Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
T-Cell Lymphoblastic Lymphoma with Cutaneous Involvement in a Child: A Rare Case Report
Authors Chen J, Tian X , Yu N, Peng L, Zhu H
Received 28 May 2022
Accepted for publication 13 August 2022
Published 23 September 2022 Volume 2022:15 Pages 2027—2033
DOI https://doi.org/10.2147/CCID.S376523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jiaoquan Chen,* Xin Tian,* Nanji Yu, Liqian Peng, Huilan Zhu
Department of Dermatology, Guangzhou Institute of Dermatology, Guangzhou, 510095, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huilan Zhu, Department of Dermatology Guangzhou Institute of Dermatology, 56 Hengfu Road, Guangzhou, 510095, People’s Republic of China, Email [email protected]
Abstract: T-cell lymphoblastic lymphoma (T-LBL) is a heterogeneous malignancy derived from T-cells that more commonly affects teens and males. Most commonly, T-LBL exhibits signs of lymph nodes, bone marrow, and mediastinal mass invasion, but in rare cases, the disease manifests cutaneously. We present a case of both cutaneous and systemic presentation of T-LBL in 9-year-old man in which the skin immunophenotype analysis showed TdT expression with positivity of CD3, CD4 and CD99. Review of all currently described cases of cutaneous T-LBL revealed that the most frequently positive tumor markers were TdT (100%), CD3 (100%), CD4 (59.1%) and CD99 (40.9%). Cutaneous involvement may be a prognostic factor in treating T-LBL with chemotherapy.
Keywords: T-cell lymphoblastic lymphoma, cutaneous lymphoma, diagnosis
Introduction
T-cell lymphoblastic lymphoma (T-LBL), the second most common subtype of non-Hodgkin lymphoma, usually presents as a mediastinal mass or lymphadenopathy in young men in their teens to early twenties1,2. Cutaneous involvement by T-LBL is a rare occurrence with a reported frequency of approximately 4.3%.3 Although cases of skin involvement are rare, it is often the first emerging manifestation of the disease and leads to misdiagnosis. We present a case in which cutaneous involvement was the first indication of T-LBL, together with a review of all relevant cases.
Case Report
A 9-year-old boy presented at our outpatient department with a 1-year history of recurrent skin lesions. One year prior, the boy developed skin lesions mainly on his scalp, face, and the distal limbs without obvious causes (Figure 1A and B). The skin lesions showed a slowly progressive relapsing course, changing from erythema, to papules, to vesicles, to necrosis and finally healing with atrophic scars and depigmentation. The child had previously been diagnosed with eczema or atopic dermatitis, with no significant improvement after antihistamine treatment or topical corticosteroids. The patient complained fever (37.8–38.4°C) and chest pain a month later. Laboratory results showed that he had a normal blood count and white blood cell differentiation, normal C-reactive protein and lactate dehydrogenase, and normal kidney and liver function tests. A skin biopsy of the left foot skin lesion was performed and histopathologic findings revealed dense lymphoid cell infiltration throughout the dermis and surrounding perivascular area, focal necrosis, and the presence of atypical lymphocytes (Figure 2A and B). Immunohistochemistry stains were positive for CD2, CD3, CD4, CD8, GrB, TIA-1, and TdT, but negative for, CD20, CD30, CD56 and CD79a (Figure 2C–E). Ki-67 was approximate 60% positive. EBV-encoded RNA were detected in skin biopsy specimens by in situ hybridization. A biopsy of the mass was performed and revealed that the tumor composed of malignant polymorphic lymphoid cells with massive necrosis (Figure 2F and G). Immunohistochemistry stains showed that the tumor cells were positive for CD2, CD3, CD4, CD8, CD10, CD99, CD1a, TdT and Bcl-2 protein (Figure 2H–J). The proliferative rate by Ki-67 was high at 90%. A CT scan revealed a large mediastinal mass with a maximum cross section of about 50 mm×37 mm (Figure 3A). The bone marrow biopsy performed was negative. The diagnosis of T-LBL with cutaneous involvement was made. The patient received 6 courses of modified Non-Hodgkin lymphoma-Berlin-Frankfurt-Munster 90 (NHL-BFM 90) protocol, including vinosic/dexamethasone/peloperoxidase/nooxorubicin (VDLD), 3 courses of cyclophosphamide/cytitidine/6-mercaptopurine (CAM), high dose methotrexate (HD-MTX) and Pirarubicin/vincristine (VCR+THP) in 9 months. A CT scan revealed the maximum cross section of the mediastinal mass was 26 mm × 21 mm, which was significantly decreased than before after the chemotherapy (Figure 3B). However, his skin lesions showed no obvious improvement after the treatment. Then the patient started treatment consisting of methotrexate (17.5 mg once a week) and 6-mercaptopurine (50 mg every night). The patient was in continued remission at 6 months of follow-up (Figure 1C and D).
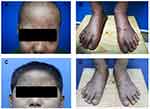 |
Figure 1 (A–D) papules, necrosis, atrophic scars and depigmentation on the scalp, face, hands, feet were significantly improved after the treatment. |
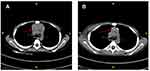 |
Figure 3 CT examination. Mediastinal mass was significantly decreased than before (A) after the chemotherapy (B) (red arrow). |
Discussion
To our knowledge, less than 7% of all cutaneous malignant lymphomas are of lymphoblastic type4,5. Though the lymphoblastic presentation is more often of T-cell lineage than B cell, cutaneous lesions have been less frequently described with a frequency of around 4.3% patients with T-LBL as compared to B cell lymphoblastic lymphoma (B-LBL) (16–33%). 4–6 As a result of a literature review, we identified total 22 cases (including our patient) of cutaneous T-LBL that either presented as a primary complaint or an associated systemic complaint3,6–17 (Table 1). The ratio of male to female was 17:5, and the patients aged from 5 to 75, only 3 patients with cutaneous T-LBL were younger than 10 years. Fourteen cases (73.6%) of cutaneous T-LBL involved nodules at the time of presentation, while the other main manifestations were papule, purpura, ecchymosis, macule, and plaque. These lesions were most commonly found on the scalp, face, and trunk, with only 7 cases (31.8%) affecting the extremities. Systemic in addition to cutaneous involvement of T-LBL was found in 95.5% (21/22) of included cases. It was reported that classical T-LBL patients usually exhibit signs of bone marrow invasion.2 However, the most common sites of involvement in the included cases were the lymph nodes (68.2%), bone marrow (59.1%), and mediastinal mass (45.5%). We suspect when T-LBL patients involve skin lesions, it indicates that other systemic diseases may already be present.
 |
Table 1 T-Cell Lymphoblastic Lymphomas with Cutaneous Involvement Reported in the Literature, Including Our Single Case |
In regards to cutaneous B-LBL, 76.3% of patients present with nodules, frequently involving the scalp and forehead.18 Thus, on cutaneous presentation, B-LBL and T-LBL are indistinguishable, and immunophenotyping is necessary for differentiation. The immunophenotype is heterogeneous, but T-LBLs express cytoplasmic or membrane-bound CD3, which is T lineage-specific. TdT is a DNA polymerase expressed in immature cells, and the marker is expressed only in LBLs, with a 95% positive rate in T-LBL.19 Consistent with the expected results of classical T-LBL, TdT and CD3 were positive expression in the histological findings of all cutaneous T-LBL cases reported in the included literature. In addition, 13/22 (59.1%) tested positive for CD4, 9/20 patients (40.9%) tested positive for CD99, 8/22 (36.4%) tested positive for CD10, and 7/22 (31.8%) tested positive for CD5. Based on these data, it is likely that most cutaneous T-LBLs are TdT+, CD3+, CD4+ and CD99+, which differ from previous reports11,15 but true in the case described herein.
Treatment of LBL with regimens typically used for nonHodgkin lymphoma is associated with poor outcomes with 58% complete remission rate and a 5-year disease free survival rate of 36%.5 The prognosis of T-LBL has improved considerably with the application of acute lymphoid leukemia (ALL)-like regimens, such as childhood chemotherapy regimens (BFM 90) and adult chemotherapy protocols (fractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone, hyper-CVAD).20,21 With ALL-type treatment regimens, the outcome of pediatric T-LBL patients has improved, with event-free survival rates of 75–90%.21 A study of the hyper-CVAD regimen in LBL reported by Thomas et al22 showed that among their patients, 30 (91%) achieved complete response, and 3 (9%) achieved partial remission. However, Zhu20 demonstrated that the BFM 90 improved the overall survival rate more than the adult regimen in patients aged <40 years. Yu et al2 found that a total of 145 T-LBL patients who underwent first-line dose-adjusted BFM-90 received 3-year overall survival and progression-free survival rates for overall were 66.8% and 58.4%, respectively. The German BFM group, who studied 105 children with T-LBL given ALL-type regimens, estimated the 5-year event-free survival rate to be 90%.23 In our case, the mediastinal tumors in the child were significantly decreased, and his conditions improved temporarily after receiving the modified NHL-BFM 90 protocol. Of the 18 cases of cutaneous T-LBL receiving chemotherapy described in the literature, 8 (44.4%) patients achieved complete remission. Of the 6 patients who died, time from diagnosis to death was an average of 14.6 months. Cutaneous T-LBL patients appear to have a worse prognosis treated with chemotherapy than those presenting with conventional T-LBL. Thus, we speculate that skin involvement may be related to mortality in T-LBL patients, and may be a poor prognostic factor.
In conclusion, we present a worthy case of cutaneous involvement of T-LBL and reviewed the current literature, highlighting the importance of an accurate diagnosis and aggressive treatment. Histopathological examination of a skin biopsy with immunohistochemical study should be established on any cases of suspected T-LBL include TdT, CD3, CD4 and CD99. More than 90% in the literature had systemic spread of the disease, suggesting that a comprehensive systemic examination is mandatory for patients with cutaneous T-LBL.Cutaneous involvement may be a poor prognostic factor in treating T-LBL with chemotherapy.
Ethics Statement
A parent of the patient gave written informed consent for publication of clinical information and photographs. No ethical committee approval was required because the data were analyzed in a retrospective manner.
Funding
This work was supported by the Health Science and technology project of Guangzhou(20191A011070) and the Natural Science Foundation of Guangdong Province (2019A1515011593).
Disclosure
The authors declare no conflict of interest in this work.
References
1. You MJ, Medeiros LJ, Hsi ED. T-lymphoblastic leukemia/lymphoma. Am J Clin Pathol. 2015;144:411–422. doi:10.1309/AJCPMF03LVSBLHPJ
2. Yu H, Mi L, Qi F, et al. Survival and prognostic analysis of T-cell lymphoblastic lymphoma patients treated with dose-adjusted BFM-90 regimen. Aging. 2022;14:3203–3215.
3. Khurana S, Beltran M, Jiang L, Ayala E, Roy V. Primary cutaneous T-Cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:1–6. doi:10.1155/2019/3540487
4. Favaro C, Bomtempo A, Dos SB, Tucunduva L, Cestari S, Filho RT. T-immunophenotype lymphoblastic lymphoma with secondary cutaneous involvement associated with rapid regression followed up with positron emission tomography. JAAD Case Rep. 2020;6:374–377. doi:10.1016/j.jdcr.2020.01.024
5. Cortelazzo S, Ponzoni M, Ferreri AJ, Hoelzer D. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2011;79:330–343. doi:10.1016/j.critrevonc.2010.12.003
6. Sander CA, Medeiros LJ, Abruzzo LV, Horak ID, Jaffe ES. Lymphoblastic lymphoma presenting in cutaneous sites. A clinicopathologic analysis of six cases. J Am Acad Dermatol. 1991;25:1023–1031.
7. Vezzoli P, Novara F, Fanoni D, et al. Three cases of primary cutaneous lymphoblastic lymphoma: microarray-based comparative genomic hybridization and gene expression profiling studies with review of literature. Leuk Lymphoma. 2012;53:1978–1987. doi:10.3109/10428194.2011.618233
8. Yaar R, Rothman K, Mahalingam M. When dead cells tell tales-cutaneous involvement by precursor T-cell acute lymphoblastic lymphoma with an uncommon phenotype. Am J Dermatopathol. 2010;32:183–186. doi:10.1097/DAD.0b013e3181b3aa1c
9. Prasad PG, Cyriac S, Sagar TG, Rathnam K. T lymphoblastic lymphoma relapsing in skin — a rare clinical scenario. Indian J Hematol Blo. 2010;26:19–20. doi:10.1007/s12288-010-0007-5
10. Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379–385. doi:10.1111/j.1600-0560.1999.tb01861.x
11. Nascimbeni C, Chantepie S, Brugiere C, Comoz F, Salaun V, Verneuil L. Localisations cutanées d’un lymphome lymphoblastique T. Ann Dermatol Venereol. 2017;144:268–274. doi:10.1016/j.annder.2017.01.001
12. Ginoux E, Julia F, Balme B, Thomas L, Dalle S. T-lymphoblastic lymphoma with cutaneous involvement. World J Clin Cases. 2015;3:727–731. doi:10.12998/wjcc.v3.i8.727
13. Zanelli M, Zizzo M, Martino G, Sanguedolce F, Ascani S. Erythematous cutaneous macules: an uncommon, challenging presentation of T-lymphoblastic lymphoma. Eur J Dermatol. 2020;30:318–320. doi:10.1684/ejd.2020.3772
14. Mansoori P, Taheri A, O’Neill SS, Sangueza OP. T-Lymphoblastic Leukemia/Lymphoma with annular skin rash and epidermotropism. Am J Dermatopathol. 2018;40:676–678. doi:10.1097/DAD.0000000000001113
15. Montes-Torres A, Llamas-Velasco M, Capusan TM, Aguado B, Adrados M. Cutaneous involvement as the first manifestation of T-lymphoblastic lymphoma and review of the literature. J Cutan Pathol. 2019;46:372–375. doi:10.1111/cup.13431
16. Chiba Y, Hirase N, Yamasaki K, Yatera K. Mediastinal t-cell lymphoblastic lymphoma diagnosed with a skin biopsy. Intern Med. 2020;59:1463–1464. doi:10.2169/internalmedicine.4390-20
17. Lee WJ, Moon HR, Won CH, et al. Precursor B- or T-lymphoblastic lymphoma presenting with cutaneous involvement: a series of 13 cases including 7 cases of cutaneous T-lymphoblastic lymphoma. J Am Acad Dermatol. 2014;70:318–325. doi:10.1016/j.jaad.2013.10.020
18. Amarasekera D, Connolly D, Gochoco A, et al. Cutaneous B-Cell lymphoblastic lymphoma. Am J Dermatopathol. 2019;41:596–601. doi:10.1097/DAD.0000000000001347
19. Terada T. TDT (-), KIT (+), CD34 (+), CD99 (+) precursor T lymphoblastic leukemia/lymphoma. Int J Clin Exp Pathol. 2012;5:167–170.
20. Zhu MY, Wang H, Huang CY, et al. A childhood chemotherapy protocol improves overall survival among adults with T-lymphoblastic lymphoma. Oncotarget. 2016;7:38884–38891. doi:10.18632/oncotarget.9144
21. Burkhardt B, Mueller S, Khanam T, Perkins SL. Current status and future directions of T-lymphoblastic lymphoma in children and adolescents. Br J Haematol. 2016;173:545–559. doi:10.1111/bjh.14017
22. Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–1630. doi:10.1182/blood-2003-12-4428
23. Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood. 2000;95:416–421.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

