effects, therapeutic drug monitoring (TDM) of tacrolimus whole-blood trough concentrations is necessary. In Europe and the United states, AUC-based tacrolimus TDM has been widely used. AUC, the best marker of exposure to tacrolimus, is calculated based on a limited sampling strategy using Bayesian estimation. The dose of tacrolimus is adjusted to reach the AUC target.18 In the PRC, most hospitals have been using trough blood concentrations for routine dose adjustment of tacrolimus. The proposed initial dose of tacrolimus is approximately 0.10–0.20 mg/kg per day in adult liver-transplant recipients, and 0.15–0.30 mg/kg per day in adult kidney-transplant recipients, and is taken orally twice daily, 1 hour before meals or 2 hours after meals.19
The recommended target trough blood concentration for Chinese renal transplant recipients is 12–15 ng/mL for the first month after transplantation, 8–12 ng/mL for the second month, 6–10 ng/mL for the third month, and 5–10 ng/mL as the sustained concentration after the third month.20 The recommended target trough blood concentration for Chinese liver–transplant recipients is 10–12 ng/mL during the first 3 months after transplantation, 8–10 ng/mL within 3–6 months, 6–8 ng/mL within 6–12 months, and 4–6 ng/mL as the sustained concentration after 1 year.21 For pediatric patients, tacrolimus dosing is based on body-surface area. The recommended starting dose for tacrolimus in Chinese pediatric renal allograft recipients is 4.7–5.6 mg/m2 per day, with a maintenance dose of 0.93–1.56 mg/m2 per day.22 Owing to interindividual variability, it can take several weeks for the dosage to achieve therapeutic efficacy; transplant recipients have a significant risk of side effects during this period. Therefore, it is paramount to achieve a stable maintenance dose as soon as possible after transplantation.23
Several factors can affect tacrolimus pharmacokinetics. In a study of 142 Chinese renal transplant recipients, tacrolimus maintenance dose negatively correlated with hematocrit, hemoglobin, total bilirubin, and indirect bilirubin, and positively correlated with body weight.18 In another study of 262 Chinese adult liver-transplant recipients, clearance of tacrolimus significantly correlated with hematocrit and total plasma protein.24 In addition, it has been reported that grapefruit juice can increase tacrolimus concentration in Chinese liver-transplant patients.25 Furthermore, drug–drug interactions can also affect tacrolimus pharmacokinetics.26,27
In addition to these factors, genetic factors play an important role in the variability of tacrolimus pharmacokinetics. A series of studies revealed that CYP3A5 6986A>G (CYP3A5*3) had a significant impact on tacrolimus pharmacokinetics in Chinese renal transplant recipients. Patients with CYP3A5*3/*3 had higher C0/D (dose-corrected tacrolimus trough concentrations) compared with patients with CYP3A5*1/*3 or CYP3A5*1/*1.28–30 Two studies found that CYP3A4*1G was significantly associated with tacrolimus pharmacokinetics in Chinese renal transplant recipients.31,32 Patients with CYP3A4*1/*1 had higher C0/D and lower tacrolimus clearance compared with patients with CYP3A4*1/*1G or CYP3A4*1G/*1G. Li et al33 found that CYP3A4*18B may be partly responsible for the large interindividual variability of tacrolimus blood levels in Chinese renal transplant patients during the first month after transplantation. However, in a study of 227 Chinese renal transplant patients, Zhu et al30 found no significant association between the C0/D of tacrolimus and CYP3A4*18B genotypes when they were classified by the different CYP3A5 genotypes.
Regarding the relationship between MDR1 polymorphisms and tacrolimus pharmacokinetics, there were inconsistent results in Chinese renal transplant recipients. Several studies revealed that the MDR1 3435C>T had a significant effect on the C0/D of tacrolimus (P<0.05). MDR1 3435CC patients displayed a lower tacrolimus level per dose than MDR1 3435CC/TT patients.34–36 Nevertheless, other studies failed to find a significant association between the MDR1 3435T>C polymorphism and tacrolimus pharmacokinetics.19,37,38 An outline of the genetic polymorphisms affecting tacrolimus pharmacokinetics in Chinese renal transplant patients is presented in Table 1.
The liver is the organ in which tacrolimus is metabolized. Therefore, donor genetics may also affect tacrolimus pharmacokinetics in Chinese liver-transplant patients. Several studies revealed significantly higher tacrolimus C0/D in donors with CYP3A5*3/*3 compared with CYP3A5*1/*3 or CYP3A5*1/*1 (P<0.05), and no significant difference of tacrolimus C0/D was observed in recipients with different CYP3A5 genotypes.39–41 However, other studies reported that recipient CYP3A5*3 was also significantly associated with tacrolimus C0/D.42–44 A series of studies reported that recipient MDR1 3435T>C significantly affected tacrolimus pharmacokinetics in Chinese liver-transplant patients. A lower daily tacrolimus dose or higher C0/D was observed in recipients carrying the MDR1 3435T allele, compared with 3435CC recipients at different times after transplantation.40,45–47 Correspondingly, two other studies found that recipient MDR1 3435T>C was not associated with tacrolimus C0/D in Chinese liver-transplant patients.41,43 Moreover, no correlation has been observed between tacrolimus daily dose or C0/D and donor MDR1 3435T>C in any of the published studies until now.40,41,45,46 Li et al41 reported that the IL-10 1082G>A of recipients can affect tacrolimus pharmacokinetics. Recipients with the homozygous variant IL-10 -1082AA genotype had higher tacrolimus C0/D compared with the IL-10 -1082GG and GA genotype, and the IL-10 1082G>A of the donors had no significant association with tacrolimus C0/D. Nevertheless, another study found that the IL-10 1082G>A of donors can affect tacrolimus C0/D, and the same variant of recipients did not significantly influence tacrolimus C0/D.46 In addition to these genetic variants, it has been reported that donor TLR4 rs1927907G>A and IL6 rs1800796 G>C were also closely associated with tacrolimus elimination in Chinese liver-transplant patients.42,44 An outline of the genetic polymorphisms affecting tacrolimus pharmacokinetics in Chinese liver-transplant patients is presented in Table 2.
  | Table 2 Effect of different gene polymorphisms on tacrolimus pharmacokinetics in Chinese liver transplant patients |
Currently, two studies have individualized the first oral dose of tacrolimus on the basis of the CYP3A5 genotype in Chinese renal transplant patients. Zhang et al48 conducted a study of 76 Chinese renal transplant recipients within two successive periods. In the first period, 0.1 mg/kg/day of tacrolimus was the initial dose prescribed for 28 recipients, regardless of their CYP3A5 genotype. In the second period, another 48 recipients were prescribed the following doses according to their genotypes: 0.08 mg/kg/day for CYP3A5*3/*3 (CYP3A5 nonexpresser) and 0.15 mg/kg/day for CYP3A5*1/*3 (CYP3A5 expresser). Adjustment of the initial dosage of tacrolimus was documented to improve the proportion of patients achieving target drug blood levels in the early postoperative stage: from 46.7% to 81.8% of the *1/*3 group, and from 46.2% to 73.1% of the *3/*3 group on the third day. Another prospective study, by Chen et al49 also confirmed the efficiency of CYP3A5 genotype-guided use of tacrolimus in Chinese renal transplant recipients. The study consisted of two parts. In the first part, 120 patients received routine initial doses of tacrolimus (0.06 mg/kg every 12 hours). Pharmacokinetic data from these patients were used to fit a regression equation that predicted tacrolimus initial dose on the basis of the CYP3A5 genotypes. In the second part, patients received initial doses of tacrolimus according to the equation derived from the first part. The results demonstrated that genotype-guided dosing could significantly decrease the incidence of out-of-range tacrolimus levels. Therefore, initial tacrolimus-dosage selection based on CYP3A5 genotype can improve the proportion of patients achieving target trough blood concentrations in Chinese renal transplant recipients.
However, besides genetic factors, other factors can also affect tacrolimus pharmacokinetics. Li et al19 developed a tacrolimus-dosing model to predict the initial dosage for Chinese renal transplant patients based on both genetic and clinical factors. However, this model has not been evaluated in clinical practice. Determining additional genetic factors of variability among Chinese individuals in response to tacrolimus and developing the tacrolimus-dosing model are very important to improve therapeutic and clinical efficacy in Chinese transplant patients.
Efficacy studies
With the improvement in technology and accumulation of clinical experience, kidney and liver transplantation has progressed, with increased survival rates of grafts and Chinese transplant recipients. Chen et al50 analyzed the data of 1,806 Chinese renal transplant recipients between February 1984 and December 2003. The overall 1-, 5-, 10-, 15-, and 20-year patient-survival rates were 92.28%, 87.20%, 78.60%, 63.45%, and 47.59%, respectively. The 1-, 5-, 10-, 15-, and 20-year graft survival rates were 84.61%, 73.64%, 57.31%, 46.77%, and 31.18%, respectively. The overall half-life of transplanted kidneys was 11.94±0.84 years. In addition, some researchers compared the clinical efficacy of kidney transplant during the different periods. From 1983 to 1989, the 1-, 5-, and 10-year patient-survival rates were 87.8%, 68.3%, and 53.4%, respectively. From 1990 to 1998, the corresponding patient-survival rates were 90.6%, 84.9%, and 79.1%, respectively. The corresponding patient-survival rates were 98.9%, 94.0%, and 89.6% between 1999 and 2012.51 Patient-survival rates increased with time. Hu et al52 analyzed 502 Chinese patients who underwent liver transplantation from 2000 to 2007, of whom 60.1% had malignant liver tumors. The overall 3-year survival rate in this group was 68.7%. Wang et al21 performed a retrospective study in a single medical center of 255 liver-transplant patients whose primary disease was hepatocellular carcinoma (HCC). The 1-, 2-, and 3-year patient-survival rates were 84.6%, 66.2%, and 52.6%, respectively; the 1-, 2-, and 3-year recurrence-free survival rates were 67.4%, 53.8%, and 47.3%, respectively. Recurrence of HCC is an important factor that influences long-term survival of Chinese liver-transplant patients.
Advancements in organ transplantation not only result from improvements in surgical technique but also benefit from the utility of immunosuppressive agents. It is particularly important for improving long-term survival of graft organs to choose a rational initial and maintenance immunosuppressive therapy regimen. So far, calcineurin inhibitors, including CsA and tacrolimus, are still the basic components of immunosuppressive regimens. There have been studies that suggested that tacrolimus is associated with a lower incidence of early stage rejection and fewer liver-toxicity events compared to CsA, therefore reducing the risk of chronic loss of graft function and cardiovascular disease while prolonging survival of patients and graft organs.53,54 The statistical data are shown in Table 3.
Yu et al20 performed a prospective study to investigate the efficacy of tacrolimus in Chinese kidney-transplant patients. Patients were randomly divided into tacrolimus (n=40) and CsA (n=50) groups. The incidence of rejection in the tacrolimus group decreased by 10% compared to the CsA group, and there were fewer toxicity events associated with tacrolimus. Acute and refractory rejection after transplant surgery is usually steroid-resistant. Favorable curative effects cannot be obtained by high-dose steroid pulse therapy in such patients. In this study, it was demonstrated that tacrolimus could reverse acute and refractory rejection in most cases. Another study also indicated the incidence of acute rejection within 1 year after renal transplantation in the tacrolimus group was 4.0%, while it was 15.6% in the CsA group (P<0.01).55
Cheung et al56 performed a prospective randomized trial with paired kidney analysis to compare the efficacy of tacrolimus and CsA in reducing the incidence of 6-year postoperative long-term rejection of 76 Chinese kidney-transplant patients. The rates of acute rejection were significantly different between groups: 18.4% (7 of 38, tacrolimus group) versus 42.1% (16 of 38, CsA group) (P=0.03). Chronic allograft nephropathy (CAN) is the main reason for graft dysfunction. Ji et al57 conducted a 3-year follow-up study in 31 patients with pathologically diagnosed CAN. The authors suggested that conversion from a CsA-based regimen to a tacrolimus-based regimen could effectively improve renal function and delay the progression of CAN. This is comparable to another study including 73 patients with CAN conducted by Peng et al58 after 1 year of conversion, the level of serum creatinine in the CsA-tacrolimus group (n=43) was significantly lower than in the CsA group (n=30) (194.8±42.5 μmol/L versus 245.4±52.8 μmol/L, P<0.01). Meanwhile, the glomerular filtration rate in the CsA-tacrolimus group was significantly higher than in the CsA group. This indicated that tacrolimus improved renal function more effectively than CsA.
The population with hepatitis B virus (HBV) accounts for about 7% of the total Chinese population. The ratio of HBV-carrier kidney-transplant patients is even higher. Liu et al59 indicated that tacrolimus treatment caused less liver function impairment than CsA in HBV-carrier kidney-transplant recipients. In addition, another study suggested that a tacrolimus-based triple regimen in kidney-transplant patients with delayed graft function was associated with fewer adverse reactions and facilitated renal function recovery more effectively.60 Tacrolimus was also associated with a lower rejection rate in liver transplantation, and could reverse refractory transplant rejections, so tacrolimus should be the first choice for Chinese patients after liver transplantation.4,61 Ye et al62 investigated the relationship between serum tacrolimus concentration and clinical efficacy. They found that tacrolimus with 10–15 ng/mL whole blood concentrations not only effectively controlled rejection but also caused fewer adverse reactions.
In conclusion, tacrolimus is a highly effective immunosuppressive agent, and is suitable for Chinese liver- and kidney-transplant patients. Tacrolimus is superior to CsA in controlling rejection, reducing adverse reactions, and prolonging graft survival.
Safety and tolerability
Tacrolimus has been used in Chinese organ-transplant practice for nearly 20 years, and has a good safety and tolerability profile. The short-term adverse effects of tacrolimus include posttransplantation diabetes mellitus, nephrotoxicity, neurotoxicity, and gastrointestinal effects. Symptoms include tremor, headache, diarrhea, and nausea; hyperglycemia and elevation of serum creatinine are additional effects. Xu et al63 analyzed data from 887 Chinese kidney-transplant patients with normoglycemia prior to transplantation. The 3-month and 1-, 3-, 5-, 10-, and 20-year cumulative incidence of hyperglycemia was 10.4%, 11.4%, 13.4%, 15.2%, 22.7%, 27.9%, and 38.3%, respectively. Among them, 61.6% of patients developed posttransplantation diabetes mellitus. The utility of tacrolimus was a risk factor for developing diabetes (relative risk 1.835, 95% confidence interval 1.181–2.851; P=0.007). Ling et al64 analyzed data from 125 Chinese liver-transplant patients without a history of diabetes. Multivariate logistic regression analysis indicated that a serum tacrolimus concentration of more than 10 ng/mL 1 month after transplantation was an independent risk factor for predicting posttransplantation diabetes mellitus (odds ratio 3.264, P=0.017). Therefore, serum glucose levels should be strictly monitored in organ-transplant patients. For patients with tacrolimus-associated diabetes, if the condition cannot be controlled by standard drug therapy, conversion to CsA should be considered. Tacrolimus is a calcineurin inhibitor with less nephrotoxicity compared to CsA. However, patients taking tacrolimus still have a risk of developing renal impairment. Lai et al65 conducted a study of 124 liver-transplant patients. Of these, 102 patients took tacrolimus as immunosuppressive drug and nine (8.8%) patients developed renal adverse effects. Calcineurin inhibitor-associated nephrotoxicity can be significantly reduced by decreasing the dose of tacrolimus and increasing the dose of MMF.
Tacrolimus has the potential to induce neurotoxicity, particularly when used in pediatric patients. Xie et al66 concluded their single-center clinical experience, and suggested that in pediatric transplant patients, tacrolimus-induced neurotoxicity is manifested by epilepsy that occurs within 2 weeks after transplant. High blood tacrolimus concentration can induce epileptic seizures; the adverse effects of tacrolimus are closely associated with blood concentrations.67
Adverse effects always occur when blood tacrolimus concentrations are higher than the therapeutic concentration − 15 ng/mL. However, most adverse effects are transient, and resolve when the treatment is withdrawn. Therefore, the TDM of tacrolimus trough concentrations is necessary. Transplant recipients require long-term immunosuppressive treatment, along with long-term survival of the graft organ. Therefore, they have a higher risk of developing infections and cancers. Chen et al50 analyzed data from 1,806 renal transplant patients; 252 patients died, with 146 (57.9%) from infection and 10 (4.0%) from malignant tumors. Pneumonia is the most common of transplant-associated infections. Zhang et al68 reported in 386 kidney transplant patients who received tacrolimus-based immunosuppression an incidence of pneumonia of 7.25%, most of which occurred within 6 months after transplant. Infections were usually associated with tacrolimus overdose. In addition, the expressive status of CYP3A5 was also an independent risk factor for the development of infections in Chinese pediatric liver-transplant patients.69
Combination therapy with steroids significantly affected infection and tumor-recurrence rates of transplant patients who received tacrolimus-based immunosuppressive therapy. In a study conducted by Hu et al52 a total of 502 liver-transplant recipients were divided into the following groups: tacrolimus with basiliximab induction and steroid-avoidance group, tacrolimus with 14 days of steroid-withdrawal group, tacrolimus with 3 months of steroid-withdrawal group, and tacrolimus with 6 months of steroid-withdrawal group. The incidence of HBV infection in these groups was 20.5%, 30.5%, 56.1%, and 62.2%, respectively. The 3-year HCC-recurrence rate was lowest in the basiliximab-induction group and the steroid-avoidance group (12.8%, P=0.037). These results indicated that a basiliximab-induction and steroid-avoidance immunosuppressive protocol can reduce HBV infection and HCC recurrence after liver transplantation.
In the PRC, calcineurin inhibitors are widely used in combination with mycophenolic acid (MPA), an antimetabolite immunosuppressant. It is reported that tacrolimus can affect the metabolism of MPA. Therefore, the MPA concentration should also be monitored when combined with calcineurin inhibitors, in order to improve the safety of transplant recipients.70
In spite of the potential adverse effects of tacrolimus, it is still the most important immunosuppressive agent for Chinese transplant patients, due to its high efficacy and low toxicity. Some strategies can be adopted to control transplant rejection effectively and to the maximum extent possible limit the toxicity of tacrolimus, including optimizing dosage and blood concentration, adopting appropriate combination regimens and personalizing regimens for each patient.
Patient satisfaction and adherence
The success of organ transplantation significantly improves quality of life in end-stage organ failure. However, patients confront certain challenges, such as economic burden and side effects, during the course of their lifetime. Psychological issues may also negatively influence treatment adherence. Approximately 20% of patients are unable to follow up, or cannot follow instructions to adjust drug dosage; some change or stop therapy on their own, resulting in adverse effects.71 Weng et al72 found that there was a significant difference in adherence between patients with once- or twice-daily dosage. The tacrolimus sustained-release capsule is administered only once daily, and its efficacy in preventing acute rejection is comparable with tacrolimus. There are no significant differences in the safety profiles between the formulations.73 The once-daily tacrolimus formulation can significantly improve patient adherence; however, in order to maintain a similar tacrolimus blood concentration, the once-daily dosage of tacrolimus should increase by 30%.74
Special features
Since the genetic backgrounds of various ethnic groups are different, the gene variations that can affect the metabolism of tacrolimus are different in various ethnic groups. It has been reported that COMT rs2239393, COMT rs4646312, and POR*28 were associated with tacrolimus trough blood concentrations in American renal transplant patients.75,76 However, the association between these variants and tacrolimus trough concentration was not found in Chinese renal transplant recipients.32 There are reports that CYP3A4*22 can affect tacrolimus pharmacokinetics in European renal transplant patients,77,78 whereas CYP3A4*22 has not yet been found in Chinese renal transplant patients.32 CYP3A5 6986A>G (CYP3A5*3) is a well-known genetic variation that can significantly affect the metabolism of tacrolimus. The gene frequency of this variant also varies with ethnic group. It was reported that the frequency of CYP3A5 expressers (CYP3A5*1/*1 and *1/*3) was 85% in African–Americans. In Asians, the frequency is 56%, and in Caucasians the frequency is about 16%.79 The variation of genetic background in different ethnic groups is a key factor leading to the different tacrolimus dosages used in Chinese and Western transplant recipients.
In Chinese adult liver-transplant patients, 78.47% have HBV, whereas HCV accounts for approximately 60% of liver transplants performed in the US.80 Due to the unprecedented proportion of patients with HBV-associated HCC, the application of the Milan criteria is limited in Chinese liver-transplant patients. Niu et al81 found that HCC-recurrence rates at postoperative years 1, 2, 3, and 4 in the sirolimus group were 13.3%, 36.7%, 43.3%, and 53.3%, respectively, and were 38.7%, 67.7%, 74.2%, and 77.4%, respectively, in the tacrolimus group. Sirolimus significantly reduced tumor recurrence and increased survival for Chinese liver transplant recipients with HCC beyond Milan criteria. Considering this situation, Jia et al82 established the Hangzhou criteria, which are suitable for Chinese transplant recipients with HCC. Based on this standard, tacrolimus is still a first-choice immunosuppressive agent for patients in the first month after liver transplantation. After the first postoperative month, sirolimus, which has antitumor effects, can be added to the immunosuppressive regimen and other immunosuppressive agents can be withdrawn gradually. Without decreasing postoperative or tumor-free survival, the Hangzhou criteria effectively expands the indication range for liver cancer-associated liver transplantation. Compared to the Milan criteria, the Hangzhou criteria increase the number of HCC patients who are eligible for liver transplantation by 37.5%. Therefore, more HCC patients can benefit from application of the Hangzhou criteria.
Conclusion
In summary, tacrolimus has been used in Chinese organ transplantation for over 10 years, and is the first-choice calcineurin inhibitor. The safety and efficacy of tacrolimus in preventing posttransplant acute rejection, as well as improving graft-organ survival, have been validated in many clinical trials. Personalized use of tacrolimus has gradually gained the attention of researchers. Owing to a narrow therapeutic range and large individual variability in its pharmacokinetics, the personalized use of tacrolimus is a challenge in clinical practice. To improve tacrolimus efficacy, reduce the incidence of side effects, and prolong graft survival, the appropriate initial and maintenance dose of tacrolimus, rational combination of tacrolimus with other agents, and optimization of immunosuppressive regimens are very important. We should evaluate patients’ immune status according to genetic background, liver function, nutritional state, complications such as infection and cancer, and TDM, to find the optimal balance between efficacy and adverse effects, and to personalize the initial and maintenance dose of tacrolimus further for Chinese patients. Conversion from a general therapy regimen for all patients to personalized regimens is the upcoming trend for tacrolimus use in Chinese organ transplantation.
Acknowledgments
This work was supported by the National Natural Science Fund of China (81101328), the Pearl River Young Talents of Science and Technology in Guangzhou (2013J2200050), the Research Fund for the Distinguished Young Teachers of Southern Medical University to Liang Li and the Research Fund for the President of Nanfang Hospital (2012B014).
Disclosure
The authors report no conflicts of interest in this work.
References
El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527–535. | ||
Bucuvalas J. Long-term outcomes in pediatric liver transplantation. Liver Transpl. 2009;15 Suppl 2:S6–S11. | ||
Yu LX, Xia RF, Deng WF, et al. 15 year clinical experience in ATG induction after renal transplantation. Zhonghua Qiguan Yizhi Zazhi. 2013;34:466–468. | ||
Lo CM, Fan ST, Liu CL, et al. Use of FK506 as primary or rescue therapy after liver transplantation in Hong Kong. Transplant Proc. 1998;30:3587–3588. | ||
Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo). 1987;40:1249–1255. | ||
Kino T, Hatanaka H, Miyata S, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo). 1987;40:1256–1265. | ||
Ochiai T, Nakajima K, Nagata M, et al. Effect of a new immunosuppressive agent, FK 506, on heterotopic cardiac allotransplantation in the rat. Transplant Proc. 1987;19:1284–1286. | ||
Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. | ||
[No authors listed]. Randomized trial comparing tacrolimus (FK506) and cyclosporine in prevention of liver allograft rejection. European FK506 Multicenter Liver Study Group. Lancet. 1994;344:423–428. | ||
[No authors listed]. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression in liver transplantation. The US Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110–1115. | ||
Pattison JM, Krensky AM. New insights into mechanisms of allograft rejection. Am J Med Sci. 1997;313:257–263. | ||
Wallemacq PE, Furlan V, Möller A, et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur J Drug Metab Pharmacokinet. 1998;23:367–370. | ||
de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther. 2012;92:366–375. | ||
Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184–198. | ||
Chen YH, Zheng KL, Chen LZ, et al. Clinical pharmacokinetics of tacrolimus after the first oral administration in combination with mycophenolate mofetil and prednisone in Chinese renal transplant recipients. Transplant Proc. 2005;37:4246–4250. | ||
Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant. 2001;16:1905–1909. | ||
Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther. 2004;75:434–447. | ||
Saint-Marcoux F, Woillard JB, Jurado C, Marquet P. Lessons from routine dose adjustment of tacrolimus in renal transplant patients based on global exposure. Ther Drug Monit. 2013;35:322–327. | ||
Li L, Li CJ, Zheng L, et al. Tacrolimus dosing in Chinese renal transplant recipients: a population-based pharmacogenetics study. Eur J Clin Pharmacol. 2011;67:787–795. | ||
Yu L, Wang Y, Fu SJ, Cheng XJ. Clinical experience with Prograf (tacrolimus, FK 506) in Chinese patients after renal transplantation. Transplant Proc. 2000;32:1709–1710. | ||
Wang ZX, Song SH, Teng F, et al. A single-center retrospective analysis of liver transplantation on 255 patients with hepatocellular carcinoma. Clin Transplant. 2010;24:752–757. | ||
Yang S, Wu Z, Wu W, et al. Characteristics of long-term immunosuppressive therapy in Chinese pediatric renal transplant patients: a single-center experience. Transplant Proc. 2009;41:4169–4171. | ||
Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31:139–152. | ||
Zhang XQ, Wang ZW, Fan JW, et al. The impact of sulfonylureas on tacrolimus apparent clearance revealed by a population pharmacokinetics analysis in Chinese adult liver-transplant patients. Ther Drug Monit. 2012;34:126–133. | ||
Liu C, Shang YF, Zhang XF, et al. Co-administration of grapefruit juice increases bioavailability of tacrolimus in liver transplant patients: a prospective study. Eur J Clin Pharmacol. 2009;65:881–885. | ||
Zuo XC, Zhou YN, Zhang BK, et al. Effect of CYP3A5*3 polymorphism on pharmacokinetic drug interaction between tacrolimus and amlodipine. Drug Metab Pharmacokinet. 2013;28:398–405. | ||
Xin HW, Li Q, Wu XC, et al. Effects of Schisandra sphenanthera extract on the blood concentration of tacrolimus in renal transplant recipients. Eur J Clin Pharmacol. 2011;67:1309–1311. | ||
Zhao Y, Song M, Guan D, et al. Genetic polymorphisms of CYP3A5 genes and concentration of the cyclosporine and tacrolimus. Transplant Proc. 2005;37:178–181. | ||
Chen JS, Li LS, Cheng DR, et al. Effect of CYP3A5 genotype on renal allograft recipients treated with tacrolimus. Transplant Proc. 2009;41:1557–1561. | ||
Zhu L, Song HT, Wang QH, Wu WZ, Yang SL, Tan JM. [Effect of CYP3A4*18B, CYP3A5*3 gene polymorphism on dosage and concentration of tacrolimus in renal transplant patients]. Yao Xue Xue Bao. 2012;47:878–883. Chinese. | ||
Zuo XC, Ng CM, Barrett JS, et al. Effects of CYP3A4 and CYP3A5 polymorphisms on tacrolimus pharmacokinetics in Chinese adult renal transplant recipients: a population pharmacokinetic analysis. Pharmacogenet Genomics. 2013;23:251–261. | ||
Li CJ, Li L, Lin L, et al. Impact of the CYP3A5, CYP3A4, COMT, IL-10 and POR genetic polymorphisms on tacrolimus metabolism in Chinese renal transplant recipients. PLoS One. 2014;9:e86206. | ||
Li DY, Teng RC, Zhu HJ, Fang Y. CYP3A4/5 polymorphisms affect the blood level of cyclosporine and tacrolimus in Chinese renal transplant recipients. Int J Clin Pharmacol Ther. 2013;51:466–474. | ||
Wu P, Ni X, Wang M, Xu X, Luo G, Jiang Y. Polymorphisms in CYP3A5*3 and MDR1, and haplotype modulate response to plasma levels of tacrolimus in Chinese renal transplant patients. Ann Transplant. 2011;16:54–60. | ||
Wang W, Zhang XD, Ma LL, et al. [Relationship between MDR1 gene polymorphism and blood concentration of tacrolimus in renal transplant patients]. Zhonghua Yi Xue Za Zhi. 2005;85:3277–3281. Chinese. | ||
Li D, Gui R, Li J, Huang Z, Nie X. Tacrolimus dosing in Chinese renal transplant patients is related to MDR1 gene C3435T polymorphisms. Transplant Proc. 2006;38:2850–2852. | ||
Rong G, Jing L, Deng-Qing L, Hong-Shan Z, Shai-Hong Z, Xin-Min N. Influence of CYP3A5 and MDR1(ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in Chinese renal transplant recipients. Transplant Proc. 2010;42:3455–3458. | ||
Zhang X, Liu ZH, Zheng JM, et al. Influence of CYP3A5 and MDR1 polymorphisms on tacrolimus concentration in the early stage after renal transplantation. Clin Transplant. 2005;19:638–643. | ||
Yu S, Wu L, Jin J, et al. Influence of CYP3A5 gene polymorphisms of donor rather than recipient to tacrolimus individual dose requirement in liver transplantation. Transplantation. 2006;81:46–51. | ||
Wei-lin W, Jing J, Shu-sen Z, et al. Tacrolimus dose requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transpl. 2006;12:775–780. | ||
Li D, Zhu JY, Gao J, Wang X, Lou YQ, Zhang GL. Polymorphisms of tumor necrosis factor-alpha, interleukin-10, cytochrome P450 3A5 and ABCB1 in Chinese liver transplant patients treated with immunosuppressant tacrolimus. Clin Chim Acta. 2007;383:133–139. | ||
Wang Z, Wu S, Chen D, et al. Influence of TLR4 rs1927907 locus polymorphisms on tacrolimus pharmacokinetics in the early stage after liver transplantation. Eur J Clin Pharmacol. 2014;70:925–931. | ||
Shi Y, Li Y, Tang J, et al. Influence of CYP3A4, CYP3A5 and MDR-1 polymorphisms on tacrolimus pharmacokinetics and early renal dysfunction in liver transplant recipients. Gene. 2013;512:226–231. | ||
Chen D, Fan J, Guo F, Qin S, Wang Z, Peng Z. Novel single nucleotide polymorphisms in interleukin 6 affect tacrolimus metabolism in liver transplant patients. PLoS One. 2013;8:e73405. | ||
Jin J, Wu LH, Wang WL, Yu SF, Yan S, Zheng SS. Impact of multidrug resistance 1 gene polymorphism on tacrolimus dose and concentration-to-dose ratio in Chinese liver transplantation recipients. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22:616–620. | ||
Zhang X, Wang Z, Fan J, Liu G, Peng Z. Impact of interleukin-10 gene polymorphisms on tacrolimus dosing requirements in Chinese liver transplant patients during the early posttransplantation period. Eur J Clin Pharmacol. 2011;67:803–813. | ||
Yu X, Xie H, Wei B, et al. Association of MDR1 gene SNPs and haplotypes with the tacrolimus dose requirements in Han Chinese liver transplant recipients. PLoS One. 2011;6:e25933. | ||
Zhang J, Zhang X, Liu L, Tong W. Value of CYP3A5 genotyping on determining initial dosages of tacrolimus for Chinese renal transplant recipients. Transplant Proc. 2010;42:3459–3464. | ||
Chen SY, Li JL, Meng FH, et al. Individualization of tacrolimus dosage basing on cytochrome P450 3A5 polymorphism – a prospective, randomized, controlled study. Clin Transplant. 2013;27:E272–E281. | ||
Chen LZ, Chen GD, Wang CX, et al. Outcome of cadaveric kidney transplantation: analysis of 1,806 cases. Chin J Urol. 2006;27:166–170. | ||
Shu KH, Ho HC, Wen MC, et al. Changing pattern of mortality in renal transplant recipients: a single-center, 30-year experience. Transplant Proc. 2014;46:442–444. | ||
Hu AB, Wu LW, Tai Q, Zhu XF, He XS. Safety and efficacy of four steroid-minimization protocols in liver transplant recipients: 3-year follow-up in a single center. J Dig Dis. 2013;14:38–44. | ||
First MR. Improving long-term renal transplant outcomes with tacrolimus: speculation vs evidence. Nephrol Dial Transplant. 2004;19 Suppl6:vi17–vi22. | ||
Beckebaum S, Klein C, Varghese J, et al. Renal function and cardiovascular risk profile after conversion from ciclosporin to tacrolimus: prospective study in 80 liver transplant recipients. Aliment Pharmacol Ther. 2009;30:834–842. | ||
Wang XH, Tang XD, Xu D. Tacrolimus vs CyA Neoral in combination with MMF and steroids after cadaveric renal transplantation. Transplant Proc. 2000;32:1702–1703. | ||
Cheung CY, Chan HW, Liu YL, Chau KF, Li CS. Long-term graft function with tacrolimus and cyclosporine in renal transplantation: paired kidney analysis. Nephrology (Carlton). 2009;14:758–763. | ||
Ji SM, Li LS, Sha GZ, Chen JS, Liu ZH. Conversion from cyclosporine to tacrolimus for chronic allograft nephropathy. Transplant Proc. 2007;39:1402–1405. | ||
Peng LK, Xie XB, Peng FH, et al. [Slowing progression of chronic allograft nephropathy by conversion from cyclosporin A to tacrolimus]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007;32:59–62. Chinese. | ||
Liu XY, Yu LX, Fu SJ, et al. [Application of tacrolimus and cyclosporine A in HBV-carrying renal transplant recipients]. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1090–1092. Chinese. | ||
Liu B, Lin ZB, Ming CS, et al. Randomized trial of tacrolimus in combination with mycophenolate mofetil versus cyclosporine with mycophenolate mofetil in cadaveric renal transplant recipients with delayed graft function. Transplant Proc. 2003;35:87–88. | ||
Lo CM, Fan ST, Liu CL, et al. More effective immunosuppression with the use of FK506 after liver transplantation. Transplant Proc. 2000;32:2269–2270. | ||
Ye QF, Ruzig MA, Gong NQ. Administration of tacrolimus in 50 liver transplantation patients. Hepatobiliary Pancreat Dis Int. 2002;1:492–494. | ||
Xu Y, Liang JX, Liu B, et al. Prevalence and long-term glucose metabolism evolution of post-transplant diabetes mellitus in Chinese renal recipients. Diabetes Res Clin Pract. 2011;92:11–18. | ||
Ling Q, Xie H, Lu D, et al. Association between donor and recipient TCF7L2 gene polymorphisms and the risk of new-onset diabetes mellitus after liver transplantation in a Han Chinese population. J Hepatol. 2013;58:271–277. | ||
Lai W, Lu SC, Wang ML, et al. Mycophenolate mofetil-based calcineurin inhibitor reduced immunosuppressive protocol for the improvement of renal dysfunction after liver transplantation. Zhonghua Yi Xue Za Zhi. 2009;89:1529–1532. | ||
Xie M, Rao W, Sun LY, et al. Tacrolimus-related seizure after pediatric liver transplantation – a single-center experience. Pediatr Transplant. 2014;18:58–63. | ||
Wu Z, Meng Q, Xia Y, Zhang F, You W. A retrospective analysis of the safety and efficacy of low dose tacrolimus (FK506) for living donor liver transplant recipients. J Biomed Res. 2013;27:305–309. | ||
Zhang YX, Yu LX, Fu SJ, Ye JS, Liu XY. [Clinical study of pulmonary infection in kidney transplantation recipients taking new immunosuppressant]. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1037–1040. Chinese. | ||
Xue F, Han L, Chen Y, et al. CYP3A5 genotypes affect tacrolimus pharmacokinetics and infectious complications in Chinese pediatric liver transplant patients. Pediatr Transplant. 2014;18:166–176. | ||
Huang HF, Yao X, Chen Y, et al. Cyclosporine A and tacrolimus combined with enteric-coated mycophenolate sodium influence the plasma mycophenolic acid concentration – a randomised controlled trial in Chinese live related donor kidney transplant recipients. Int J Clin Pract Suppl. 2014:4–9. | ||
Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77:769–776. | ||
Weng FL, Israni AK, Joffe MM, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol. 2005;16:1839–1848. | ||
Posadas Salas MA, Srinivas TR. Update on the clinical utility of once-daily tacrolimus in the management of transplantation. Drug Des Devel Ther. 2014;8:1183–1194. | ||
Ma MK, Kwan LP, Mok MM, Yap DY, Tang CS, Chan TM. Significant reduction of tacrolimus trough level after conversion from twice daily Prograf to once daily Advagraf in Chinese renal transplant recipients with or without concomitant diltiazem treatment. Ren Fail. 2013;35:942–945. | ||
Jacobson PA, Oetting WS, Brearley AM, et al. Novel polymorphisms associated with tacrolimus trough concentrations: results from a multicenter kidney transplant consortium. Transplantation. 2011;91:300–308. | ||
Elens L, Hesselink DA, Bouamar R, et al. Impact of POR*28 on the pharmacokinetics of tacrolimus and cyclosporine A in renal transplant patients. Ther Drug Monit. 2014;36:71–79. | ||
Elens L, van Schaik RH, Panin N, et al. Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors’ dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics. 2011;12:1383–1396. | ||
Kurzawski M, Dąbrowska J, Dziewanowski K, Domański L, Perużyńska M, Droździk M. CYP3A5 and CYP3A4, but not ABCB1 polymorphisms affect tacrolimus dose-adjusted trough concentrations in kidney transplant recipients. Pharmacogenomics. 2014;15:179–188. | ||
Zan SJ, Zhu LQ, Duan WY, Zhang Y. Effects of race on the pharmacokinetics of tacrolimus in transplantation patients. China Pharm. 2013;24:2438–2441. | ||
Saidi RF. Current status of liver transplantation. Arch Iran Med. 2012;15:772–776. | ||
Niu YJ, Liu Y, Wang LT, et al. Impact of sirolimus and tacrolimus on the tumor recurrence after liver transplantation due to HCC beyond Milan criteria: randomized controlled clinical trial. Chin J Organ Transplant. 2014;35:99–102. | ||
Jia JJ, Lin BY, He JJ, et al. “Minimizing tacrolimus” strategy and long-term survival after liver transplantation. World J Gastroenterol. 2014;20:11363–11369. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.