Back to Journals » International Journal of General Medicine » Volume 14
Systemic Immune-Inflammation Index (SII) Can Be an Early Indicator for Predicting the Severity of Acute Pancreatitis: A Retrospective Study
Authors Liu X, Guan G, Cui X, Liu Y, Liu Y, Luo F
Received 7 October 2021
Accepted for publication 26 November 2021
Published 8 December 2021 Volume 2021:14 Pages 9483—9489
DOI https://doi.org/10.2147/IJGM.S343110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Xingming Liu, Guoxin Guan, Xinye Cui, Yaqing Liu, Yinghan Liu, Fuwen Luo
Department of General Surgery, The Second Affiliated Hospital, Dalian Medical University, Dalian, 116023, People’s Republic of China
Correspondence: Fuwen Luo Email [email protected]
Objective: Systemic immune-inflammation index (SII) is a new systemic inflammatory prognostic indicator associated with outcomes in patients with different tumors. Studies have shown an association between SII and many chronic/acute inflammatory diseases. This study aimed at exploring whether SII can be used as an effective parameter for predicting the severity of acute pancreatitis (AP).
Methods: A total of 101 acute pancreatitis patients were enrolled in this study (mild acute pancreatitis (MAP): n = 73 and severe acute pancreatitis (SAP): n = 28). Patient demographics and SII were analyzed using the chi-square test, Student’s t-test, and Mann–Whitney U-test. A receiver operating characteristic curve was generated to test the potential of using neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and SII to predict AP’s severity. Logistic regression analysis was performed to determine major risk factors.
Results: Patients with SII value ≥ 2207.53 had a higher probability of having SAP (sensitivity = 92.9%, specificity = 87.7%, and AUC = 0.920), and SII was a significantly better predictive value than PLR and NLR. Logistic regression analysis results showed SII could differentiate MAP from SAP as a major risk factor.
Conclusion: This study has shown that SII is a potential indicator for predicting the severity of acute pancreatitis. The findings suggested that SII is more sensitive and specific than NLR and PLR in predicting the severity of acute pancreatitis.
Keywords: acute pancreatitis, severity, systemic immune inflammation index
Key Messages
Systemic immune-inflammation index (SII) is a new systemic inflammatory prognostic indicator. SII has been used as an indicator for predicting and assessing neurological disease, inflammatory disease, and carcinomas. In this research, SII was proved to be an index for evaluating the severity of acute pancreatitis, and its sensitivity, specificity, and predictive values were better than those of PLR and NLR.
Introduction
Acute pancreatitis (AP) is an inflammatory disease that activates a cascade-like response of inflammatory factors, thereby inducing Systemic inflammatory response syndrome (SIRS).1,2 Evidence suggests that severe acute pancreatitis (SAP), characterized by multiple organ failure, has a high mortality rate compared to mild acute pancreatitis (MAP).3,4 Therefore, increased attention has been subjected to SAP research, with molecular studies focusing on the activation of cytokine, macrophage-mediated inflammatory response, and neutrophil infiltration.5–7 Clinical indicators such as Ranson criteria, Glasgow Coma Scale (GCS), bedside index for severity in acute pancreatitis (BISAP) score, computed tomography severity index (CTSI) score, and Balthazar score also play an essential role in the diagnosis, treatment, and prognosis of AP.8–10 However, there is a need to identify early and easy diagnosis indicators for distinguishing SAP from MAP with the overarching goal of reducing mortality.
According to the Atlanta classification system, acute pancreatitis is divided into mild, moderate, and severe based on the severity.11 SAP has more systemic complications than MAP, such as persistent multiple organ failure (respiratory, renal, and liver) and local complications, which are observed through imageological examination.12,13 The use of traditional pancreatitis severity scores, including Acute Physiology and Chronic Health Evaluation (APACHE), Sepsis-related Organ Failure Assessment (SOFA), Ranson criteria, GCS, BISAP, and CTSI (which mainly evaluate the systemic condition and local pancreatic condition), is limited by time costs and manipulation complexity.14 Therefore, this calls for identifications of fast, effective, and sensitive biomarkers for predicting AP severity.
Several previous studies have revealed that peripheral blood cells (neutrophils, lymphocytes, and platelets) are associated with malignant tumors and inflammatory disease.15–17 For example, Fox18 showed a relationship between increased neutrophil levels and platelet counts with a poor prognosis of advanced renal cell carcinoma patients. Moreover, the systemic inflammation scores, calculated by a formula using the counts of inflammatory cells, such as neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR), were associated with outcomes in many inflammatory diseases.19 However, although these scores are very convenient, quick, and effective, more relevant studies should be conducted to confirm this conclusion.
Red blood cell distribution width (RDW), which represents the variability in the size of circulating erythrocytes, has been widely used in the clinical field.20,21 Previous studies have shown that RWD is a parameter associated with the activity, predictive, and risk factor of many diseases, including acute kidney injury,22 coronary heart disease,21 and acute respiratory failure.23
There is an urgent need to identify easy-to-assess biomarkers to differentiate the severity of acute pancreatitis. Systemic immune-inflammation index (SII), combined with neutrophils, lymphocytes, and platelets, was first used in 2014 by Hu24 to evaluate the prognosis of hepatocellular carcinoma (HCC). In recent years, SII has been used as an indicator for predicting and assessing neurology disease, inflammatory disease, and carcinomas.25–27 This study aimed at exploring whether SII is an effective biomarker for predicting the severity of acute pancreatitis.
Methods
We retrospectively analyzed 101 out of the 106 adult acute pancreatitis patients hospitalized at the Second Affiliated Hospital of Dalian Medical University. The patients were continuously followed up from January 2020 to August 2021 at the acute abdomen, intensive care, and gastrointestinal surgery department. Patients who had chronic renal function failure (2 cases), malignant tumors (2 cases), and acute pancreatitis in pregnancy (1 case) were excluded from the study. Patients were then divided into two groups: MAP (n = 73, 65.7%) and SAP (including moderate-severe pancreatitis and severe acute pancreatitis) (n = 28) according to the Atlanta classification of acute pancreatitis. Demographic and clinical characteristics were retrieved from patient files.
Acute pancreatitis was diagnosed using the clinical, laboratory, and radiological examination results, including epigastric pain, high amylase-lipase levels (at least three times greater than the upper limit of normal), and pancreatic inflammation checked from the computer-tomography scan. The BISAP 0 h score was calculated by fitting the patient files. CTSI for pancreatitis was evaluated from a CT scan taken on admission or in the emergency room. With regard to laboratory measurements, blood was collected and used for routine blood tests and biochemical tests within 72 hours post the clinical onset of AP.
Statistical Analysis
The statistical analyses were performed using IBM SPSS version 25.0 (released by IBM Corp. in 2017). Normally distributed values were expressed as mean ± SD, whereas categorical variables were expressed as percentages. On the other hand, non-normally distributed (conclude between-group variance) data were expressed as median (interquartile range (IQR)). Independent sample t-test analysis was performed in instances where two groups had normal distribution, whereas the Mann–Whitney U-test was used to compare two groups with non-normal distribution or uneven variance data. In addition, Pearson chi-square and Fisher’s exact test were used for categorical cross-tab analysis. Receiver operating characteristic (ROC) analysis was performed to determine the appropriate cut-off point for independent indicators and calculate sensitivity and specificity values. Notably, the cut-off point was calculated based on Youden Index. Logistic regression was used to calculate the main factors of the independent variables at 95% confidence intervals (CI). Positive LR was calculated as sensitivity/(1 − specificity), whereas negative LR was calculated as (1 − sensitivity)/specificity. P-value < 0.05 was considered statistically significant.
Results
A total of 101 patients were enrolled in this study. Table 1 shows the baseline demographic results and clinical characteristics of patients in the study group. The data showed that 63% of patients in the MAP group were male (52/73), with a median age of 52 (38~66.5) years old. In the SAP group, only 39.3% of the patients were male (11/28), and the median age was 55.5 (39.25~66) years old.
 |
Table 1 Demographical Characteristics and Clinical Data of the Patients |
Inpatient days and the possibility of ICU admission were significantly higher in the SAP group compared to the MAP group (P < 0.001). For laboratory markers, WBC, neutrophil, lymphocyte, PLR, NLR, SII, BISAP 0 h score, and CTSI score increased significantly (P < 0.001) in the SAP group compared to the MAP group, whereas LMR decreased significantly (P < 0.001). Moreover, higher AST (P = 0.02), ALP (P = 0.041), and platelet count (P = 0.023) were observed in the SAP group. With regard to other results, including etiology of AP, urea, creatinine, ALT, TB, GGT, amylase, lipase, monocyte, and RDWSD, there was no significant difference between the two groups (P > 0.05).
ROC curves were generated to compare the predictive values of SII, NLR, PLR, and LMR for the severity of acute pancreatitis. Table 2 shows the sensitivity, specificity, AUC, and the best cut-off points. Results showed that the best cut-off points of NLR were 9.68 (sensitivity = 82.1% and specificity = 82.2%), and PLR was 195.08 (sensitivity = 82.1% and specificity = 84.9%). Patients with SII value ≥ 2207.53 had a higher probability of having SAP with a sensitivity of 92.9%. Although these three values were good in predicting the severity of AP, results indicated that the predictive value of SII was better than that of NLR and PLR (AUC = 0.920 vs 0.811 and 0.877) (Figure 1).
 |
Table 2 Diagnostic Performances of SII, PLR, and NLR for Distinguishing Between MAP & SAP |
 |
Figure 1 Receiver operating characteristic (ROC) curve analysis for assessing the performance of the SII, PLR, and NLR in determining the severity of acute pancreatitis. |
Furthermore, multivariate logistic regression analysis showed that RDW-SD, Age, WBC, Platelet, Neutrophil, Monocyte, and Gender (male) had no ability to distinguish MAP and SAP. SII and Bisap 0h score were identified as the risk factors to differentiate MAP from SAP (SII: OR = 1.001, 95% CI 1.000–1.002, p = 0.037; Bisap 0h score: OR = 2.774, 95% CI 1.003–7.448, p = 0.043) (Table 3).
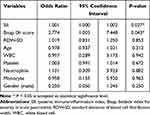 |
Table 3 Multivariate Logistic Regression for Severe Acute Pancreatitis |
Discussion
This study evaluated the predictive value of SII and other indicators for the severity of acute pancreatitis. SII is a newly defined, simple, inexpensive index that reflects the balance between the inflammatory and immune responses. The expression of SII consists of neutrophils, lymphocytes, and platelets, which play a role in the pathogenesis of acute pancreatitis. Our results showed that SII could be used as an index to assess the severity of pancreatitis. Its sensitivity, specificity, and predictive values were better than those of PLR and NLR. To the best of our knowledge, this is the first study that has shown the predictive value of SII for diagnosing the severity of pancreatitis.
Nearly one hundred years ago, Moynihan28 revealed that acute pancreatitis is the most devastating disease among all the conditions associated with abdominal organs and is characterized by a sudden onset, followed by endless pain and ultimately death. Over the years, acute pancreatitis, especially severe acute pancreatitis, is still a significant challenge for clinicians worldwide. To date, the incidence of severe pancreatitis continues to increase, and the mortality rate has not yet been significantly reduced (15%~20%) despite the continuous advancement of clinical pharmacy and critical care medicine.29,30 It is worth noting that pancreatitis is initially an aseptic inflammation, but as the disease progresses, bacterial infections, peritonitis, and shock can occur in advanced stages. To determine the prognosis and severity of pancreatitis, clinicians combine clinical and laboratory indicators such as the BISAP score, APACHE score, and Ranson score. In addition, independent CT imaging is used to analyze the severity of pancreatitis, among which CTSI is the most commonly used.31,32 These scores are the most widely used for AP severity assessment, and many clinical data are needed to be calculated for evaluating these indicators. However, in some cases where medical conditions are scarce, some fast and straightforward evaluation indexes may be required at the first mention of a pancreatitis diagnosis.29,33 In recent years, studies have explored the use of new types of severity and predictive scores such as NLR and PLR for predicting the severity of pancreatitis.34,35
It is well known that inflammation is involved in the occurrence and development of pancreatitis. In the early period of severe acute pancreatitis, immunosuppression could be involved in the complex inflammation and infection caused by gut mucosal barrier dysfunction.36 One study proposed that the crosstalk among damaged cells, neutrophils, and reactive oxygen species (ROS) appears to promote the process of pancreatitis synergistically.37 Platelets are directly involved in the systemic inflammatory process of acute pancreatitis, thereby leading to consumption, which is compensated by an immediate bone marrow response.38 For many decades, the contributions of neutrophils to the pathology of SAP were traditionally thought to involve the chemokine and cytokine cascades that accompany inflammation.39 Thus, the scores and indicators based on these inflammatory cells, including neutrophils, macrophages, lymphocytes, and plasma cells, were used to reflect the immunologic balance in acute pancreatitis.
NLR and PLR scores have been used as diagnostic indicators in many inflammatory and neoplastic diseases.40–42 In a previous study on the relationship between hepatocellular carcinoma and NLR, the NLR cut-off value was about 3.43 Zhang44 reported that an increased NLR is an independent risk factor for persistent organ failure (POF), prolonged ICU stays, and higher in-hospital mortality in AP. In our research, the cut-off value of NLR was 9.68, similar to the previous values for severe pancreatitis.33,34 Elevated neutrophils and depleted lymphocytes make “High NLR”, which may be caused by sepsis. Kaplan45 showed that NLR and PLR values were significant in severe acute pancreatitis. Therefore, combining the two scores would result in a better predictive value for determining the severity of AP compared to other scoring systems. In this study, PLR and NLR scores were significantly higher in the SAP group than in the MAP group (both P < 0.001). RDW-CV (coefficient of variation of RDW) and RDW-SD (standard deviation of RDW), respectively, both of which can imply RDW (blood cell distribution width) indicators of inhomogeneity of red blood cells. RDW-CV and RDW-SD were demonstrated to be independent risk factors predictive of mortality in SAP patients.46 A previous study showed that RDW is positively associated with AP severity and may be a helpful indicator for predicting AP severity. However, the results obtained herein showed that there was no significant difference in RDW values between the two groups (P = 0.349), same with the previous article.34
This study has shown that AP patients with SII value ≥ 2207.53 have a higher probability of having SAP (sensitivity = 92.9%, specificity = 87.7%, and AUC = 0.920). The predictive capability of SII for the severity of acute pancreatitis is more specific than PLR (sensitivity = 82.1%, specificity = 84.9%, and AUC = 0.877) and NLR (sensitivity = 82.1%, specificity = 82.2%, and AUC = 0.811).
However, this study had some limitations. First, the data used was obtained from a single center. Second, SII was only calculated at a one-time point. Therefore, the changes in SII over time and during the onset need to be studied further. Third, the number of samples was small. Future studies should increase the number of samples to verify our results.
Conclusion
The findings of this study suggest that SII is more specific and sensitive than NLR and PLR in distinguishing between MAP and SAP. Thus, it can be used as an early indicator to determine the severity of acute pancreatitis. However, large-scale, prospective, and well-designed studies should be conducted to validate the results.
Ethics Statement
The local ethics committee of the Second Affiliated Hospital of Dalian Medical University approved the study protocol. This study was conducted in accordance with the declaration of Helsinki. The requirement for written informed consent was waived because of the retrospective design of this study. Demographic information and laboratory analysis were collected from medical records. We confirmed that all the data was anonymized and maintained with confidentiality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Starr ME, Ueda J, Takahashi H, et al. Age-dependent vulnerability to endotoxemia is associated with reduction of anticoagulant factors activated protein C and thrombomodulin. Blood. 2010;115(23):4886–4893. doi:10.1182/blood-2009-10-246678
2. Wang J, Chen G, Gong H, Huang W, Long D, Tang W. Amelioration of experimental acute pancreatitis with Dachengqi Decoction via regulation of necrosis-apoptosis switch in the pancreatic acinar cell. PLoS One. 2012;7(7):e40160. doi:10.1371/journal.pone.0040160
3. Feng C, Li B, Wang LL, et al. Effect of peritoneal lavage with ulinastatin on the expression of NF-κB and TNF-α in multiple organs of rats with severe acute pancreatitis. Exp Ther Med. 2015;10(6):2029–2034. doi:10.3892/etm.2015.2802
4. Li YY, Li XJ, Lv S, et al. Ascitic fluid and serum from rats with acute pancreatitis injure rat pancreatic tissues and alter the expression of heat shock protein 60. Cell Stress Chaperones. 2010;15(5):583–591. doi:10.1007/s12192-010-0170-5
5. Liu Y, Wang L, Cai Z, et al. The decrease of peripheral blood CD4+ T cells indicates abdominal compartment syndrome in severe acute pancreatitis. PLoS One. 2015;10(8):e0135768. doi:10.1371/journal.pone.0135768
6. Jung KH, Son MK, Yan HH, et al. ANGPTL4 exacerbates pancreatitis by augmenting acinar cell injury through upregulation of C5a. EMBO Mol Med. 2020;12(8):e11222. doi:10.15252/emmm.201911222
7. Guo HY, Cui ZJ. Extracellular histones activate plasma membrane toll-like receptor 9 to trigger calcium oscillations in rat pancreatic acinar tumor cell AR4-2J. Cells. 2018;8(1):3. doi:10.3390/cells8010003
8. Balthazar EJ, Jung KH, Son MK. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002;223(3):603–613. doi:10.1148/radiol.2233010680
9. Yi KQ, Yang T, Yang YM, et al. Appraisal of the diagnostic procedures of acute pancreatitis in the guidelines. Syst Rev. 2021;10(1):17. doi:10.1186/s13643-020-01559-4
10. Ong Y, Shelat VG. Ranson score to stratify severity in Acute Pancreatitis remains valid - Old is gold. Expert Rev Gastroenterol Hepatol. 2021;15(8):865–877. doi:10.1080/17474124.2021.1924058
11. Petrov MS, Windsor JA. Classification of the severity of acute pancreatitis: how many categories make sense? Am J Gastroenterol. 2010;105(1):74–76. doi:10.1038/ajg.2009.597
12. Tan JH, Cao RC, Zhou L, et al. ATF6 aggravates acinar cell apoptosis and injury by regulating p53/AIFM2 transcription in severe acute pancreatitis. Theranostics. 2020;10(18):8298–8314. doi:10.7150/thno.46934
13. Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg. 2016;59(2):128–140. doi:10.1503/cjs.015015
14. Silva-Vaz P, Abrantes AM, Morgado-Nunes S, et al. Evaluation of prognostic factors of severity in acute biliary pancreatitis. Int J Mol Sci. 2020;21(12):4300. doi:10.3390/ijms21124300
15. Xie H, Wei B, Shen H, Gao Y, Wang L, Liu H. BRAF mutation in papillary thyroid carcinoma (PTC) and its association with clinicopathological features and systemic inflammation response index (SIRI).. Am J Transl Res. 2018;10(8):2726–2736.
16. Hong YM, Yoon KT, Cho M. Systemic immune-inflammation index predicts prognosis of sequential therapy with sorafenib and regorafenib in hepatocellular carcinoma. BMC Cancer. 2021;21(1):569. doi:10.1186/s12885-021-08124-9
17. Gungor H, Babu AS, Zencir C, et al. Association of preoperative platelet-to-lymphocyte ratio with atrial fibrillation after coronary artery bypass graft surgery. Med Princ Pract. 2017;26(2):164–168. doi:10.1159/000453614
18. Fox P, Hudson M, Brown C, et al. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer. 2013;109(1):147–153. doi:10.1038/bjc.2013.300
19. Nathan SD, Mehta J, Stauffer J, et al. Changes in neutrophil-lymphocyte or platelet-lymphocyte ratios and their associations with clinical outcomes in idiopathic pulmonary fibrosis. J Clin Med. 2021;10(7):1427. doi:10.3390/jcm10071427
20. Park S, Kim YH, Kim YC, et al. Association between post-transplant red cell distribution width and prognosis of kidney transplant recipients. Sci Rep. 2017;7(1):13755. doi:10.1038/s41598-017-13952-6
21. Zhang W, Wang Y, Wang J, Wang S. Association between red blood cell distribution width and long-term mortality in acute respiratory failure patients. Sci Rep. 2020;10(1):21185. doi:10.1038/s41598-020-78321-2
22. Jia L, Cui S, Yang J, et al. Red blood cell distribution width predicts long-term mortality in critically ill patients with acute kidney injury: a retrospective database study. Sci Rep. 2020;10(1):4563. doi:10.1038/s41598-020-61516-y
23. Su C, Liao LZ, Song Y, Xu ZW, Mei WY. The role of red blood cell distribution width in mortality and cardiovascular risk among patients with coronary artery diseases: a systematic review and meta-analysis. J Thorac Dis. 2014;6(10):1429–1440. doi:10.3978/j.issn.2072-1439.2014.09.10
24. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
25. Dionisie V, Filip GA, Manea MC, et al. Neutrophil-to-lymphocyte ratio, a novel inflammatory marker, as a predictor of bipolar type in depressed patients: a quest for biological markers. J Clin Med. 2021;10(9):1924. doi:10.3390/jcm10091924
26. Bartl T, Bekos C, Postl M, et al. The systemic immune-inflammation index (SII) is an independent prognostic parameter of survival in patients with invasive vulvar cancer. J Gynecol Oncol. 2021;32(1):e1. doi:10.3802/jgo.2021.32.e1
27. Erdogan T. Role of systemic immune-inflammation index in asthma and NSAID-exacerbated respiratory disease. Clin Respir J. 2021;15(4):400–405. doi:10.1111/crj.13314
28. Moynihan B. ACUTE PANCREATITIS. Ann Surg. 1925;81(1):132–142. doi:10.1097/00000658-192501010-00013
29. Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):
30. Kiat TTJ, Gunasekaran SK, Junnarkar SP, Low JK, Woon W, Shelat VG. Are traditional scoring systems for severity stratification of acute pancreatitis sufficient? Ann Hepatobiliary Pancreat Surg. 2018;22(2):105–115. doi:10.14701/ahbps.2018.22.2.105
31. Papachristou GI, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105(2):
32. Harshit Kumar A, Singh Griwan M. A comparison of APACHE II, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol Rep. 2018;6(2):127–131. doi:10.1093/gastro/gox029
33. Ranson JH, Pasternack BS. Statistical methods for quantifying the severity of clinical acute pancreatitis. J Surg Res. 1977;22(2):79–91. doi:10.1016/0022-4804(77)90045-2
34. Liu G, Tao J, Zhu Z, Wang W. The early prognostic value of inflammatory markers in patients with acute pancreatitis. Clin Res Hepatol Gastroenterol. 2019;43(3):330–337. doi:10.1016/j.clinre.2018.11.002
35. Zhou H, Mei X, He X, Lan T, Guo S. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: a retrospective study. Medicine. 2019;98(16):e15275. doi:10.1097/MD.0000000000015275
36. Li JP, Yang J, Huang JR, et al. Immunosuppression and the infection caused by gut mucosal barrier dysfunction in patients with early severe acute pancreatitis. Front Biosci. 2013;18:892–900. doi:10.2741/4150
37. Huang C, Chen S, Zhang T, et al. TLR3 ligand PolyI:C prevents acute pancreatitis through the Interferon-β/Interferon-α/β receptor signaling pathway in a caerulein-induced pancreatitis mouse model. Front Immunol. 2019;10:980. doi:10.3389/fimmu.2019.00980
38. Mimidis K, Papadopoulos V, Kotsianidis J, et al. Alterations of platelet function, number and indexes during acute pancreatitis. Pancreatology. 2004;4(1):22–27. doi:10.1159/000077024
39. Yang ZW, Meng XX, Xu P. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J Cell Mol Med. 2015;19(11):2513–2520. doi:10.1111/jcmm.12639
40. Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–2290. doi:10.2147/COPD.S141760
41. Wang D, Bai N, Hu X, et al. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ. 2019;7:e7132. doi:10.7717/peerj.7132
42. Fang T, Wang Y, Yin X, et al. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res. 2020;2020:9146042. doi:10.1155/2020/9146042
43. Shelat VG. Role of inflammatory indices in management of hepatocellular carcinoma-neutrophil to lymphocyte ratio. Ann Transl Med. 2020;8(15):912. doi:10.21037/atm-2020-90
44. Zhang Y, Wu W, Dong L, Yang C, Fan P, Wu H. Neutrophil to lymphocyte ratio predicts persistent organ failure and in-hospital mortality in an Asian Chinese population of acute pancreatitis. Medicine. 2016;95(37):e4746. doi:10.1097/MD.0000000000004746
45. Kaplan M, Ates I, Oztas E, et al. A new marker to determine prognosis of acute pancreatitis: PLR and NLR combination. J Med Biochem. 2018;37(1):21–30. doi:10.1515/jomb-2017-0039
46. Zhang FX, Li ZL, Zhang ZD, Ma XC. Prognostic value of red blood cell distribution width for severe acute pancreatitis. World J Gastroenterol. 2019;25(32):4739–4748. doi:10.3748/wjg.v25.i32.4739
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
