Back to Journals » Journal of Pain Research » Volume 10
Systemic alterations in plasma proteins from women with chronic widespread pain compared to healthy controls: a proteomic study
Authors Wåhlén K, Olausson P, Carlsson A, Ghafouri N, Gerdle B , Ghafouri B
Received 24 November 2016
Accepted for publication 8 February 2017
Published 5 April 2017 Volume 2017:10 Pages 797—809
DOI https://doi.org/10.2147/JPR.S128597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Karin Wåhlén, Patrik Olausson, Anders Carlsson, Nazdar Ghafouri, Björn Gerdle, Bijar Ghafouri
Pain and Rehabilitation Centre, Department of Medical and Health Sciences, Linköping University, Linköping, Sweden
Abstract: Chronic widespread pain (CWP) is a complex pain condition that is difficult to treat. The prevalence of CWP approximates ~10% of the general population, with higher prevalence in women. Lack of understanding of molecular mechanisms has been a challenge for diagnosis and treatment of chronic pain. The aim of this study was to explore the systemic protein changes in CWP compared to those in healthy controls (CON). By applying 2-dimensional gel electrophoresis, we analyzed the protein pattern of plasma samples from women with CWP (n=16) and healthy women (n=23). The proteomic data were analyzed using multivariate statistical models, and altered proteins were identified using mass spectrometry. The proteome analysis was further validated by gel-free Western blot. Multivariate statistical data analysis of quantified proteins revealed 22 altered proteins in women with CWP, compared to CON group. Many of the identified proteins are previously known to be involved in different parts of the complement system and metabolic and inflammatory processes, e.g., complement factor B, vitamin D-binding protein, ceruloplasmin, transthyretin and alpha-2-HS-glycoprotein. These results indicate that important systemic protein differences exist between women with CWP and healthy women. Further, this study illustrates the potential use of proteomics to detect biomarkers that may provide new insights into the molecular mechanism(s) of chronic pain. However, further larger investigations are required in order to confirm these findings before it will be possible to identify proteins as potential pain biomarkers for clinical use.
Keywords: inflammation, biomarker, painomics, complement system, GC protein
Introduction
Chronic widespread pain (CWP) is a complex pain condition that is difficult to treat. The prevalence of CWP in the European population is ~10%,1–3 whereof 2–4% is further diagnosed with fibromyalgia syndrome (FMS).4–6 According to the American College of Rheumatology (ACR) criteria from 1990, CWP is defined as pain present in at least 3 of 4 body quadrants, including axial skeleton pain, and persists for at least 3 months.7 Individuals diagnosed with CWP/FMS often experience tiredness, sleeping disorders and other psychological and somatic disorders, e.g., irritable bowel syndrome and stiffness in muscle and joints.1,8–11 There is evidence that CWP/FMS is more prominent among women with increased age, obesity, depression and low physical activity.4,12
Central alterations in the pain matrix in the brain and central hyperexcitability are known to be part of the underlying mechanism in CWP/FMS.13–15 There are several studies suggesting that peripheral factors and alterations could have a prominent role and therefore contribute to the maintenance of pain and increased pain sensitivity in CWP/FMS, e.g., alterations in muscle16–19 and peripheral nociceptors.20–22 However, the molecular mechanisms behind CWP are not fully elucidated.
Applying proteomics as an approach to study new biomarkers in different body tissues, from individuals with different diseases, has been used in several studies. The proteome of serum from rheumatoid arthritis (RA) patients,23 cerebrospinal fluid (CSF) from patients with neuropathic pain24 and the interstitial fluid of the trapezius muscle from patients with CWP/FMS25 have been investigated. A preliminary proteomic study of human serum from FMS patients found upregulated levels of 3 proteins, α1-antitrypsin, transthyretin and retinol-binding protein 4, compared to healthy controls (CON).26 Further, the metabolic profile in serum from women with localized and widespread pain has recently been investigated, suggesting systemic changes in processes related to lipid metabolism and energy consumption.27
We have previously performed proteomics on muscle biopsies from patients with CWP/FMS and found protein alterations involved in metabolic pathways, inflammation and muscle recovery.28 Further, the pain intensity and pressure pain threshold correlated significantly with specific proteomic patterns.29 In this study, from the same cohort, we aimed to analyze the protein pattern, and potential new biomarkers, in plasma sample from female CWP/FMS patients compared to healthy CON.
Materials and methods
Subjects
The recruitment process, including the inclusion and exclusion criteria for the patients with CWP and healthy CON, has been described in detail previously.30 Briefly, according to the exclusion criteria, none of the included participants in either group used any type of anticoagulatory, opioid or steroidal medication. Further, if a medical history record of bursitis, tendonitis, capsulitis, postoperative conditions in the neck/shoulder area, previous neck trauma, disorder of the spine, neurological disease, RA or any other systemic diseases, metabolic disease, malignancy, severe psychiatric illness or pregnancy or difficulties understanding the Swedish languages were present, participants were excluded from the study. The healthy CON group was recruited through local newspaper advertisement and consisted of 23 women aged between 20 and 65 years. Female patients with CWP were recruited to this project by either being among former patients with CWP at the Pain and Rehabilitation Centre of the University Hospital, Linköping, Sweden, or from an organization for FMS patients. The ACR criteria from 1990 were used for classification of FMS.7 The recruitment process resulted in 19 CWP patients. As it has been reported previously30 based on the concentration of lactate using power and sample size calculation (a=0.05, power=0.8), it was found that 17 subjects in each group were needed.
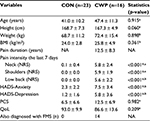
To confirm the individual eligibility, all participants (CON and CWP) received a standardized clinical examination. The examination was followed by a health questionnaire concerning chronic pain,30 which included questions about pain intensity in the neck–shoulder and low back regions using numeric rating scale (NRS, 0=no pain and 10=worst possible pain), anxiety and depressive symptoms (Hospital Anxiety and Depression Scale [HADS; cutoff values for both subscales >10]), catastrophizing (Pain Catastrophizing Scale [PCS]; cutoff >37) and quality of life (QoL).
All participants signed a written consent before the start of the study, after receiving verbal and written information about the objectives and procedures of the study. The study was approved by the Regional Ethical Review Board in Linköping, Sweden (Dnr: M10–08, M233–09, Dnr: 2010/164–32) and followed the guidelines according to the Declaration of Helsinki. All methods were carried out in accordance with the approved ethical application.
Sample collection
The participants were asked not to take nonsteroidal anti-inflammatory drugs for 7 days and/or paracetamol medication for 12 hours prior to the sampling. Venous blood samples were collected in EDTA vacutainer and centrifuged at 1000× g for 15 minutes, plasma was collected, aliquoted and stored at −70°C until use. All samples were blinded before analysis. There were some difficulties with blood sampling for 2 subjects, and the sample from 1 subject was not sufficient for further proteomic analysis. Therefore, plasma samples from 16 women with CWP were included in this study.
Proteomics
The procedure for 2-dimensional gel electrophoresis (2-DE) has been described in previous studies.24,28 Briefly, 40 µL plasma sample from each subject was depleted of albumin and immunoglobulin G (IgG) using ProteoPrep (Sigma-Aldrich Co, St Louis, MO, USA), followed by protein concentration measurement using 2D-Quant Kit (GE Healthcare, Little Chalfont, UK). Samples were further desalted with PD-10 columns (GE Healthcare) and lyophilized prior to use in the first dimension. Lyophilized proteins were resolved in 2-DE urea sample buffer according to Gorg et al,31 and 100 µg of total protein from each subject was applied in the first dimension and further run in second dimension using Ettan™ DALTsix Electrophoresis unit (Amersham, Pharmacia Biotech, Uppsala, Sweden).24,28
Separated proteins were fluorescently stained with SYPRO Ruby® (Bio-Rad Laboratories, Hercules, CA, USA), and gels were visualized using a charged coupled device (CCD) camera (VersaDoc™ Imaging system 4000 MP; Bio-Rad Laboratories). 2-DE protein patterns were analyzed and quantified using software PDQuest Advanced version 8.0.1 (Bio-Rad Laboratories). Protein spots of interest were excised from the gel and, after tryptic digestion, were analyzed by mass spectrometry using ultrafleXtreme™ matrix-assisted laser desorption/ionization – time of flight (MALDI-TOF; Bruker Daltronik GmbH, Bremen, Germany). Database search was performed in ProteinProspector MS-Fit version 5.14.4 including Swiss-Prot database version 2015.3.5 as described in previous studies.28,32
Immunological analysis of vitamin D-binding protein (VDBP)
Plasma samples were analyzed using Peggy Simple Western size assay (ProteinSimple, Santa Clara, CA, USA) according to the user manual and as described previously.33 Briefly, plasma samples were mixed with master mix containing 80 mM dithiothreitol and fluorescent molecular markers and heated at 95°C for 5 minutes. The samples, blocking reagents, primary antibody, secondary antibody and chemiluminescence substrate were loaded onto a 384-well plate according to the user manual. The primary antibody (VDBP, monoclonal mouse anti-human, LS-B3318; Nordic BioSite, Täby, Sweden) was diluted in antibody diluent in a 1:50 dilution. The chemiluminescence signals were digitized using a CCD camera. The digital images were analyzed with Compass software version 2.7.1 (ProteinSimple). To evaluate the concentration of VDBP in plasma, a 6-point standard curve, consisting of recombinant VDBP (Nordic BioSite), was run simultaneously with the plasma samples. The concentration of VDBP was calculated from the standard curve (R2=0.99), and the data are presented as median ± min–max µg/µL.
Vitamin D (25-hydroxyvitamin D) concentration in plasma
Analysis of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 was performed with high-performance liquid chromatography (HPLC) according to a method previously described.34 The HPLC system consisted of a JASCO PU-2089 pump and a JASCO UV-975 detector set on the wavelength 265 nm (both from Japan Spectroscopic Company, Tokyo, Japan). The column was a Grace Smart RP 18 (100 × 2.1 mm, 3 μm), and the mobile phase consisted of methanol:water (80:20, v/v) and the flow rate was 0.4 mL/min. Twenty microliters of the samples were injected, and the concentration was determined based on a standard curve that was prepared by an in-house reference. Standards of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Together with standards with known concentration, a reference was analyzed together with the samples. The inter-assay and intra-assay coefficient of variation was 3.9% and 5.7%, respectively. As a quality control, we used plasma references from Vitamin D External Quality Control Scheme (DEQAS; http://www.deqas.org). Because sampling was performed on different occasions over the year, the concentrations of vitamin D were adjusted for what time of the year it was drawn against a standard curve, obtained from results of a British study of 7437 individuals.35
Statistics
All statistics regarding the anthropometric, vitamin D and VDBP data were calculated in IBM SPSS version 23 (IBM Corporation, Armonk, NY, USA). For normally distributed data, a Student t-test was applied, and for non-normally distributed data, a Mann–Whitney U test was applied. p≤0.05 was considered significant. Data are presented as mean values ± 1 standard deviation (SD) if not stated otherwise.
Multivariate correlation between membership of groups and quantified proteins were analyzed with orthogonal partial least square discriminant analysis (OPLS-DA) using SIMCA-P+ version 13.0 (UMETRICS, Umeå, Sweden). The procedure to compute multivariate correlation models has been described earlier24,28 and is in accordance with Wheelock and Wheelock.36 Briefly, prior to OPLS-DA analysis, a principle component analysis (PCA) was created to control for multiple outliers. No detectable multivariate outliers were identified in this study. The OPLS-DA model contains data variables (quantified proteomic data) that were mean centered and scaled for unified variance (UV-scaled). The variable influence of projection (VIP value) is a value that describes the importance and relevance of each X variable (quantified proteins), pooled over all dimensions, and Y variables (CWP or CON) that include the group of variables that best explain Y. In this study, a VIP>1.0 with a jacked-knifed 95% confidence interval was considered significant as reported in previous study.28 The OPLS-DA model was performed in a 2-step analysis. First, a pre-model was built including all proteins. From this model, proteins with a VIP value>1.0 including the jacked-knifed 95% confidence interval, not including zero, were further used in a new regression model. R2 describes the goodness of fit – the fraction of sum of squares of all the variables explained by a principal component. Q2 describes the goodness of prediction – the fraction of the total variation of the variables that can be predicted by a principal component using cross-validation methods. R2 should not be considerably higher than Q2. A difference >0.2–0.3 implies overfitting meaning that the robustness of the model is poor. To validate the model, we used cross-validated analysis of variance (CV-ANOVA), which was considered of significant importance when p<0.05.
Results
Clinical and anthropometric data
In total, plasma samples were retrieved from 16 CWP patients (14 of 16 were diagnosed with FMS) and 23 healthy women (CON). No significant differences were found between the groups regarding age, height, weight, body mass index (BMI) or pain duration. As expected, CWP had higher pain intensities than CON for neck, shoulder and low back (Table 1). The CWP group reported significant higher HADS scores for the anxiety and depression subscales compared to CON, but on group level, these values were below the cutoffs indicating clinically depressive or anxiety states. No significant differences existed in PCS or QoL (Table 1).
Multivariate regression analysis to assess group belonging
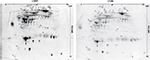
To identify systemic differences in plasma between CWP and CON, a proteomic approach was used in combination with PCA and OPLS-DA models. In total, 414 ± 21 (CWP: 423 ± 20, CON: 408 ± 20) protein spots from each gel were detected in the 2-DE analysis. Out of these proteins, 325 protein spots in each plasma gel were further quantified, matched and initially analyzed with an unsupervised PCA model. No detectable strong, or moderate, outliers were identified in either group in this study. Further, an OPLS-DA model was applied consisting of 1 predictive and 1 orthogonal component. The model showed a clear separation between groups with a good fit (R2=0.84) and predictivity (Q2=0.60) and presented a significant CV-ANOVA, p=2.31e−06 (Figure 1).
Altered proteins
Twenty-two proteins, including several different isoforms, had a VIP value >1.0 and were considered to be important for group belongings and were significantly altered in the CWP group compared to CON. The identified proteins were divided into 4 classes according to their involvement in different biological processes found in Uniprot Database (uniprot.org), as follows: iron ion hemostasis and immunity, metabolic and inflammatory processes (Figure 2; Table 2).
Two proteins belonged to cellular iron ion hemostasis (ceruloplasmin [CERU], 9 upregulated isoforms; serotransferrin, 1 downregulated isoform). Eight proteins belonged to immunity process/complement activation (complement C1r subcomponent, 6 upregulated isoforms; complement factor B, 1 upregulated isoform; complement C1s subcomponent, 1 upregulated isoform; Ig alpha-2 chain C region, 1 downregulated isoform; complement C3 alpha chain fragment 2, 1 upregulated isoform; complement factor I light chain, 1 upregulated isoform; ficolin-3, 1 upregulated isoform, Ig kappa chain C region, 1 upregulated isoform and 1 downregulated). Eight proteins belonged to metabolic processes (gelsolin, 2 upregulated isoforms; plasminogen, 6 upregulated isoforms; coagulation factor XIII B chain, 1 upregulated isoform; prothrombin, 1 upregulated isoform; alpha-2-antiplasmin, 2 upregulated isoforms; fibrinogen gamma chain, 3 upregulated isoforms; apolipoprotein A-I, 1 downregulated isoform; transthyretin, 1 downregulated isoform). Three proteins belonged to inflammatory response (VDBP, 3 upregulated isoforms; alpha-2-HS-glycoprotein, 1 upregulated isoform; haptoglobin beta chain, 1 upregulated isoform) and 1 unidentified protein was upregulated. A more detailed table over the mass spectrometry result is shown in Table S1.
VDBP and vitamin D concentration
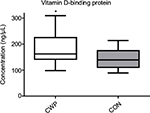
The VDBP result from 2-DE was further verified using gel-free Western blot. The VDBP concentration in plasma samples from CWP (n=14) and CON (n=22) was analyzed, and significant elevated concentrations of VDBP were found in CWP group compared to CON (CWP=162.4 ± 98.2–309.8 ng/µL; CON=138.8 ± 89.3–214.3 ng/µL, p=0.034; Figure 3). Additionally, significant higher concentration of vitamin D (25-hydroxyvitamin D) in plasma was found in CWP group compared to CON (CWP=67.7 ± 18.8 nmol/L; CON=48.5 ± 16.8 nmol/L, p=0.002).
Discussion
In this novel study, significant differences were found between CWP and CON in several plasma proteins belonging to inflammatory, immunity, and metabolic processes using proteomics in combination with multivariate data analysis.
There are limited studies that have been conducted exploring systemic changes of plasma proteins in CWP. Balfoussia et al37 investigated the plasma proteome of male elite runners before and after performing a running race of 246 km within 36 hours. They found several proteins that are similar in significance and alteration as in our study, e.g., upregulated levels of complement factor B and VDBP and downregulated levels of transthyretin, Ig kappa chain C region and apolipoprotein A-I.37 In a recent plasma proteomic study of male farmers with musculoskeletal disorders from our group,38 increased levels of complement factor B, haptoglobin and serotransferrin and decreased levels of alpha-2-HS-glycoprotein, VDBP and apolipoprotein A-1 were found, suggesting that these farmers may be subjected to systemic inflammation. In this study, the same proteins were found significant, however, differentially altered, e.g., increased levels of alpha-2-HS-glycoprotein and VDBP and decreased levels of serotransferrin. It is interesting to reflect upon that both studies share the same type of altered proteins even though they are differently expressed. This could be due to different pain disorders, gender or type of environmental exposures. In contrast to our results, significantly decreased levels of VDBP and fibrinogen gamma chain among others have been reported in serum from patients with carpal tunnel syndrome.39 All the abovementioned studies have one thing in common, ie, the altered proteins are mainly involved in inflammation, either by themselves or through activation of different pathways of the complement system or metabolic processes.37–39
Regarding our study, reflecting plasma protein changes in women with CWP, a vast amount of the proteins belong to different parts of the complement system. The complement system is characterized by a cascade of proteolytic cleavages after recognition of different molecular patterns from mainly microorganisms, impaired host cells or other molecules, leading to production of different components mainly regarded as pro-inflammatory proteins, through the recruitment of different immune cells.40,41 Six proteins were significantly upregulated and belong to the complement system activation, through either of the 3 pathways; the classical pathway (complement C1r subcomponent, C1s subcomponent, complement C3c alpha chain fragment 2, complement factor I light chain), alternative pathway (complement factor B) or the lectin pathway (ficolin-3). Complement C3c alpha chain fragment 2 (CO3), a component of C3, is one of the central proteins of both the classical and alternative pathways of the complement system.40 After cleavage of CO3, pro-inflammatory fragments of C3a are produced leading to activation of several different cell types, mainly leukocytes, as well as increased influx of Ca2+ in these cells that induces an inflammatory response.42 Furthermore, complement factor B has been shown to be involved in muscle inflammation.41 Since a majority of the proteins involved in the complement and coagulation cascade in this study were significantly upregulated and were among the ones most important for group separation with highest VIP values, it is tempting to speculate that an increased activity of these proteins leads to a larger production and activation of pro-inflammatory substances that contribute to an overall increased inflammation in the muscles of chronic pain patients.
Several isoforms of CERU were found upregulated in CWP (2 isoforms were among the ones with highest VIP value). CERU is primarily produced in the liver and secreted out in the circulation and is known to be one of the larger copper-carrier proteins in the body. Arner et al43 found elevated levels of CERU secreted from adipose cells and circulating levels in obese females compared to non-obese subjects. The detected isoforms of CERU in our study were found elevated. Although, there were no significant differences in weight or BMI in this cohort, it is generally known that one of the risk factors is obesity in females with CWP/FMS.44,45 Several studies have also found a correlation between higher levels of CERU, haptoglobin and complement proteins in plasma and serum to be associated with major depressive disorders.46–50 In this study, the CWP group reported statistically significant higher HADS scores (anxiety and depression) compared to CON. However, their HADS scores were <10, and they showed no clinical signs of depression or anxiety. Hence, the findings in this study are not primarily related to depression or anxiety in females with CWP and could therefore not explain the increased levels of these proteins in the CWP group compared to CON.
VDBP main function is as a carrier and transporter of vitamin D in the circulation. It also acts as an actin scavenger, forming complexes with G-actin and gelsolin, primarily by depolarization of free actin that is released upon injury to prevent damage to the microcirculation.51 VDBP further works as a macrophage-activating factor (MAF), initiating macrophage activity.52 In this study, both VDBP and gelsolin were upregulated in the CWP group. We also verified the 2-DE findings of VDBP with an additional method. So far, to our knowledge, no one has investigated the VDBP levels in plasma from CWP patients. However, it is more common to analyze vitamin D metabolites, and several studies have found decreased levels in chronic low back pain53 and FMS/CWP patients.54,55 This is in conflict with our result, showing increased levels of both vitamin D and VDBP. The cause of elevated levels of VDBP in the CWP group is unknown; one theory could be physiological, since the levels of vitamin D is increased, this could follow with increased levels of VDBP, due to elevated transportation of the protein is required. Another theory could be VDBP’s function as a cofactor, together with one of the by-products from the alternative and classical pathway of complement, c5a, which essentially needs VDBP to be able to work as chemoattractant for neutrophils.56 One could speculate that since several proteins from the complement system are upregulated in the CWP group, it could possibly lead to elevated levels of complement by-products. Therefore, excessive levels of VDPB together with complement by-product, e.g., c5a, can lead to more recruitment of neutrophils and in turn activate and/or prolong an immune response. Additionally, studies have suggested that vitamin D supplement could be an alternative for decreased pain among women with CWP/FMS.54,57,58 Hence, in our study, the higher levels of both vitamin D and VDBP cannot explain the reported pain in the CWP patients.
Finally, the coagulation system interacts with the complement system via thrombin activation of C5.59 Coagulation factor XIII B chain, fibrinogen gamma chain and prothrombin are proteins involved in the coagulation/fibrinolytic system. In this study, these proteins were all upregulated in the CWP group compared to CON. It has been shown in several studies that patients suffering from chronic pain states have impaired microcirculation.16,60,61 We have earlier in the same cohort also found significantly increased concentration of lactate and glutamate, in the interstitial muscle and plasma, in the CWP group compared to CON.30 Impaired microcirculation in the peripheral tissue might affect the presence of circulating factors and cells in the muscle tissue in patients with chronic pain, which could be an explanation to the altered levels of proteins seen in the CWP group compared to CON.
This study demonstrates that there are systemic changes in women with chronic pain that might contribute to central nervous system alterations. However, one should keep in mind that this proteomic study only reflects the plasma proteome at one explicit time point, where precautions of its significance should be reflected upon. Further, the number of participants might be considered as low and should in future studies be increased although the number of subjects in this study compared to other proteomic studies reporting biomarker candidates for different pain conditions is relatively high.39,62
Conclusion
This study illustrates the potential use of proteomics for identifying systemic protein changes associated with CWP. We have found significantly altered levels of inflammatory, immunity, and metabolic proteins in plasma samples from women with CWP compared to healthy CON, suggesting that patients with CWP are subjected to a low-grade systemic inflammation. The identified differences in the plasma proteome in this study provide novel information about the biological mechanisms in CWP, which, together with earlier proteome studies in biopsies from the same cohort, might contribute to better understanding of the involved nociceptive and pain mechanisms in CWP.
Acknowledgments
This study was supported by the Swedish Council for Working Life and Social Research (2010-0913), the Swedish Research Council (K2015-99X-21874-05-04), the Medical Research Council of Southeast Sweden (159031), AFA Insurance (Dnr-140341), Region Östergötland research fund (LIO-445161; SC-2013-00395-36) and Åke Wiberg foundation.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):173–183. | ||
Papageorgiou AC, Silman AJ, Macfarlane GJ. Chronic widespread pain in the population: a seven year follow up study. Ann Rheum Dis. 2002;61(12):1071–1074. | ||
Bergman S, Herrstrom P, Hogstrom K, Petersson IF, Svensson B, Jacobsson LT. Chronic musculoskeletal pain, prevalence rates, and sociodemographic associations in a Swedish population study. J Rheumatol. 2001;28(6):1369–1377. | ||
Lindell L, Bergman S, Petersson IF, Jacobsson LT, Herrstrom P. Prevalence of fibromyalgia and chronic widespread pain. Scand J Prim Health Care. 2000;18(3):149–153. | ||
Gerdle B, Bjork J, Coster L, Henriksson K, Henriksson C, Bengtsson A. Prevalence of widespread pain and associations with work status: a population study. BMC Musculoskelet Disord. 2008;9:102. | ||
Coster L, Kendall S, Gerdle B, Henriksson C, Henriksson KG, Bengtsson A. Chronic widespread musculoskeletal pain – a comparison of those who meet criteria for fibromyalgia and those who do not. Eur J Pain. 2008;12(5):600–610. | ||
Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. | ||
Mourao AF, Blyth FM, Branco JC. Generalised musculoskeletal pain syndromes. Best Pract Res Clin Rheumatol. 2010;24(6):829–840. | ||
Mannerkorpi K, Burckhardt CS, Bjelle A. Physical performance characteristics of women with fibromyalgia. Arthritis Care Res. 1994;7(3):123–129. | ||
Yang TY, Chen CS, Lin CL, Lin WM, Kuo CN, Kao CH. Risk for irritable bowel syndrome in fibromyalgia patients: a national database study. Medicine. 2015;94(10):e616. | ||
Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. | ||
Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003;17(4):547–561. | ||
Bosma RL, Mojarad EA, Leung L, Pukall C, Staud R, Stroman PW. FMRI of spinal and supra-spinal correlates of temporal pain summation in fibromyalgia patients. Hum Brain Mapp. 2016;37(4):1349–1360. | ||
Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. | ||
Staud R, Smitherman ML. Peripheral and central sensitization in fibromyalgia: pathogenetic role. Curr Pain Headache Rep. 2002;6(4):259–266. | ||
Sandberg M, Larsson B, Lindberg LG, Gerdle B. Different patterns of blood flow response in the trapezius muscle following needle stimulation (acupuncture) between healthy subjects and patients with fibromyalgia and work-related trapezius myalgia. Eur J Pain. 2005;9(5):497–510. | ||
Gerdle B, Lemming D, Kristiansen J, Larsson B, Peolsson M, Rosendal L. Biochemical alterations in the trapezius muscle of patients with chronic whiplash associated disorders (WAD)--a microdialysis study. Eur J Pain. 2008;12(1):82–93. | ||
Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136(pt 6):1857–1867. | ||
Staud R. The role of peripheral input for chronic pain syndromes like fibromyalgia syndrome. J Musculoskelet Pain. 2008;16(1–2):67–74. | ||
Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154(11):2310–2316. | ||
Serra J, Collado A, Sola R, et al. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol. 2014;75(2):196–208. | ||
Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145(1–2):96–104. | ||
Serada S, Fujimoto M, Ogata A, et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis. 2010;69(4):770–774. | ||
Backryd E, Ghafouri B, Carlsson AK, Olausson P, Gerdle B. Multivariate proteomic analysis of the cerebrospinal fluid of patients with peripheral neuropathic pain and healthy controls – a hypothesis-generating pilot study. J Pain Res. 2015;8:321–333. | ||
Olausson P, Gerdle B, Ghafouri N, Larsson B, Ghafouri B. Identification of proteins from interstitium of trapezius muscle in women with chronic myalgia using microdialysis in combination with proteomics. PLoS One. 2012;7(12):e52560. | ||
Ruggiero V, Era B, Cacace E, et al. A preliminary study on serum proteomics in fibromyalgia syndrome. Clin Chem Lab Med. 2014;52(9):e207–e210. | ||
Hadrevi J, Bjorklund M, Kosek E, et al. Systemic differences in serum metabolome: a cross sectional comparison of women with localised and widespread pain and controls. Sci Rep. 2015;5:15925. | ||
Olausson P, Gerdle B, Ghafouri N, Sjostrom D, Blixt E, Ghafouri B. Protein alterations in women with chronic widespread pain – an explorative proteomic study of the trapezius muscle. Sci Rep. 2015;5:11894. | ||
Olausson P, Ghafouri B, Ghafouri N, Gerdle B. Specific proteins of the trapezius muscle correlate with pain intensity and sensitivity – an explorative multivariate proteomic study of the trapezius muscle in women with chronic widespread pain. J Pain Res. 2016;9:345–356. | ||
Gerdle B, Larsson B, Forsberg F, et al. Chronic widespread pain: increased glutamate and lactate concentrations in the trapezius muscle and plasma. Clin J Pain. 2014;30(5):409–420. | ||
Gorg A, Drews O, Luck C, Weiland F, Weiss W. 2-DE with IPGs. Electrophoresis. 2009;30(suppl 1):S122–S132. | ||
Ghafouri B, Karlsson H, Mortstedt H, Lewander A, Tagesson C, Lindahl M. 2,5-Dihydroxybenzoic acid instead of alpha-cyano-4-hydroxycinnamic acid as matrix in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for analyses of in-gel digests of silver-stained proteins. Anal Biochem. 2007;371(1):121–123. | ||
Chen JQ, Heldman MR, Herrmann MA, et al. Absolute quantitation of endogenous proteins with precision and accuracy using a capillary Western system. Anal Biochem. 2013;442(1):97–103. | ||
Turpeinen U, Hohenthal U, Stenman UH. Determination of 25-hydroxyvitamin D in serum by HPLC and immunoassay. Clin Chem. 2003;49(9):1521–1524. | ||
Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85(3):860–868. | ||
Wheelock AM, Wheelock CE. Trials and tribulations of ’omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol Biosyst. 2013;9(11):2589–2596. | ||
Balfoussia E, Skenderi K, Tsironi M, et al. A proteomic study of plasma protein changes under extreme physical stress. J Proteomics. 2014;98:1–14. | ||
Ghafouri B, Carlsson A, Holmberg S, Thelin A, Tagesson C. Biomarkers of systemic inflammation in farmers with musculoskeletal disorders; a plasma proteomic study. BMC Musculoskelet Disord. 2016;17(1):206. | ||
Oh YM, Ma TZ, Kwak YG, Eun JP. Proteomic evaluation to identify biomarkers for carpal tunnel syndrome: a comparative serum analysis. Connect Tissue Res. 2013;54(1):76–81. | ||
Janssen BJC, Huizinga EG, Raaijmakers HCA, et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437(7058):505–511. | ||
Frenette J, Cai B, Tidball JG. Complement activation promotes muscle inflammation during modified muscle use. Am J Pathol. 2000;156(6):2103–2110. | ||
Norgauer J, Dobos G, Kownatzki E, et al. Complement fragment C3a stimulates Ca2+ influx in neutrophils via a pertussis-toxin-sensitive G protein. Eur J Biochem. 1993;217(1):289–294. | ||
Arner E, Forrest AR, Ehrlund A, et al. Ceruloplasmin is a novel adipokine which is overexpressed in adipose tissue of obese subjects and in obesity-associated cancer cells. PLoS One. 2014;9(3):e80274. | ||
Neumann L, Lerner E, Glazer Y, Bolotin A, Shefer A, Buskila D. A cross-sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin Rheumatol. 2008;27(12):1543–1547. | ||
Okifuji A, Bradshaw DH, Olson C. Evaluating obesity in fibromyalgia: neuroendocrine biomarkers, symptoms, and functions. Clin Rheumatol. 2009;28(4):475–478. | ||
Ruland T, Chan MK, Stocki P, et al. Molecular serum signature of treatment resistant depression. Psychopharmacology. 2016;233(15–16):3051–3059. | ||
Song C, Dinan T, Leonard BE. Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls. J Affect Disord. 1994;30(4):283–288. | ||
Kaya MC, Bez Y, Selek S, et al. No effect of antidepressant treatment on elevated serum ceruloplasmin level in patients with first-episode depression: a longitudinal study. Arch Med Res. 2012;43(4):294–297. | ||
La Rubia M, Rus A, Molina F, Del Moral ML. Is fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status? Clin Exp Rheumatol. 2013;31(6 suppl 79):S121–S127. | ||
Lee J, Joo EJ, Lim HJ, et al. Proteomic analysis of serum from patients with major depressive disorder to compare their depressive and remission statuses. Psychiatry Investig. 2015;12(2):249–259. | ||
Jorgensen CS, Christiansen M, Laursen I, et al. Large-scale purification and characterization of non-glycosylated Gc globulin (vitamin D-binding protein) from plasma fraction IV. Biotechnol Appl Biochem. 2006;44(pt 1):35–44. | ||
Yamamoto N, Kumashiro R. Conversion of vitamin D3 binding protein (group-specific component) to a macrophage activating factor by the stepwise action of beta-galactosidase of B cells and sialidase of T cells. J Immunol. 1993;151(5):2794–2802. | ||
Lodh M, Goswami B, Mahajan RD, Sen D, Jajodia N, Roy A. Assessment of vitamin D status in patients of chronic low back pain of unknown etiology. Indian J Clin Biochem. 2015;30(2):174–179. | ||
Wepner F, Scheuer R, Schuetz-Wieser B, et al. Effects of vitamin D on patients with fibromyalgia syndrome: a randomized placebo-controlled trial. Pain. 2014;155(2):261–268. | ||
Kuru P, Akyuz G, Yagci I, Giray E. Hypovitaminosis D in widespread pain: its effect on pain perception, quality of life and nerve conduction studies. Rheumatol Int. 2015;35(2):315–322. | ||
Trujillo G, Zhang J, Habiel DM, et al. Cofactor regulation of C5a chemotactic activity in physiological fluids. Requirement for the vitamin D binding protein, thrombospondin-1 and its receptors. Mol Immunol. 2011;49(3):495–503. | ||
von Kanel R, Muller-Hartmannsgruber V, Kokinogenis G, Egloff N. Vitamin D and central hypersensitivity in patients with chronic pain. Pain Med. 2014;15(9):1609–1618. | ||
Knutsen KV, Brekke M, Gjelstad S, Lagerlov P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: a cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand J Prim Health Care. 2010;28(3):166–171. | ||
Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–687. | ||
Jeschonneck M, Grohmann G, Hein G, Sprott H. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology. 2000;39(8):917–921. | ||
Shang Y, Gurley K, Symons B, et al. Noninvasive optical characterization of muscle blood flow, oxygenation, and metabolism in women with fibromyalgia. Arthritis Res Ther. 2012;14(6):R236. | ||
Liu XD, Zeng BF, Xu JG, Zhu HB, Xia QC. Proteomic analysis of the cerebrospinal fluid of patients with lumbar disk herniation. Proteomics. 2006;6(3):1019–1028. |
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.