Back to Journals » Psoriasis: Targets and Therapy » Volume 6
Tacrolimus for the management of psoriasis: clinical utility and place in therapy
Received 12 July 2016
Accepted for publication 18 October 2016
Published 7 December 2016 Volume 2016:6 Pages 153—163
DOI https://doi.org/10.2147/PTT.S101233
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Uwe Wollina
Nina Malecic,1,2 Helen Young2
1Manchester Medical School, 2The Dermatology Research Centre, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK
Abstract: Psoriasis affects 1%–3% of the population in the United Kingdom and can convey significant detriment to the physical and mental health of sufferers. Plaques of psoriasis typically affect the extensor skin surfaces and scalp. Less frequently inverse psoriasis can affect more sensitive skin such as the face, genitals, and intertriginous areas. Psoriasis is incurable, but there are a range of treatment modalities that can be used to manage the condition. Treatment options include topical preparations, phototherapy, systemic therapy, and biological agents. Tacrolimus is a macrolide calcineurin inhibitor licensed for immunosuppression in transplant patients and topical administration in atopic dermatitis. Tacrolimus administered orally and in topical form has been shown to produce successful outcomes in patients with psoriasis. Topical tacrolimus is particularly effective for inverse psoriasis, which is likely to be due to the reduced level of induration seen in these psoriatic lesions, which allows greater skin penetrance, compared with hyperkeratotic plaques of psoriasis on the body. It is also notable that the areas affected by inverse psoriasis are more susceptible to adverse effects of topical corticosteroid therapy, and thus a topical preparation without the risk of skin atrophy, telangiectasia, and striae could be a valuable addition to current topical treatment options. Oral tacrolimus has shown efficacy in the treatment of severe, refractory psoriasis. Compared to ciclosporin, systemic tacrolimus may be more suited to a patient population with increased cardiovascular risk. This review will draw together the current literature on topical and oral tacrolimus for the treatment of psoriasis. Efficacy and safety have been evaluated by case reports and randomized controlled trials and comparisons have been made between tacrolimus therapy and standard treatment.
Keywords: tacrolimus, psoriasis, topical therapy, oral treatment
Introduction
Psoriasis is a complex, chronic inflammatory skin condition that is common in individuals of European descent, affecting 1%–3% of the population.1 The disease has a multifactorial etiology; environmental influences, such as infection or trauma, can trigger a helper T-cell (Th)1/17 inflammatory process, which encompasses an interaction between endothelial cells, keratinocytes, dendritic cells, monocytes, neutrophils, and various cytokines, in a genetically susceptible individual.2 The molecular genetics that contribute to development of psoriasis are complex and appear to be polygenic.3 Key histological findings include hyperproliferation of keratinocytes in the stratum corneum, infiltration of the epidermis with numerous types of inflammatory cells and cytokines, particularly Th17 cells, and abnormal angiogenesis.4
The most commonly diagnosed subtype of psoriasis is chronic plaque psoriasis. Its cutaneous manifestation is variable, but classically psoriasis is described as thick, raised, well-demarcated, erythematous plaques. Extensor surfaces, scalp, and nails are most commonly affected in a localized or widespread, symmetrical distribution.5 Inverse or flexural psoriasis is less common and usually presents in the axillae, genitofemoral area, and submammory folds. Psoriasis follows a relapsing–remitting course and shows significant variability in clinical severity between patients. The impact upon quality of life depends upon disease severity and individual factors relating to the patient. There are well-established extracutaneous comorbidities of psoriasis, including psoriatic arthritis, cardiovascular disease, and psychosocial sequelae.
Current treatment for psoriasis is not curative; however, there are a range of treatment options for management of the disease. The majority of patients with psoriasis have mild disease, which can often be effectively managed with topical preparations.6 However, adequate compliance can be a significant burden for many patients due to inconvenience of application and lack of cosmetic acceptability.7 For moderate or severe psoriasis, treatments can include phototherapy, systemic therapies, small-molecule inhibitors, or biological therapies. Topical therapy plays a role throughout the disease severity spectrum in psoriasis, and in moderate and severe disease, topical treatment may lessen the requirement for systemic and phototherapy.
Tacrolimus is a macrolide calcineurin inhibitor that is produced by Streptomyces tsukubaensis. Although tacrolimus and ciclosporin inhibit calcineurin leading to suppression of T-cell activation, tacrolimus has been found to have a more potent immunological effect with a diminished vasoconstrictive and fibrinogenic effect. Systemic tacrolimus is now widely used for rejection prophylaxis post solid organ transplantation, in preference to ciclosporin, which is associated with a less favorable side effect profile; serious side effects of ciclosporin include hypertension and nephrotoxicity.
There has been a significant amount of research conducted not only with regard to the licensed indications of tacrolimus, but also for off-license indications such as psoriasis. This review paper will evaluate the current literature about use of tacrolimus as management strategy in psoriasis.
Pharmacology
Tacrolimus inhibits dephosphorylation of the transcription factor nuclear factor of activated T-cells by calcineurin, and therefore there is suppressed activity of the genes that code for interleukin 2 (IL-2) in the nucleus. Tacrolimus also causes decreased transcription and release of other T-cell-derived cytokines including IL-3, IL-4, IL-8, TNF-α, INF-γ, and granulocyte macrophage colony-stimulating factor; it has also been found that exocytosis of in cytotoxic T-cells is inhibited by tacrolimus.8 In vitro research has indicated that p53 levels in psoriatic skin are diminished; Lemster et al8 describe augmentation of p53 gene expression by tacrolimus treatment, resulting in a reduced rate of epidermal hyperproliferation.
Orally administered ciclosporin is an effective treatment for psoriasis; however, topical application of the drug is ineffective due to inadequate skin penetrance. In light of this, topical pimecrolimus and tacrolimus preparations were developed. Topical tacrolimus penetrates the skin at 0.03% and 0.1% strength; however, topical corticosteroids have a superior skin penetrance than topical calcineurin inhibitors. Due to a more selective mechanism of action that does not alter collagen synthesis, topical calcineurin inhibitors can be utilized as corticosteroid-sparing agents as they are not associated with agenesis of the skin; this has been found to be of particular usefulness in facial, genital, and intertriginous areas.
Pimecrolimus is a structurally similar molecule to tacrolimus; however, pimecrolimus has greater lipophilicity. The implication of this is that there is a high level of pimecrolimus retained within the skin following application and thus systemic absorption is minimal. Systemic absorption of topical tacrolimus is reported to be highest through skin that has compromised barrier function and it is not absorbed systemically through intact skin. No systemic side effects have been reported following topical tacrolimus treatment; however, there is a lack of evidence about long-term usage.
Topical treatment with tacrolimus
Evidence of efficacy for psoriasis
Since 1990, 9 double-blind and 13 open studies have demonstrated the efficacy of topical tacrolimus in psoriasis, especially for facial, genital, and intertriginous disease. These are summarized in Table 1.
  | Table 1 Summary of clinical studies investigating the efficacy of topical tacrolimus for the management of psoriasis Abbreviations: RCT, randomized control trial; UVA1, ultraviolet A1. |
The first investigation into the efficacy of topical tacrolimus for the treatment of psoriasis was a vehicle-controlled trial of 70 participants who were randomized into three possible treatment arms including twice-daily application of calcipotriol 0.005% ointment, once-daily application of placebo ointment, or once-daily application of 0.3% tacrolimus ointment. At the end of the 6-week trial, those treated with tacrolimus had improvement in psoriasis, which was both significantly inferior to treatment with calcipotriol, 33.3% versus 62.5% (p<0.005) respectively, and comparable to treatment with placebo, 42.9% (p=0.77).9 Nevertheless, the investigators suggested that topical tacrolimus might be ineffective for treatment of thick psoriatic plaques due to its molecular weight of 822.05 Da, which is relatively large for a topical formulation. The finding of this study prompted further work to examine whether the use of a skin penetration enhancer in combination with topical tacrolimus could improve treatment efficacy.9
The effect of combined administration of 0.1% tacrolimus ointment and 6% salicylic acid gel to act as a penetration enhancer for treatment of chronic plaque psoriasis was investigated during a double-blind randomized vehicle-controlled trial involving 30 participants. Participants were instructed to apply the salicylic acid gel first to all plaques, allow it to dry, and then apply the ointment to plaques on one side of the body only. Treatment was given for 8 weeks, with follow-up at 1, 2, 4, 8, and 12 weeks after commencement of the drug. The results demonstrated greater improvement in patients treated with tacrolimus ointment compared with placebo (p<0.05). There was a statistically significant decline in erythema, scaling, and pruritus in the treated group.10
The clinical efficacy and safety of 0.3% tacrolimus gel and 0.5% tacrolimus cream was measured against calcipotriol 0.005% ointment in 124 participants with mild-to-moderate chronic plaque psoriasis in a 12-week multicenter randomized observer blinded trial. Efficacy was measured by the percentage change in local psoriasis severity index between baseline and week 12. The results did not show a statistically significant difference between the preparations. However, clinical improvement was observed after 1 week of treatment and an improvement was noted throughout the study duration. Patients treated with the tacrolimus preparations more frequently reported a mild burning sensation at the application site (31% of patients in both tacrolimus groups, compared with 7.5% in the calcipotriol group [p=0.011]); however, this was mostly self-limiting, fading after the first week of treatment.11
With the objective of investigating the efficacy and safety profile of topical tacrolimus cream, a randomized, double-blind placebo-controlled trial was carried out involving 128 participants. Participants were randomized into three equally sized groups: one group to apply vehicle cream, one group to apply tacrolimus 0.1% cream, and the other group to apply tacrolimus 0.5% cream; all participants were instructed to apply the cream twice daily for 8 weeks and were followed up at regular intervals during the treatment period and for 2 weeks after cessation of treatment.12 About 33.3% of patients who applied tacrolimus 0.1% cream and 34.1% patients using tacrolimus 0.5% cream achieved near or complete clearance. This was significantly greater than the 11.9% patients who achieved skin clearance in the vehicle-treated group (p<0.05). Blood levels of tacrolimus were measured through the study, and all of the participants treated with tacrolimus 0.1% cream and 82% of patients treated with 0.5% cream had plasma levels of <1 μg/mL for the duration of the study. This study demonstrated that tacrolimus cream is effective in the treatment of chronic plaque psoriasis when used as a twice-daily regimen. The lower dose preparation may be preferable due to similar efficacy but has reduced systemic absorption than the higher 0.5% cream.12
A multicenter open-label study randomized 18 patients with mild-to-moderate chronic plaque psoriasis and demonstrated that tacrolimus 0.3% gel, tacrolimus 0.5% cream, and calcipotriol 0.005% ointment improved the clinical features of erythema and induration observed with psoriasis. However, histological assessment showed that calcipotriol ointment had a more potent effect on abnormal keratinization than tacrolimus.13
In a Phase II, double-blind trail of 16 patients with chronic plaque psoriasis, patients were randomized to one of the following six treatment options: 0.3% tacrolimus ointment with 1% diisopropyl adipate as a penetration enhancer, 0.3% tacrolimus ointment without penetration enhancer, tacrolimus ointment base, 0.1% betamethasone 17 alpha valerate ointment, 0.005% calcipotriol ointment applied under occlusion, and betamethasone ointment base. The results were assessed by a composite score of erythema, infiltration, and superficial blood flow (used as a surrogate marker of erythema) within the plaques. Superficial blood flow was measured by Doppler ultrasound, and skin biopsies were taken from the plaques to determine epidermal thickness. Overall, betamethasone demonstrated greater efficacy than tacrolimus, which was, in turn, more effective than treatment with calcipotriol. Tacrolimus ointment produced significant improvement in the plaques, when used in conjunction with the penetration enhancer, which increased its efficacy. The level of erythema and infiltration was diminished (p<0.001), superficial blood flow was decreased (p<0.01), and there was a reduction in epidermal thickness (p≤0.001). This study suggested that tacrolimus may be useful for psoriasis affecting thinner skin and as an alternative to topical corticosteroids and calcipotriol, which can cause agenesis of the skin and local irritation, respectively. However, the preparation of tacrolimus used in this study was stronger than those currently available for clinical use. This and other studies highlight the need to develop a formulation that allows for sufficient cutaneous penetration of the drug and an effective application regimen.14,15
Utility for face and flexural skin
In numerous case studies, topical tacrolimus has shown promise for the treatment of facial psoriasis.16–19 Subsequently, 10 long-term psoriasis sufferers were included in a trial to examine the efficacy of tacrolimus 0.1% ointment used on anogenital and facial lesions. Tacrolimus 0.1% ointment was applied twice daily for 10 days, and follow-up continued for 12 weeks in total. There was notable improvement in all subjects by the end of the first week, and no adverse effects were reported.20
Following this, a multicenter randomized double-blind placebo-controlled trail investigated the utility of tacrolimus 0.1% ointment in 167 patients with inverse psoriasis. The participants were instructed to apply the ointment twice daily to areas of facial or intertriginous psoriasis for 8 weeks. Observers noted that from day 8 of the trial, more patients in the active treatment arm had completely cleared or achieved marked improvement than in the vehicle group. By the end of the trail, 65.2% of patients treated with tacrolimus 0.1% ointment were clear or almost clear of their psoriasis, compared to 31.5% treated with placebo (p<0.0001).21
Then an open-label single-arm clinical trial was conducted that included 21 patients who had psoriasis affecting the face or intertriginous areas, or both. The participants applied tacrolimus 0.1% ointment twice daily for 8 weeks. Two participants reported mild pruritus at the application site on the first day of treatment, and a transient warm sensation was also reported, lasting for 1 hour after application for the initial few days of treatment. Seventeen (out of 21) participants achieved complete skin clearance by the end of the study – supporting the use of topical tacrolimus in inverse psoriasis.22
In 2007, a double-blind randomized controlled trial was carried out in 50 individuals to compare the efficacy and tolerability of calcitriol 3 μg/g and tacrolimus 0.3 mg/g ointment in psoriasis affecting facial and genitofemoral areas. Both drugs were well tolerated; however, 55% of patients treated with calcitriol suffered from perilesional erythema at 6 weeks, compared with 16% in the tacrolimus group (p<0.05).23
An open-label clinical trial evaluated the efficacy of tacrolimus 0.1% ointment in 15 patients with psoriasis affecting the face, genital area, intertriginous areas, and on typical psoriasis plaques affecting the body. By the end of the trial, there was statistically significant improvement in clinical assessment of erythema, desquamation, and infiltration compared to baseline. In addition, the mean Psoriasis Area and Severity Index (PASI) had decreased from 12 at baseline to 2.2. The only reported adverse effects were of two participants who experienced a mild warm sensation on facial lesions post application, but this did not require discontinuation or dose adjustment and resolved without intervention.24
An open-label pilot study investigated the safety and efficacy of tacrolimus ointment in the management of male genital psoriasis. The study included 12 participants, who were administered tacrolimus 0.1% ointment twice daily for 8 weeks; the participants were followed up for a further 4 weeks after the end of the treatment period. The mean male genital PASI was significantly reduced from 15.8 at the beginning of the trial to 1.2 at week 8 (p<0.001). The formulation was well tolerated; a mild and self-limiting pruritic or burning sensation was the only adverse effect reported.25
Treatment of pediatric psoriasis
Steele et al26 published a promising retrospective case study of 13 pediatric patients, aged from 22 months to 16 years. Twelve of the participants achieved complete clearance of psoriatic skin lesions affecting the face and intertriginous areas within 2 weeks. These participants were treated with tacrolimus 0.1% ointment twice daily and were instructed to stop application of the ointment once their psoriasis had cleared. Patients were followed up for 2 years after the start of the study and instructed to apply the ointment if there was any recurrence of skin lesions.26 More recently, a pilot study of 11 pediatric patients between the age of 6 and 15 years demonstrated the efficacy of tacrolimus 0.1% ointment for treatment of facial and inverse psoriasis. All patients had either complete clearance or had excellent improvement of psoriasis after 30 days of treatment, and there was an unacceptable degree of pruritus experienced by only one patient when using the ointment in the genital region. Several participants experienced a relapse of their condition following cessation of treatment, but after recommencing treatment adequate control was regained within 7 days. When surveyed at the end of trial, caregivers rated the treatment regimen as easy or very easy for them to use and all but one caregiver stated that the treatment provided complete control of the disease.27
These are important findings as one-third of patients with psoriasis have childhood onset, and facial and inverse psoriasis is more prevalent in this population of patients. The use of topical calcineurin inhibitors for facial and intertriginous psoriasis could allow patients to have greater psoriasis control and overcome the adverse effects of long-term topical corticosteroid use. Although oral administration of tacrolimus has lead to concerns about the side effect profile, the systemic absorption from topical application is highly likely to be negligible. Topical tacrolimus is currently licensed for use in pediatric patients with atopic dermatitis, and because of the thickness of psoriasis lesions, systemic absorption will be probably be less in patients with psoriasis than those with atopic dermatitis, although no research has been carried out to confirm this.
Treatment of nail and pustular psoriasis
Tacrolimus 0.1% ointment also produced promising treatment results when used in a randomized controlled open-label study, involving 21 patients with nail psoriasis. Participants were randomized to either the treatment or placebo group and were instructed to apply the ointment to their nails once daily at bedtime and to avoid washing their hands until the morning. Severity of nail psoriasis was measured using the Nail Psoriasis Severity Index (NAPSI). NAPSI score can range from 0 in a nail with normal matrix and bed, to a score of 8 when nails signs are present in all four quadrants on the nail in both the matrix and the bed. At the end of the 12-week trial, the participants who received treatment had a mean significant reduction in NAPSI score of 13, compared with a mean reduction of 3 points in the placebo group (p<0.001).28
A number of case reports and small case series have suggested that topical tacrolimus treatment is effective for generalized pustular psoriasis,29,30 palmoplantar pustular psoriasis,31 and oral psoriasis.32,33 Topical tacrolimus and excimer laser treatment was effective for a patient with inverse psoriasis; it was postulated that the laser therapy decreased the thickness of the epidermis and thus facilitated penetration of the topical preparation, which lead to a reduction in the number of cutaneous nerve fibers and diminished pruritus.34 Tacrolimus ointment 0.1% has also been reported as effective when used as part of a combined topical treatment regimen in a patient with psoriasis lesions affecting the lips.33
Side effects of topical treatment
Overall, topical tacrolimus preparations have been well tolerated by patients with psoriasis. The most common adverse effects reported in clinical trials/studies include mild, self-limiting pruritus and a sensation of warmth at the application site at the onset of treatment, which subsides relatively quickly with continuation of treatment. Unlike corticosteroids, topical calcineurin inhibitors are not associated with skin atrophy, striae, or telangectasia and may therefore be more appropriate for use on skin areas that are more susceptible to these effects. In addition, use of topical tacrolimus is less likely to be associated with perilesional erythema and local irritation than use of topical vitamin D analogs.
Oral treatment with tacrolimus
Studies have also demonstrated the efficacy of oral tacrolimus in treating psoriasis, and these are summarized in Table 2.
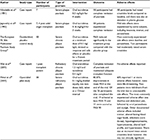  | Table 2 Summary of clinical studies investigating the efficacy of oral tacrolimus for the management of psoriasis Abbreviation: PASI, Psoriasis Area and Severity Index. |
In 1991, Nikolaidis et al35 published a report of seven patients with severe chronic plaque psoriasis who had been refractory to treatment with conventional therapies and who had been treated with oral tacrolimus for a trial period. The paper demonstrated promising results as all patients had complete remission following treatment. Participants were started on a dose of 0.3 mg/kg/d given as an oral capsule. There were some metabolic changes noted in the patients during the treatment period; all seven patients had increased serum creatinine compared with pretreatment levels; however, the levels decreased following a dose reduction. Uric acid levels also increased in the early part of the trial, but this effect was transient and only slightly above normal parameters. After 4 weeks of treatment, raised plasma glucose levels were observed. These effects were considered potentially significant drawbacks in clinical practice, especially in the psoriasis population where patients may have preexisting altered metabolic function and increased cardiovascular risk.35
In 1992, a case series of seven patients was published that documented complete remission of psoriasis after 3 weeks of oral tacrolimus therapy. The side effects noted during the study were insomnia, tremor, and paraesthesia; however, these were mild and no dose adjustment was required. This case series provided the foundation for further research into the usefulness of tacrolimus in psoriasis.36
The first report of a placebo-controlled trial that evaluated the efficacy of systemic tacrolimus in patients with moderate-to-severe psoriasis was published in 1996. Fifty participants received either oral tacrolimus treatment or placebo for 9 weeks and were then followed up for a further 3 weeks. At the end of the 9-week treatment period, the average PASI of participants in the treatment arm of the trial was reduced by 70%, compared to a 50% reduction in the placebo group. The main side effects reported were diarrhea and paraesthesia; significant change in blood pressure was not recorded during the study. However, 2 participants had moderately elevated serum creatinine. The authors concluded that tacrolimus was effective in patients with psoriasis at a dose of at least 0.1 mg/kg/d.37
Recently, a case report documented the experience of a 55-year-old male patient with long-standing psoriasis who received antirejection therapy subsequent to a cadaveric renal transplant. The patient experienced an acute exacerbation of psoriasis 2 months post transplant for which topical treatment was ineffective. The antirejection regimen was adjusted from tacrolimus 0.08 mg/kg/d and prednisolone 5 mg/d to everolimus 1.5 mg/d and tacrolimus 0.5 mg/d. This combination successfully prevented transplant rejection and induced complete remission of the psoriasis. The patient was maintained on this therapy and had no subsequent recurrences of psoriasis over 18 months. This study represents the first report of everolimus and tacrolimus being used concurrently to treat refractory psoriasis. The authors proposed that there may be a synergistic effect between the two structurally similar molecules, as tacrolimus exerts its effect upstream in the target immunological cascade, to everolimus, which inhibits the IL-2 receptor-mediated signal transduction pathway.38
In 2015, research was carried out to investigate the efficacy and safety of oral tacrolimus treatment in adults with severe recalcitrant psoriasis. The open-label prospective study included 30 patients who had previously tried at least one standard systemic agent for a minimum of 3 months without a 50% or more improvement in PASI. Subjects were given oral tacrolimus at a dose of 0.1 mg/kg for 12 weeks, titrated throughout the trial based upon response and side effects, to be taken twice daily at a 12-hour interval. Three patients were withdrawn from the study in total and 18 participants (60%) experienced at least one adverse effect, which was mainly dose dependent and self-limiting. At the end of the trial, the average PASI had improved by 80.37% (p<0.001). Nineteen (out of 26) patients achieved PASI 75, and 11 patients were scored as PASI 90. This study supported the findings from previously conducted research, suggesting that oral tacrolimus is efficacious for short-term treatment of severe chronic plaque psoriasis.39
One of the principal extracutaneous manifestations of psoriasis is psoriatic arthritis, which is evident in up to 30% of suffers.40 Tacrolimus has significant utility in a number of rheumatological conditions including rheumatoid arthritis41 and systemic sclerosis.42 Two case reports have described the efficacy of tacrolimus in the treatment of psoriatic arthritis.43,44 The most recently published case specifically illustrated the successful use of tacrolimus in a patient refractory to treatment with traditional disease-modifying antirheumatic drugs and anti-TNF-α biological therapy.44 Further studies to establish the positioning of tacrolimus in PsA management are required.
Side effects of oral tacrolimus
When administered to organ transplant recipients, the most frequently reported side effects of oral or intravenous tacrolimus have been insomnia, tremors, headache, paraesthesia, myalgia, pruritus, fatigue, photophobia, and gastrointestinal effects. Significant adverse effects can include infection, hypertension, hyperglycemia, hyperkalaemia, nephrotoxicity, neurotoxicity, and increased risk of neoplasia. Transplant patients may experience different toxic effects of tacrolimus compared to patients with an autoimmune disease, and dosage and duration of treatment may also be different in a post transplant tacrolimus treatment regimen. However, there appear to be safety advantages to treatment with tacrolimus in comparison to ciclosporin, based on both the clinical results in psoriasis and the experience in transplantation medicine.45 A recently published study in children with glomerulonephritis demonstrated a similar side effect profile for both drugs. However, reports of hypertrichosis, hypertension, and gum hypertrophy were significantly less frequently reported in the tacrolimus-treated group.46
Approved use of tacrolimus
Topical calcineurin inhibitors have demonstrated efficacy in atopic dermatitis, and as a result topical tacrolimus and pimecrolimus are licensed for use in this inflammatory skin disease. Topical tacrolimus is available in 0.1% and 0.03% potency – the weaker preparation most commonly prescribed to children aged 2–16 years. Topical tacrolimus is prescribed off-license for the treatment of psoriasis, lichen planus, lichen sclerosis, cutaneous lupus, seborrhoeic dermatitis, vitiligo, and pityriasis alba.
Oral tacrolimus is licensed for prophylaxis of transplant rejection in recipients of liver, kidney, or heart allografts and in cases of transplant rejection that are inadequately managed by standard immunosuppressive therapy.
Discussion
Both topically and orally administered tacrolimus have demonstrated promising efficacy for the treatment of psoriasis; however, neither has been approved for use in psoriasis management. With regard to topical tacrolimus, two double-blind trials concluded that the effect of topical tacrolimus was significantly more potent than placebo and another trial showed that tacrolimus had statistically significant benefit over calcipotriol. Open trials have suggested specific subtypes of psoriasis for which topical tacrolimus could provide adequate disease control without the risk of developing skin agenesis, telangiectasia, and striae, which can be associated with long-term use of topical steroids. The key areas of interest were facial, genital, and intertriginous psoriasis; these are body sites that are particularly susceptible to the deleterious effects of corticosteroids. There have been mixed reports of the efficacy of topical tacrolimus for treatment of classically distributed chronic plaque psoriasis due to inadequate skin penetrance through the plaques. There may be a role for topical tacrolimus for these patients when used in conjunction with a skin penetration enhancer or with application under occlusion. Furthermore, studies of topical tacrolimus used in combination with other therapeutics for the management of psoriasis have demonstrated enhanced efficacy.33,47–50
Oral tacrolimus has demonstrated efficacy as a short-term treatment for recalcitrant psoriasis. There have been two trials and two case reports documenting the effect and safety profile of oral tacrolimus in psoriasis management. Treatment of psoriasis with oral ciclosporin is associated with a less favorable side effect profile than treatment with oral tacrolimus. However, ciclosporin is frequently used in the management of psoriasis. Further investigation is required to identify a suitable dose regimen for oral tacrolimus for effective treatment of psoriasis while mitigating the potential for adverse treatment effects.
Conclusion
Topical and oral administration of tacrolimus has demonstrated considerable efficacy in the treatment of psoriasis. With regard to topical tacrolimus therapy, particular utility has been observed in cases of inverse psoriasis and psoriasis affecting the face, genitalia, and nails. Topical tacrolimus appears to have a role as a corticosteroid-sparing agent and may be associated with fewer side effects than other topical preparations. Efficacy and safety have been evaluated in a number of promising case reports and studies. However, further investigation to compare the efficacy with placebo and with standard therapy would be useful to identify the most appropriate role of tacrolimus in patient care.
Oral tacrolimus therapy has also produced successful outcomes in patients with severe recalcitrant plaque psoriasis. The side effect profile of systemic tacrolimus is more favorable than ciclosporin, and it may be more suitable for patients with increased cardiovascular and metabolic comorbidities; however, larger-scale randomized control trials must be conducted before oral administration of tacrolimus can become a standard component of the treatment armamentarium for psoriasis.
Acknowledgment
Helen Young orcid.org/0000-0003-1538-445X.
Disclosure
Helen Young has received grant support from Biogen Idec, Galderma, LEO Pharma, Novartis, Schering-plough, Stiefel, and Wyeth/Pfizer. She has acted as a consultant for Teva Pharmaceuticals and on advisory boards for Abbott/Abbvie, Amgen, Eli Lilly, Janssen-Cilag, and LEO Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. | ||
Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol. 2012;51(4):389–395; quiz 95–98. | ||
Hebert HL, Ali FR, Bowes J, Griffiths CE, Barton A, Warren RB. Genetic susceptibility to psoriasis and psoriatic arthritis: implications for therapy. Br J Dermatol. 2012;166(3):474–482. | ||
Barker JN. The pathophysiology of psoriasis. Lancet. 1991;338(8761):227–230. | ||
Christophers E. Psoriasis – epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. | ||
Laws PM, Young HS. Topical treatment of psoriasis. Expert Opin Pharmacother. 2010;11(12):1999–2009. | ||
Albrecht L, Bourcier M, Ashkenas J, et al. Topical psoriasis therapy in the age of biologics: evidence-based treatment recommendations. J Cutan Med Surg. 2011;15(6):309–321. | ||
Lemster BH, Carroll PB, Rilo HR, Johnson N, Nikaein A, Thomson AW. IL-8/IL-8 receptor expression in psoriasis and the response to systemic tacrolimus (FK506) therapy. Clin Exp Immunol. 1995;99(2):148–154. | ||
Zonneveld IM, Rubins A, Jablonska S, et al. Topical tacrolimus is not effective in chronic plaque psoriasis. A pilot study. Arch Dermatol. 1998;134(9):1101–1102. | ||
Carroll CL, Clarke J, Camacho F, Balkrishnan R, Feldman SR. Topical tacrolimus ointment combined with 6% salicylic acid gel for plaque psoriasis treatment. Arch Dermatol. 2005;141(1):43–46. | ||
Ortonne JP, van de Kerkhof PC, Prinz JC, et al. 0.3% Tacrolimus gel and 0.5% Tacrolimus cream show efficacy in mild to moderate plaque psoriasis: results of a randomized, open-label, observer-blinded study. Acta Derm Venereol. 2006;86(1):29–33. | ||
Maloney JM, Flores J, Sheehan M, Schlessinger J. Efficacy and safety of 0.1% and 0.5% tacrolimus cream versus vehicle for treatment of mild to moderate plaque psoriasis in adults. J Am Acad Dermatol. 2007;56(2, Suppl 2):AB10. | ||
Vissers WH, van Vlijmen I, van Erp PE, de Jong EM, van de Kerkhof PC. Topical treatment of mild to moderate plaque psoriasis with 0.3% tacrolimus gel and 0.5% tacrolimus cream: the effect on SUM score, epidermal proliferation, keratinization, T-cell subsets and HLA-DR expression. Br J Dermatol. 2008;158(4):705–712. | ||
Omland SH, Gniadecki R. Psoriasis inversa: a separate identity or a variant of psoriasis vulgaris? Clin Dermatol. 2015;33(4):456–461. | ||
Remitz A, Reitamo S, Erkko P, Granlund H, Lauerma AI. Tacrolimus ointment improves psoriasis in a microplaque assay. Br J Dermatol. 1999;141(1):103–107. | ||
Yamamoto T, Nishioka K. Topical tacrolimus is effective for facial lesions of psoriasis. Acta Derm Venereol. 2000;80(6):451. | ||
Yamamoto T, Nishioka K. Topical tacrolimus: an effective therapy for facial psoriasis. Eur J Dermatol. 2003;13(5):471–473. | ||
Clayton TH, Harrison PV, Nicholls R, Delap M. Topical tacrolimus for facial psoriasis. Br J Dermatol. 2003;149(2):419–420. | ||
Kroft EB, Erceg A, Maimets K, Vissers W, van der Valk PG, van de Kerkhof PC. Tacrolimus ointment for the treatment of severe facial plaque psoriasis. J Eur Acad Dermatol Venereol. 2005;19(2):249–251. | ||
Rallis E, Nasiopoulou A, Kouskoukis C, et al. Successful treatment of genital and facial psoriasis with tacrolimus ointment 0.1%. Drugs Exp Clin Res. 2005;31(4):141–145. | ||
Lebwohl M, Freeman AK, Chapman MS, Feldman SR, Hartle JE, Henning A. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51(5):723–730. | ||
Freeman AK, Linowski GJ, Brady C, et al. Tacrolimus ointment for the treatment of psoriasis on the face and intertriginous areas. J Am Acad Dermatol. 2003;48(4):564–568. | ||
Liao YH, Chiu HC, Tseng YS, Tsai TF. Comparison of cutaneous tolerance and efficacy of calcitriol 3 microg g(-1) ointment and tacrolimus 0.3 mg g(-1) ointment in chronic plaque psoriasis involving facial or genitofemoral areas: a double-blind, randomized controlled trial. Br J Dermatol. 2007;157(5):1005–1012. | ||
Martin Ezquerra G, Sanchez Regana M, Herrera Acosta E, Umbert Millet P. Topical tacrolimus for the treatment of psoriasis on the face, genitalia, intertriginous areas and corporal plaques. J Drugs Dermatol. 2006;5(4):334–336. | ||
Bissonnette R, Nigen S, Bolduc C. Efficacy and tolerability of topical tacrolimus ointment for the treatment of male genital psoriasis. J Cutan Med Surg. 2008;12(5):230–234. | ||
Steele JA, Choi C, Kwong PC. Topical tacrolimus in the treatment of inverse psoriasis in children. J Am Acad Dermatol. 2005;53(4):713–716. | ||
Brune A, Miller DW, Lin P, Cotrim-Russi D, Paller AS. Tacrolimus ointment is effective for psoriasis on the face and intertriginous areas in pediatric patients. Pediatric Dermatol. 2007;24(1):76–80. | ||
De Simone C, Maiorino A, Tassone F, D’Agostino M, Caldarola G. Tacrolimus 0.1% ointment in nail psoriasis: a randomized controlled open-label study. J Eur Acad Dermatol Venereol. 2013;27(8):1003–1006. | ||
Nagao K, Ishiko A, Yokoyama T, Tanikawa A, Amagai M. A case of generalized pustular psoriasis treated with topical tacrolimus. Arch Dermatol. 2003;139(9):1219. | ||
Rodriguez Garcia F, Fagundo Gonzalez E, Cabrera-Paz R, et al. Generalized pustular psoriasis successfully treated with topical tacrolimus. Br J Dermatol. 2005;152(3):587–588. | ||
Laino L, DiCarlo A. Palmoplantar pustular psoriasis: clinical and video thermographic evaluation before and after topical tacrolimus treatment. Arch Dermatol. 2011;147(6):760. | ||
Yamamoto T, Nishioka K. Successful treatment with topical tacrolimus for oral psoriasis. J Eur Acad Dermatol Venereol. 2006;20(9):1137–1138. | ||
Sehgal VN, Sehgal S, Verma P, Singh N, Rasool F. Exclusive plaque psoriasis of the lips: efficacy of combination therapy of topical tacrolimus, calcipotriol, and betamethasone dipropionate. Skinmed. 2012;10(3):183–184. | ||
Carrascosa JM, Soria X, Domingo H, Ferrandiz C. Treatment of inverse psoriasis with excimer therapy and tacrolimus ointment. Dermatol Surg. 2007;33(3):361–363. | ||
Nikolaidis NL, Abu-Elmagd K, Thomson AW, et al. Metabolic effects of FK 506 in patients with severe psoriasis: short-term follow-up of seven cases. Transpl Proc. 1991;23(6):3325–3327. | ||
Jegasothy BV, Ackerman CD, Todo S, Fung JJ, Abu-Elmagd K, Starzl TE. Tacrolimus (FK 506) – a new therapeutic agent for severe recalcitrant psoriasis. Arch Dermatol. 1992;128(6):781–785. | ||
Group TEFMPS. Systemic tacrolimus (FK 506) is effective for the treatment of psoriasis in a double-blind, placebo-controlled study. Arch Dermatol. 1996;132(4):419–423. | ||
Wei KC, Lai PC. Combination of everolimus and tacrolimus: a potentially effective regimen for recalcitrant psoriasis. Dermatol Ther. 2015;28(1):25–27. | ||
Mittal A, Dogra S, Narang T, Sharma A. Pilot study to evaluate the efficacy and safety of oral tacrolimus in adult patients with refractory severe plaque psoriasis. J Cutan Med Surg. 2016;20(3):228–232. | ||
Kaltwasser JP, Nash P, Gladman D, et al. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2004;50(6):1939–1950. | ||
Yocum DE, Furst DE, Kaine JL, et al. Efficacy and safety of tacrolimus in patients with rheumatoid arthritis: a double-blind trial. Arthritis Rheum. 2003;48(12):3328–3337. | ||
Morton SJ, Powell RJ. Cyclosporin and tacrolimus: their use in a routine clinical setting for scleroderma. Rheumatology. 2000;39(8):865–869. | ||
Yoon KH. Successful usage of tacrolimus (FK506) in resistant/relapsed rheumatic diseases. APLAR J Rheumatol. 2004;7(1):44–48. | ||
Lythgoe M, Abraham S. Tacrolimus: an effective treatment in refractory psoriatic arthritis following biologic failure. Clin Exp Rheumatol. 2016;34(1 Suppl 95):S12–S13. | ||
Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 2013;191(12):5785–5791. | ||
Shah SS, Hafeez F. Comparison of efficacy of tacrolimus versus cyclosporine in childhood steroid-resistant nephrotic syndrome. J Coll Physicians Surg Pak. 2016;26(7):589–593. | ||
Weidemann AK, Crawshaw AA, Byrne E, Young HS. Vascular endothelial growth factor inhibitors: investigational therapies for the treatment of psoriasis. Clin Cosmet Investig Dermatol. 2013;6:233–244. | ||
Tirado-Sanchez A, Ponce-Olivera RM. Preliminary study of the efficacy and tolerability of combination therapy with calcipotriene ointment 0.005% and tacrolimus ointment 0.1% in the treatment of stable plaque psoriasis. Cutis. 2012;90(3):140–144. | ||
Buder K, Knuschke P, Wozel G. Evaluation of methylprednisolone aceponate, tacrolimus and combination thereof in the psoriasis plaque test using sum score, 20-MHz-ultrasonography and optical coherence tomography. Int J Clin Pharmacol Ther. 2010;48(12):814–820. | ||
Rivard J, Janiga J, Lim HW. Tacrolimus ointment 0.1% alone and in combination with medium-dose UVA1 in the treatment of palmar or plantar psoriasis. J Drugs Dermatol. 2006;5(6):505–510. | ||
Kleyn CE, Woodcock D, Sharpe GR. The efficacy of 0.1% tacrolimus ointment compared with clobetasone butyrate 0.05% ointment in patients with facial, flexural or genital psoriasis. Br J Dermatol. 2005;153:33. | ||
Rajzer L, Wojas-Pelc A, Obtulowicz A. The efficacy and safety of an ointment containing 0.1% tacrolimus in facial and flexural psoriasis. J Eur Acad Dermatol. 2005;19:178. | ||
Nelson A, Krejci-Manwaring J, Fleischer A, et al. A randomized, double-blind, vehicle-controlled, right/left comparative study of the efficacy of actitretin with and without the co-administration of 0.1% tacrolimus topical ointment in the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2007;56:AB181. | ||
He Y. Clinical efficacy of 0.1% tacrolimus ointment on plaque psoriasis of scalp and face. J Clin Dermatol. 2008;37:254–255. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
