Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 11
Systematic Review of Kingella kingae Musculoskeletal Infection in Children: Epidemiology, Impact and Management Strategies
Authors Wong M , Williams N , Cooper C
Received 29 May 2019
Accepted for publication 7 December 2019
Published 24 February 2020 Volume 2020:11 Pages 73—84
DOI https://doi.org/10.2147/PHMT.S217475
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Maria Wong, 1 Nicole Williams, 1, 2 Celia Cooper 3
1Department of Orthopaedic Surgery, Women and Children’s Hospital, Adelaide, SA, Australia; 2Center for Orthopaedic and Trauma Research, University of Adelaide, Adelaide, SA, Australia; 3Department of Infectious Diseases, Women and Children’s Hospital, Adelaide, SA, Australia
Correspondence: Nicole Williams
Department of Orthopaedic Surgery, Women and Children’s Hospital, 72 King William Rd, North Adelaide, SA 5006, Australia
Tel +61 8 8161 7223
Fax +61 8 8161 057
Email [email protected]
Abstract: Kingella kingae, a pathogen often responsible for musculoskeletal infections in children is the most common cause of septic arthritis and osteomyelitis in children 6 to 36 months of age. The aim of this study was to perform a systematic review of previous studies to determine the proportion of K. kingae in bacteriologically proven musculoskeletal infections among the pediatric population. A secondary objective was to describe the diagnostic strategies and outcome of patients with musculoskeletal infections caused by K. kingae. A systematic review was conducted to identify publications that report on musculoskeletal infections caused by K. kingae in the pediatric population (patients 0 to < 18 years old with microbiologic culture and/or polymerase chain reaction (PCR) confirmation of K. kingae and a description of the musculoskeletal infection involved). Of 144 studies included in this review, we sought to determine the proportion of K. kingae pediatric musculoskeletal infections. A total of 711 (30.8%) out of 2308 pediatric cases with culture and/or PCR proven musculoskeletal infections had K. kingae successfully identified from twenty-nine studies. Of the 1070 patients who were aged less than 48 months, K. kingae was the organism identified in 47.6% of infections. We found the average age from the collated studies to be 17.73 months. Of 520 pediatric musculoskeletal patients in which K. kingae infections were identified and where the studies reported the sites of infection, a large proportion of cases (65%) were joint infections. This was followed by 18.4% osteoarticular infection (concomitant bone and joint involvement), with isolated bone and spine at 11.9% and 3.5%, respectively. Twenty-one papers reported clinical and laboratory findings in children with confirmed K. kingae infection. The median temperature reported at admission was 37.9°C and mean was 38.2°C. Fourteen studies reported on impact and treatment, with the majority of children experiencing good clinical outcome and function following antibiotic treatment with no serious orthopaedic sequelae.
Keywords: Kingella kingae, osteoarticular infection, septic arthritis, osteomyelitis, spondylodiscitis
Introduction
Since the 1990s there has been increasing recognition of Kingella kingae as an emerging cause of osteoarticular infections in young children. This, coupled with the decline of skeletal infections caused by Haemophilus influenzae due to widespread vaccination has resulted in an increased proportion of musculoskeletal infections in young children caused by K. kingae.1,2
Previously known as Moraxella kingii, K. kingae was first described in the 1960s. It is a beta-hemolytic encapsulated coccobacillus belonging to the Neisseriaceae family of gram-negative bacteria found colonizing the oropharynx of healthy young children.3,4 The pathogen is transmitted by close contact between young children with a recent reported increase in oropharyngeal carriage rates and outbreaks of invasive disease involving the skeletal system in daycare centers.5–8 The colonized oropharyngeal surfaces are the source of droplet transmission and the respiratory mucosa is the gateway for the bacterium into the bloodstream where it disseminates to distant sites. Bone and joint infections including spondylodiscitis are the most commonly reported K. kingae infections in the paediatric population. However, soft tissue infections, occult bacteraemia, endocarditis and rarely lower respiratory tract, meningeal and ocular infections have also been reported in children.5,9–12 Epithelium breach may facilitate invasion of K. kingae into the circulation.13,14 K. kingae invasive infections have been associated with hand-foot-mouth disease, herpes simplex virus causing stomatitis, varicella zoster virus and human rhinovirus.13,15,16
K. kingae exhibits virulence factors which include RTX (repeat-in-toxin), polysaccharide capsule and pili. Pili enable adherence of the organism to the respiratory and synovial epithelium.17 The polysaccharide capsule protects the organism from phagocytosis and the cell-mediated immune system of the host.5 Munoz et al highlighted a novel interplay between the capsule and exopolysaccaride, where the capsule interfered with neutrophil response and the exopolysaccaride promotes evasion of neutrophil killing through complementary mechanisms while also promoting virulence.18 Kehl-Fie et al suggested that K. kingae may damage the epithelial layer of the upper respiratory tract due to the broad spectrum cytotoxicity of RTX that lyses epithelial cells, leukocytes and facilitates upper respiratory colonization and induction of buccal ulcers, enabling survival of the bacterium in the bloodstream and invasion of skeletal tissues. The toxin also damages synoviocytes to allow seeding into joint spaces.19
K. kingae frequently colonises the oropharynx (but not the nasopharynx) of young children from 6–48 months.20 While there are significant variations in reported carriage rates which can be attributed to both host and technical factors, age is a major predictor of carriage in all studied populations.21 Carriage rates greatly exceed rates of invasive disease with <1% of carriers developing invasive disease over a one-year period.22
K. kingae is fastidious, slow growing and exhibits preferred growth at 35°C under aerobic conditions. Growth is enhanced in a 5% CO2 atmosphere.23 It is notoriously difficult to culture from synovial fluid and bone aspirate.11 Recent knowledge has initiated the use of blood culture vials inoculated with synovial fluid or bone aspirate. Inoculation of the synovial fluid into a large volume of nutrient broth dilutes the concentration of harmful factors in the aspirates, thus enhancing the recovery of K. kingae.24 The addition of broad spectrum polymerase chain reaction (PCR) use since the mid-90s in laboratory analysis has increased the diagnosis of musculoskeletal infections caused by K. kingae especially in affluent countries where more advanced laboratory technology is available. Nucleic acid amplification (NAA) assays that target genes encoding the RTX toxin (rtxA and rtxB) are highly sensitive and can be used on multiple clinical specimens. NAA techniques enable the identification of a significant number of additional cases even when using the most sensitive conventional culture techniques.25 Molecular tests that only target the RTX locus do not distinguish between K. kingae and the recently described K. negevensis species.26 A novel K. kingae specific RT-PCR assay that amplify the more specific cpn60 and malate dehydrogenase genes has demonstrated high specificity and sensitivity.27
Over the last decade, K. kingae has gained a reputation as being the major pathogen of osteoarticular infection in children 6–36 months following the optimization of conventional culture methods and the development of current molecular techniques such as real-time PCR.28,29
The aim of this study was to perform a systematic review to determine the proportion of K. kingae in bacteriology proven musculoskeletal infections in the pediatric population. A secondary objective was to describe the clinical findings, diagnostic strategies and outcome of patients with musculoskeletal infections caused by K. kingae.
Methods
Search Strategy
A systematic review was conducted to identify publications that report on musculoskeletal infections caused by K. kingae in the paediatric population (patients 0 to <18 years old with microbiologic culture and/or PCR confirmation of K. kingae and a description of the musculoskeletal infection involved). An exhaustive, comprehensive search without data restriction was conducted up to March 6th 2019 using Ovid Medline, Embase, Cinahl, PubMed and Cochrane Central. Search items included “Kingella kingae” OR “Moraxella kingii” AND “musculoskeletal infection” OR “bone” OR “joint” OR “skeletal” OR “osteoarticular” OR “osteoarthritis” OR “osteomyelitis” OR “spondylodiscitis” OR “tenosynovitis” AND “infant” or “children” or “adolescent” or “pediatrics” or “juvenile” NOT (animals or adults).
Inclusion and Exclusion Criteria
Studies were included in the critical appraisal process if they were written in English and retrievable through the South Australian Health Library Service. The authors used the CoCoPop (condition, context, population) to identify the included articles. Citations selected were included in the final analysis if these following data were available: documentation of musculoskeletal infection in children (septic arthritis, osteomyelitis, osteoarticular, spondylodiscitis, tenosynovitis) and confirmation of K. kingae detection by cultures or molecular methods (PCR test). Studies were excluded if they were of the adult population, literature synthesis or expert reviews. Two reviewers were involved in the article selection process, and full agreement was reached on the included articles.
Critical Appraisal Process
The critical appraisal tool known as the critical appraisal instrument for studies reporting prevalence data from Joanna Briggs Institute was used to review the methodology and summarize the findings of the selected studies.30 A set of nine questions with “yes”, “no” or “unclear” responses are used to guide the reviewer which then leads to the final decision to “include” or “exclude” a study if a minimum score of five out of nine is achieved for each study.
Results
Search Findings
A total of 682 studies were located (Ovid-265, Embase-339, Cochrane Central-3, Cinahl-46, PubMed-27, other-2). All 682 identified citations were loaded into EndNote X9.1.1 (2019) and 299 duplicates removed. Titles and abstracts were screened for assessment against the inclusion criteria for the review and another 239 screened abstracts removed, leaving a final result of 144 studies to be critically appraised independently by two reviewers. These 144 potentially relevant papers were retrieved in full text and assessed in detail against the inclusion criteria by two independent reviewers. Studies were excluded if they were of the adult population, literature synthesis or expert reviews. Any disagreements that arose between the reviewers at each stage of the selection process were resolved through discussion. The results of the search were reported in full in the final report and presented according to the Preferred Reporting Items for Systematic Reviews (PRISMA) flow diagram (Figure 1).31
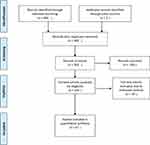 |
Figure 1 Flow chart of the systematic review search result. Notes: Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.31 |
Twenty-nine articles were considered relevant with respect to identifying the number of confirmed infections with K. kingae in bacteriologically proven musculoskeletal infections in children (Table 1).2,22,28,29,32–51,66–70 This was further studied to summarize the frequency of K. kingae infections among children under 48 months old (Table 2). Additional epidemiological data extracted and collated included musculoskeletal infection sites, gender, age and seasonality. Twenty-one articles reported on clinical findings (Table 3)4,7,35,36,39,41,51–64,71 and fourteen articles reported on the impact or outcome as well as management strategies (Table 4)4,25,35,51,52,55–61,65,71 in children who had K. kingae musculoskeletal infection.
 |
Table 1 Summary of Characteristics of Kingella kingae (KK) Musculoskeletal Infection in Children |
 |
Table 2 Fraction of K. kingae (KK) infections among children aged <48 months |
 |
Table 3 Clinical Features of Kingella kingae Musculoskeletal Infection in Children |
 |
Table 4 Impact and Management Strategies of Kingella kingae Musculoskeletal Infection in Children |
Study Characteristics
Epidemiology
To determine the proportion of K. kingae cases, we collected all studies which reported on bacteriologically confirmed (positive culture and/or PCR test) musculoskeletal infection in children, from any organism. Out of 2308 cases of paediatric (below 18 years old) musculoskeletal infections collated from 29 reports (see Table 1), a total of 711 (30.8%) patients had K. kingae successfully identified. These reports were further examined to summarize the frequency of K. kingae infections among children aged below 48 months (Table 2). We found 47.6% of 1070 children under 48 months old were diagnosed with K. kingella infections. Moumile et al reported 14% K. kingae in a retrospective study of a French cohort of diagnosed bacterial osteoarticular infection in children (n=74). The authors noted Staphylococcus aureus was the most isolated bacterium among older children who were over 36 months whereas K. kingae was isolated only from children under 36 months old.49 In a separate French paediatric centre, Verdier et al reported 14% K. kingae osteoarticular infection, with Staphylococcus aureus (47%) and Streptococcus pyogenes (16%) in children whose age ranges from 9–90 months with a median of 17.9 months. The authors also confirmed the classical occurrence of osteoarticular infection due to K. kingae in children between 6 months and 4 years of age.4,28,57,72 More recent studies using both conventional culture and PCR combined showed K. kingae to be the most common cause of osteoarticular infection in children <4 years of age. We found the average age from the collated studies to be 17.73 months.
The sites of musculoskeletal infections included joint and/or bone, spine and tenosynovitis. Of 520 pediatric musculoskeletal patients in which K. kingae infections were identified and where the studies reported the sites of infection, a large proportion of reported cases (65%) involved joint infections. This was followed by 18.4% osteoarticular infection (concomitant bone and joint involvement), with isolated bone and spine at 11.9% and 3.5%, respectively. French studies have reported K. kingae as the most common pathogen in septic arthritis (Chometon et al 45%, Ilharreborde et al 52% and Aupiais et al 69%) in young children.2,29,39
Most studies which identified gender reported a male preponderance (60.5%). A large proportion of K. kingae patients were also diagnosed in the autumn to winter season. Dubnov-Raz et al confirmed previous observations of a consistent seasonal pattern, characterized by higher incidences between May and January with a nadir between February and April in the northern hemisphere.4 As respiratory epithelial carriage of K. kingae is fairly stable throughout the year, the seasonal variation is likely to reflect an association with upper respiratory viral infections and stomatitis, allowing for passage of the colonizing K. kingae through the breached epithelium.
Management Strategies and Impact
Out of a collection of twenty-one papers which reported on clinical and laboratory findings (Table 3), a total of 569 paediatric patients with musculoskeletal infections caused by K. kingae were identified. The median temperature reported at admission was 37.9°C and mean temperature was 38.2°C. The range of temperature for patients on admission ranged between 36.2°C and 40.0°C. A trend could not be identified in the white blood cell count, C-reactive protein test (CRP) and the erythrocyte sedimentation rate (ESR) diagnostic tests of patients upon admission; however, some studies based on patients from France and Israel generally reported a milder clinical picture with normal to mildly raised inflammatory markers.4,35,71
Fourteen papers, including 382 pediatric patients reported the duration of antibiotic treatment and the outcome of treatment in children with K. kingae infections (Table 4). The antibiotic treatment ranged from 7 to 21 days both for median and mean for both intravenous (IV) and oral treatments. IV antibiotics were administered between 7 to 14 days whereas oral antibiotics between 14 and 21 days. Most of the studies report good clinical and functional outcome following antibiotic treatment with no serious orthopaedic sequelae.
Discussion
The increasing knowledge surrounding musculoskeletal infection caused by K. kingae in paediatric patients reflects increasing awareness of the organism and ability to isolate it in the laboratory. Studies published mainly from Western Europe, North America as well as Australia have placed the bacterium as a major pathogen causing osteoarticular infection in healthy children below the age of 4 years.2,4 K. kingae is the leading pathogen for septic arthritis in this age group as reported in Europe.2,29,39,71 Soft tissue infection caused by K. kingae has also been reported, including a Swedish report of a fluctuant mass anterior to tendon Achilles insertion and 6 cases of pyogenic hand tenosynovitis from Switzerland.9,47,73 Three centers from France, Israel and Switzerland collaborated in 2018 to report on ten children (ages 6–22 months, mean 12.1) with K. kingae hand and wrist tenosynovitis. The authors recommended children aged below 48 months with at least one Kanavel sign, a recent viral infection and no prior penetrating hand injury should be highly suspected for K. kingae tenosynovitis. Seven children were confirmed by culture or PCR of the synovial fluid or blood, while three were presumed infected because of positive oropharyngeal PCR.73 The authors suggested that the index of presumptive diagnosis for K. kingae infection in non-operated cases were high if the pathogen is detected in the oropharyngeal swabs by PCR. This was earlier reported (Ceroni et al, 2013) that detection of K. kingae in the oropharynx of ill children has a positive predictive value of 91% for K. kingae infection.38
K. kingae is a recognized commensal in the oropharynx of young children particularly in ages 6 to 48 months, which is considered a prerequisite for invasive disease which is commonly triggered by viral infections.74 Children aged less than 6 months benefited from maternal protective antibodies; whereas at 6–48 months, susceptibility is due to relative immaturity of the immune system particularly with respect to the development of antibodies directed against the polysaccharide capsule.8 This observation also indicates that isolation of K. kingae from a normally sterile site in older children should alert the clinician to the possibility of underlying immunodeficiency or endocarditis indicating the need for cardiologic evaluation.75
Yagupsky et al stressed the importance of blood cultures in a young child with unexplained skeletal system complaints even in the absence of fever or constitutional symptoms in areas of high prevalence of K. kingae infections.76 Increasing awareness regarding this elusive emerging pathogen has led Lebel et al adopt a more aggressive approach in an attempt to aspirate and culture all suspected joint fluid in this age group.63
In general, K. kingae musculoskeletal infections are reported to present with a milder clinical picture as compared to typical pathogens which present with high fever, elevated leukocyte count and inflammatory markers.35,71 Ceroni et al in their attempt to identify predictors for K. kingae osteoarticular infection when compared to typical pathogens found that 13 out of 30 (43.3%) of proven K. kingae cases remained afebrile with normal CRP and only two (15.4%) had an elevated leukocyte count. The authors concluded that a normal body temperature at admission, a normal WBC count and CRP do not exclude the diagnosis of K. kingae.35 This is similar to Dubnov-Raz et al who reported that children frequently presented with body temperature of <38°C and lacked objective signs of osteoarthritis and leukocytosis.4 Chometon et al reported only 33.3% of K. kingae skeletal infection cases had fever on admission.39 This current review found both mean and median temperature at presentation at 38.2°C and 37.9°C, respectively.
Experienced centers stress the importance of sampling and inoculation of aspirates into blood culture vials, ie, BACTEC blood culture system before transport to the laboratory. Successful isolation of K. kingae from bone and joint aspirates is strongly dependent on bacteriologic methodology used and PCR identifies multiple cases of K. kingae infection in “culture negative” cases.24,57 K. kingae is reportedly susceptible to most antibiotics including penicillin and cephalosporin, with resistance to vancomycin, clindamycin and fusidic acid.61 It is interesting to note there were four children who had K. kingae proven osteoarticular infection and recovered completely despite never receiving antibiotics.63 There were also two irritable hip cases with K. kingae isolated from blood but were self-limiting, without antibiotic treatment.64
This raises the question about the implications of misdiagnosing a suppurative joint infection caused by K. kingae since it appears to be self-limiting in certain cases. Some authors have even questioned the need to treat K. kingae due to these self-limiting reports, and it has been suggested that invasive procedures are not necessary. In contrast, there have been reported cases of severe infections which involved abscess formation, growth plate damage and valgus deformity of lower limbs. Narrowed disc spaces in the spine were reported but without any functional or clinical morbidity.24,55,77 In another report, five children had more than one presentation to emergency department while one child required two arthroscopic knee washouts in order to gain control of the infection. These reports suggest that infection with K. kingae should not be generally considered benign or self-limiting.51 ß-lactamase production is a survival strategy shared by many pathogens of respiratory origin in order to colonize the human mucosal surface. Common pathogens of the upper respiratory tract, ie, Staphylococcus aureus, Haemophilus influenzae are repeatedly exposed to antimicrobials resulting in high rates of therapy resistance. K. kingae which is usually susceptible to common antibiotics ß-lactamase production and spread has been detected in the USA since 1993, Iceland since 1995 and Israel since 2001.78 Production of ß-lactamase has important therapeutic implications as this may cause severe skeletal system morbidity and fatal endocarditis.
Despite the recognition of self-limiting cases, reports of significant complications would indicate that at a minimum, appropriate antibiotics should be administered to patients when the organism is recovered from a normally sterile body site. Patients with K. kingae isolated from blood should be immediately suspected of bacterial endocarditis and treated at once with antibiotics. Septicaemia with endocarditis is the most severe form of infection caused by K. kingae with reported fatalities.4,79
K. kingae is resistant to clindamycin, fusidic acid and vancomycin, which are used in the treatment of osteomyelitis and MRSA related infections.61 Hence, the further relevance of identifying K. kingae in order to commence an appropriate antibiotic.
A limitation of this current review is that most data were sourced from affluent countries with access to routine PCR investigation. Microbiological confirmation of skeletal infection enables early diagnosis, identification of the pathogen and antibiotic susceptibility to achieve a good outcome. Molecular technology has reduced the number of false-negative cases in cases of transient synovitis and reactive arthritis which are frequently treated at home with rest and analgesia, usually without antibiotics.64 A high index of suspicion is required for K. kingae infection in children 6–48 months with suggestive clinical features and aspirate samples should be inoculated and transported in blood culture vials if PCR is not available. Some authors suggest total antibiotic duration 2–3 weeks for septic arthritis, 3–6 weeks for osteomyelitis and 3–12 weeks for spondylodiscitis in cases where K. kingae is isolated.72,80 These were accumulated experiences based on collection of individual case reports or case series with several expert reviews regarding K. kingae. Experience from other parts of the world is required to examine the changing epidemiology of this musculoskeletal pathogen to complete a worldwide prevalence picture. Treatment strategies lack solid recommendations, duration of treatment is not well defined and all these should be studied in larger prospective studies.
Conclusion
The practical implication of this study is that a high index of suspicion is needed due to the subtle presentation of K. kingae musculoskeletal infection in young children below 48 months. Increased clinician awareness of this age group, enhanced laboratory protocols, and communication between clinicians and laboratory staff is critical to achieve a better understanding of the true burden of musculoskeletal infections caused by K. kingae. The need for appropriate culture media that can detect K. kingae namely blood culture bottles and the availability of molecular testing cannot be overemphasized. Failure to take this approach may lead to inappropriate diagnosis of culture-negative arthritis in cases of K. kingae infection.
Acknowledgments
The authors thank Natalie Dempster for assistance with the database search.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yagupsky P, Dagan R. Kingella kingae: an emerging cause of invasive infections in young children. Clini Infect Dis. 1997;24(5):860–866. doi:10.1093/clinids/24.5.860
2. Aupiais C, Ilharreborde B, Doit C, et al. Aetiology of arthritis in hospitalised children: an observational study. Arch Dis Child. 2015;100(8):742–747. doi:10.1136/archdischild-2014-307490
3. Yagupsky P, Bar-Ziv Y, Howard CB, Dagan R. Epidemiology, etiology, and clinical features of septic arthritis in children younger than 24 months. Archiv Pediatr Adolesc Med. 1995;149(5):537–540. doi:10.1001/archpedi.1995.02170180067010
4. Dubnov-Raz G, Ephros M, Garty BZ, et al. Invasive pediatric Kingella kingae infections: a nationwide collaborative study. Pediatr Infect Dis J. 2010;29(7):639–643. doi:10.1097/INF.0b013e3181d57a6c
5. Yagupsky P. Kingella kingae: carriage, transmission, and disease. Clin Microbiol Rev. 2015;28(1):54–79. doi:10.1128/CMR.00028-14
6. Yagupsky P, Ben-Ami Y, Trefler R, Porat N. Outbreaks of invasive Kingella kingae infections in closed communities. J Pediatr. 2016;169:135–139e131. doi:10.1016/j.jpeds.2015.10.025
7. El Houmami N, Minodier P, Dubourg G, et al. Patterns of Kingella kingae disease outbreaks. Pediatr Infect Dis J. 2016;35(3):340–346. doi:10.1097/INF.0000000000001010
8. Valaikaite R, El Houmami N, Spyropoulou V, Braendle G, Ceroni D. Kingella kingae: from oropharyngeal carriage to paediatric osteoarticular infections. Expert Rev Anti Infect Ther. 2018;16(2):85–87. doi:10.1080/14787210.2018.1421944
9. Claesson B, Falsen E, Kjellman B. Kingella kingae infections: a review and a presentation of data from 10 Swedish cases. Scand J Infect Dis. 1985;17(3):233–243. doi:10.3109/inf.1985.17.issue-3.01
10. de Groot R, Glover D, Clausen C, Smith AL, Wilson CB. Bone and joint infections caused by Kingella kingae: six cases and review of the literature. Rev Infect Dis. 1988;10(5):998–1004. doi:10.1093/clinids/10.5.998
11. Yagupsky P, Dagan R, Howard CB, Einhorn M, Kassis I, Simu A. Clinical features and epidemiology of invasive Kingella kingae infections in southern Israel. Pediatrics. 1993;92(6):800–804.
12. Goutzmanis JJ, Gonis G, Gilbert GL. Kingella kingae infection in children: ten cases and a review of the literature. Pediatr Infect Dis J. 1991;10(9):677–683. doi:10.1097/00006454-199109000-00011
13. Amir J, Yagupsky P. Invasive Kingella kingae infection associated with stomatitis in children. Pediatr Infect Dis J. 1998;17(8):757–758. doi:10.1097/00006454-199808000-00021
14. El Houmami N, Mirand A, Dubourg G, et al. Hand, foot and mouth disease and Kingella kingae infections. Pediatr Infect Dis J. 2015;34(5):547–548. doi:10.1097/INF.0000000000000607
15. Yagupsky P, Press J. Arthritis following stomatitis in a sixteen-month-old child. Pediatr Infect Dis J. 2003;22(6):
16. Bidet P, Collin E, Basmaci R, et al. Investigation of an outbreak of osteoarticular infections caused by Kingella kingae in a childcare center using molecular techniques. Pediatr Infect Dis J. 2013;23:558–560.
17. Kehl-Fie TE, Miller SE, St Geme JW
18. Munoz VL, Porsch EA, St Geme JW
19. Kehl-Fie TE, St Geme JW
20. Yagupsky P, Dagan R, Prajgrod F, Merires M. Respiratory carriage of Kingella kingae among healthy children. Pediatr Infect Dis J. 1995;14(8):673–678. doi:10.1097/00006454-199508000-00005
21. Amit U, Flaishmakher S, Dagan R, Porat N, Yagupsky P. Age-dependent carriage of Kingella kingae in young children and turnover of colonizing strains. J Pediatric Infect Dis Soc. 2014;3(2):160–162. doi:10.1093/jpids/pit003
22. Ceroni D, Dubois-Ferriere V, Anderson R, et al. Small risk of osteoarticular infections in children with asymptomatic oropharyngeal carriage of Kingella kingae. Pediatr Infect Dis J. 2012;31(9):983–985. doi:10.1097/INF.0b013e31825d3419
23. <Chapter 35. Aggregatibacter, Capnocytophaga, Eikenella, Kingella, Pasteurella, and Other Fastidious or Rarely Encountered Gram-Negative Rods_.pdf>.
24. Yagupsky P, Dagan R, Howard CW, Einhorn M, Kassis I, Simu A. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J Clin Microbiol. 1992;30(5):1278–1281.
25. Cherkaoui A, Ceroni D, Emonet S, Lefevre Y, Schrenzel J. Molecular diagnosis of Kingella kingae osteoarticular infections by specific real-time PCR assay. J Med Microbiol. 2009;58(Pt 1):65–68. doi:10.1099/jmm.0.47707-0
26. El Houmami N, Bzdreng J, Durand GA, et al. Molecular tests that target the RTX locus do not distinguish between Kingella kingae and the recently described Kingella negevensis species. J Clin Microbiol. 2017;55(10):3113–3122. doi:10.1128/JCM.00736-17
27. El Houmami N, Durand GA, Bzdrenga J, et al. A new highly sensitive and specific real-time PCR assay targeting the malate dehydrogenase gene of Kingella kingae and application to 201 pediatric clinical specimens. J Clin Microbiol. 2018;56(8). doi:10.1128/JCM.00505-18
28. Ceroni D, Cherkaoui A, Ferey S, Kaelin A, Schrenzel J. Kingella kingae osteoarticular infections in young children: clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J Pediatr Orthop. 2010;30(3):301–304. doi:10.1097/BPO.0b013e3181d4732f
29. Ilharreborde B, Bidet P, Lorrot M, et al. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J Clin Microbiol. 2009;47(6):1837–1841. doi:10.1128/JCM.00144-09
30. Munn ZMS, Lisy K, Riitano D, Tufanara C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Based Healthc. 2015. doi:10.1097/XEB.0000000000000054
31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
32. Alcobendas R, Remesal A, Murias S, Nunez E, Calvo C. Outpatients with acute osteoarticular infections had favourable outcomes when they received just oral antibiotics without intravenous antibiotics. Acta Paediatr. 2018;107(10):1792–1797. doi:10.1111/apa.2018.107.issue-10
33. Calvo C, Nunez E, Camacho M, et al. Epidemiology and management of acute, uncomplicated septic arthritis and osteomyelitis spanish multicenter study. Pediatr Infect Dis J. 2016;35(12):1288–1293. doi:10.1097/INF.0000000000001309
34. Carter K, Doern C, Chan-Hee J, Copley LAB, Jo C-H. The clinical usefulness of polymerase chain reaction as a supplemental diagnostic tool in the evaluation and the treatment of children with septic arthritis. J Pediatr Orthop. 2016;36(2):167–172. doi:10.1097/BPO.0000000000000411
35. Ceroni D, Cherkaoui A, Combescure C, Francois P, Kaelin A, Schrenzel J. Differentiating osteoarticular infections caused by Kingella kingae from those due to typical pathogens in young children. Pediatr Infect Dis J. 2011;30(10):906–909. doi:10.1097/INF.0b013e31821c3aee
36. Ceroni D, Belaieff W, Kanavaki A, et al. Possible association of Kingella kingae with infantile spondylodiscitis. Pediatr Infect Dis J. 2013;32(11):1296–1298. doi:10.1097/INF.0b013e3182a6df50
37. Ceroni D, Belaieff W, Cherkaoui A, et al. Primary epiphyseal or apophyseal subacute osteomyelitis in the pediatric population: a report of fourteen cases and a systematic review of the literature. J Bone Joint Surg. 2014;96(18):1570–1575. doi:10.2106/JBJS.M.00791
38. Ceroni D, Dubois-Ferriere V, Cherkaoui A, et al. Detection of Kingella kingae osteoarticular infections in children by oropharyngeal swab PCR. Pediatrics. 2013;131(1):e230–e235. doi:10.1542/peds.2012-0810
39. Chometon S, Benito Y, Chaker M, et al. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J. 2007;26(5):377–381. doi:10.1097/01.inf.0000259954.88139.f4
40. Dayer R, Alzahrani MM, Saran N, et al. Spinal infections in children. Bone Joint J. 2018;100-B(4):542–548. doi:10.1302/0301-620X.100B4.BJJ-2017-1080.R1
41. Ferroni A, Al Khoury H, Dana C, et al. Prospective survey of acute osteoarticular infections in a French paediatric orthopedic surgery unit. Clin Microbiol Infect. 2012. doi:10.1111/clm.12031
42. Gene Giralt A, Ludwig Sanz-Orrio G, Munoz-Almagro C, Noguera-Julian A. Osteoarticular infections in pediatric patients: the aetiological importance of Kingella kingae. Enferm Infecc Microbiol Clin. 2019;37(3):209–210. doi:10.1016/j.eimc.2018.03.014
43. Gravel J, Ceroni D, Lacroix L, et al. Association between oropharyngeal carriage of Kingella kingae and osteoarticular infection in young children: a case-control study. Cmaj. 2017;189(35):. doi:10.1503/cmaj.170127
44. Hernandez-Ruperez MB, Suarez-Arrabal MDC, Villa-Garcia A, et al. Kingella kingae as the main cause of septic arthritis: importance of molecular diagnosis. Pediatr Infect Dis J. 2018;37(12):1211–1216. doi:10.1097/INF.0000000000002068
45. Juchler C, Spyropoulou V, Wagner N, et al. The contemporary bacteriologic epidemiology of osteoarticular infections in children in Switzerland. J Pediatr. 2018;194:190–196.e191. doi:10.1016/j.jpeds.2017.11.025
46. Kanavaki A, Ceroni D, Tchernin D, Hanquinet S, Merlini L. Can early MRI distinguish between Kingella kingae and Gram-positive cocci in osteoarticular infections in young children? Pediatr Radiol. 2012;42(1):57–62. doi:10.1007/s00247-011-2220-2
47. Lironi C, Steiger C, Juchler C, Spyropoulou V, Samara E, Ceroni D. Pyogenic tenosynovitis in infants: a case series. Pediatr Infect Dis J. 2017;36(11):1097–1099. doi:10.1097/INF.0000000000001673
48. Lundy DW, Kehl DK. Increasing prevalence of Kingella kingae in osteoarticular infections in young children. J Pediatr Orthop. 1998;18(2):262–267. doi:10.1097/01241398-199803000-00025
49. Moumile K, Merckx J, Glorion C, Pouliquen JC, Berche P, Ferroni A. Bacterial aetiology of acute osteoarticular infections in children. Acta Paediatr. 2005;94(4):419–422. doi:10.1080/08035250410023278
50. Nguyen JC, Rebsamen SL, Tuite MJ, Davis JM, Rosas HG. Imaging of Kingella kingae musculoskeletal infections in children: a series of 5 cases. Emerg. 2018;16:16.
51. Williams N, Cooper C, Cundy P. Kingella kingae septic arthritis in children: recognising an elusive pathogen. J Child Orthop. 2014;8(1):91–95. doi:10.1007/s11832-014-0549-4
52. Garron E, Viehweger E, Launay F, Guillaume JM, Jouve JL, Bollini G. Nontuberculous spondylodiscitis in children. J Pediatr Orthop. 2002;22(3):321–328. doi:10.1097/01241398-200205000-00010
53. Gene A, Garcia-Garcia JJ, Sala P, Sierra M, Huguet R. Enhanced culture detection of Kingella kingae, a pathogen of increasing clinical importance in pediatrics. Pediatr Infect Dis J. 2004;23(9):886–888. doi:10.1097/01.inf.0000137591.76624.82
54. Luegmair M, Chaker M, Ploton C, Berard J. Kingella kingae: osteoarticular infections of the sternum in children: a report of six cases. J Child Orthop. 2008;2(6):443–447. doi:10.1007/s11832-008-0144-7
55. Mallet C, Ceroni D, Litzelmann E, et al. Unusually severe cases of Kingella kingae osteoarticular infections in children. Pediatr Infect Dis J. 2014;33(1):1–4. doi:10.1097/INF.0b013e3182a22cc6
56. Moumile K, Merckx J, Glorion C, Berche P, Ferroni A. Osteoarticular infections caused by Kingella kingae in children: contribution of polymerase chain reaction to the microbiologic diagnosis. Pediatr Infect Dis J. 2003;22(9):837–839. doi:10.1097/01.inf.0000083848.93457.e7
57. Verdier I, Gayet-Ageron A, Ploton C, et al. Contribution of a broad range polymerase chain reaction to the diagnosis of osteoarticular infections caused by Kingella kingae: description of twenty-four recent pediatric diagnoses. Pediatr Infect Dis J. 2005;24(8):692–696. doi:10.1097/01.inf.0000172153.10569.dc
58. Basmaci R, Ilharreborde B, Doit C, et al. Two atypical cases of Kingella kingae invasive infection with concomitant human rhinovirus infection. J Clin Microbiol. 2013;51(9):3137–3139. doi:10.1128/JCM.01134-13
59. Moylett EH, Rossmann SN, Epps HR, Demmler GJ. Importance of Kingella kingae as a pediatric pathogen in the United States. Pediatr Infect Dis J. 2000;19(3):263–265. doi:10.1097/00006454-200003000-00023
60. Birgisson H, Steingrimsson O, Gudnason T. Kingella kingae infections in paediatric patients: 5 cases of septic arthritis, osteomyelitis and bacteraemia. Scand J Infect Dis. 1997;29(5):495–498. doi:10.3109/00365549709011861
61. Dubnov-Raz G, Scheuerman O, Chodick G, Finkelstein Y, Samra Z, Garty B. Invasive Kingella kingae infections in children: clinical and laboratory characteristics. Pediatrics. 2008;122(6):1305–1309. doi:10.1542/peds.2007-3070
62. Gamble JG, Rinsky LA. Kingella kingae infection in healthy children. J Pediatr Orthop. 1988;8(4):445–449. doi:10.1097/01241398-198807000-00012
63. Lebel E, Rudensky B, Karasik M, Itzchaki M, Schlesinger Y. Kingella kingae infections in children. J Pediatr Orthop B. 2006;15(4):289–292. doi:10.1097/01202412-200607000-00011
64. Yagupsky P, Dubnov-Raz G, Gene A, Ephros M, Israeli-Spanish Kingella kingae Research G. Differentiating Kingella kingae septic arthritis of the hip from transient synovitis in young children. J Pediatr. 2014;165(5):985–989.e981. doi:10.1016/j.jpeds.2014.07.060
65. Dodman T, Robson J, Pincus D. Kingella kingae infections in children. J Paediatr Child Health. 2000;36(1):87–90. doi:10.1046/j.1440-1754.2000.00447.x
66. Nielsen AB, Nygaard U, Hoffmann T, Kristensen K. Short individualised treatment of bone and joint infections in Danish children. Arch Dis Child. 2019;104(2):205–206. doi:10.1136/archdischild-2018-315734
67. O’Rourke S, Meehan M, Bennett D, et al. The role of real-time PCR testing in the investigation of paediatric patients with community-onset osteomyelitis and septic arthritis. Ir J Med Sci. 2019;31:31.
68. Rasmont Q, Yombi JC, Van der Linden D, Docquier PL. Osteoarticular infections in Belgian children: a survey of clinical, biological, radiological and microbiological data. Acta Orthop Belg. 2008;74(3):374–385.
69. Slinger R, Moldovan I, Bowes J, Chan F. Polymerase chain reaction detection of Kingella kingae in children with culture-negative septic arthritis in eastern Ontario. Paediatrics & Child Health. 2016;21(2):79–82. doi:10.1093/pch/21.2.79
70. Spyropoulou V, Dhouib Chargui A, Merlini L, et al. Primary subacute hematogenous osteomyelitis in children: a clearer bacteriological etiology. J Child Orthop. 2016;10(3):241–246. doi:10.1007/s11832-016-0739-3
71. Basmaci R, Lorrot M, Bidet P, et al. Comparison of clinical and biologic features of Kingella kingae and Staphylococcus aureus arthritis at initial evaluation. Pediatr Infect Dis J. 2011;30(10):902–904. doi:10.1097/INF.0b013e31821fe0f7
72. Yagupsky P, Porsch E, St Geme JW. Kingella kingae: an emerging pathogen in young children. Pediatrics. 2011;127(3):557–565. doi:10.1542/peds.2010-1867
73. El Houmami N, Yagupsky P, Ceroni D. Kingella kingae hand and wrist tenosynovitis in young children. J Hand Surg Eur Vol. 2018;43(9):1001–1004. doi:10.1177/1753193418764818
74. El Houmami N, Minodier P, Dubourg G, et al. An outbreak of Kingella kingae infections associated with hand, foot and mouth disease/herpangina virus outbreak in Marseille, France, 2013. Pediatr Infect Dis J. 2015;34(3):246–250. doi:10.1097/INF.0000000000000572
75. Yagupsky P. Diagnosing Kingella kingae infections in infants and young children. Expert Rev Anti Infect Ther. 2017;15(10):925–934. doi:10.1080/14787210.2017.1381557
76. Yagupsky P, Press J. Unsuspected Kingella kingae infections in afebrile children with mild skeletal symptoms: the importance of blood cultures. Eur J Pediatr. 2004;163(9):563–564. doi:10.1007/s00431-004-1496-8
77. Lacour M, Duarte M, Beutler A, Auckenthaler R, Suter S. Osteoarticular infections due to Kingella kingae in children. Eur J Pediatr. 1991;150(9):612–618. doi:10.1007/BF02072618
78. Basmaci R, Bonacorsi S, Bidet P, et al. Genotyping, local prevalence and international dissemination of beta-lactamase-producing Kingella kingae strains. Clin Microbiol Infect. 2014;20(11):O811–O817. doi:10.1111/1469-0691.12648
79. Verbruggen AM, Hauglustaine D, Schildermans F, et al. Infections caused by Kingella kingae: report of four cases and review. J Infect. 1986;13(2):133–142. doi:10.1016/S0163-4453(86)92841-0
80. Yagupsky P. Kingella kingae infections of the skeletal system in children: diagnosis and therapy. Expert Rev Anti Infect Ther. 2004;2(5):787–794. doi:10.1586/14789072.2.5.787
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
