Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Systematic literature review on patterns of pharmacological treatment and adherence among patients with bipolar disorder type I in the USA
Authors Greene M , Paladini L , Lemmer T, Piedade A, Touya M, Clark O
Received 27 February 2018
Accepted for publication 11 May 2018
Published 14 June 2018 Volume 2018:14 Pages 1545—1559
DOI https://doi.org/10.2147/NDT.S166730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Mallik Greene,1 Luciano Paladini,2 Teresa Lemmer,2 Alexandra Piedade,2 Maelys Touya,3 Otavio Clark2
1Health Economics & Outcomes Research, Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ, USA; 2Evidências – Kantar Health, Campinas, Brazil; 3Lundbeck Pharmaceuticals Services, LLC, Deerfield, IL, USA
Background: Bipolar disorder type I (BD-I) is a chronic condition characterized by mania episodes followed by syndromic recovery periods, usually permeated by depressive symptomatology and recurring acute manic episodes. It requires long-term pharmacological treatment; thus, it is critical to understand the patterns of drug therapy use and medication compliance to better plan health care policies and needs. This systematic literature review aims to study these data among patients with BD-I in the USA, focusing on medications to treat mania.
Methods: Articles published in the last 10 years to October 2016 were searched on MEDLINE and Embase. Studies on patterns of drug therapy, concordance of prescription with clinical practice guidelines, and adherence and persistence with pharmacological treatments for BD-I in the USA under observational conditions, with focus on treatments for mania, were selected.
Results: Treatment prevalence for BD-I is low in the USA, with the most current study showing a 46% 12-month rate. There is a lack of studies addressing the use of long-acting injectable (LAI) antipsychotics. Second-generation antipsychotics (SGAs) have been used by nearly all patients receiving oral antipsychotics since the 2000s. However, 30%–60% of individuals with BD do not receive appropriate treatment, and adherence to oral therapies is poor, with medication possession ratios ≥80% seen in only approximately 60% of patients. For persistence rates, results suggest that treatment duration is short for a condition with recommendation for at least 6 months of maintenance therapy. Literature indicates that LAI SGAs may be related to better adherence and persistence.
Conclusion: There is a need for studies addressing specifically patterns of therapy and adherence to pharmacological treatment in BD-I patients in the USA to better understand the value of current standards, and an urgent need to improve the rates of adherence and persistence to BD-I pharmacotherapy and to increase the understanding of LAI SGAs’ potential to address this issue.
Keywords: polypharmacy, clinical practice guidelines, oral treatment, bipolar mania, treatment patterns, persistence
Background
Bipolar disorder (BD) is a chronic condition defined by the type of mood episodes that patients may present (mania, hypomania, and/or depression).1 BD type I (BD-I) is defined by the presence of at least one manic episode, although major depression and subthreshold depressive symptoms often present as well.1,2 A large study assessing surveys conducted between 2001 and 2007, and covering 11 countries in the Americas, Europe, and Asia, found a global lifetime prevalence of 0.6% for BD-I in adults (in the survey conducted in the USA, the lifetime prevalence of BD-I was reported as 1%).3 As BD-I is a highly prevalent and disabling condition, clinical development has brought a number of different pharmacological agents into the market, particularly atypical antipsychotics in the early 2000s.4
The goals of the treatment in patients with BD-I are to provide rapid control of symptoms of mania, in the acute phase, hence enabling patients to return to their previous level of psychosocial functioning, and then to prevent relapse and recurrence of mood episodes in the maintenance phase. Clinical practice guidelines recommend long-term pharmacological management for patients with BD-I, even after achieving symptomatic remission,5–8 with the most current USA guideline recommending maintenance therapy for at least 6 months.9
One of the greatest challenges faced when dealing with chronic illnesses and prescription of long-term therapies is treatment adherence, which is usually described as being poor among these patients in the literature.10 For serious mental illness, mean rates of treatment adherence have been reported as 40%–60%, although there are large variations in these figures found in the available research.11,12
Since drug therapy patterns and patient compliance to pharmacological treatment may differ among countries, this systematic literature review aimed to study these two objectives among patients with BD-I in the USA, with focus on medications to treat mania. The scenario of a given country or global area is valuable for multiple purposes, from helping to forecast the clinical benefit and predict the economic impact expected to accompany the introduction of new health technologies to identifying gaps in health care policies and drive further research.
Methods
Search strategy and eligibility criteria
The search strategy was designed to answer the objective of this systematic literature review on patterns of drug therapy, concordance of prescription patterns with clinical practice guidelines, and adherence and persistence with pharmacological treatments for BD-I in the USA under observational, naturalistic conditions. Studies including patients with BDs as a whole but describing the use of agents to treat mania were also eligible. Randomized controlled trials, naturalistic studies in which the intervention was determined by the study protocol, and studies addressing interventions for improving adherence to treatment (leading to “nonnatural” adherence patterns), safety, quality of life, heterogeneity of treatment effects, burden of disease, and cost of illness were excluded. Treatments considered were pharmacological therapies involving at least one agent to treat mania (antipsychotics, typical or atypical; and/or mood stabilizers [MSs], such as lithium and anticonvulsants). Studies should report data specific to the USA population. Search for studies was limited to those published in the last 10 years, in order to obtain data better reflecting the current standards of care in BD-I. For studies dealing with patterns of treatment, only those reporting data on patients treated since the year 2000 were addressed (corresponding to the date of approval of olanzapine as the first second-generation antipsychotic [SGA] approved for BD by the US Food and Drug Administration [FDA]). Studies with sample size <100 patients were considered not representative and were excluded.
Scientific electronic databases were searched and included MEDLINE via PubMed and Embase. The search strings used for each database are detailed in Table 1.
  | Table 1 Search terms used |
Study selection
All references were obtained from each database, and duplicates were removed. Study selection was conducted in two stages: initial screening of titles and abstracts consistent with the inclusion criteria was followed by screening of the full papers or posters identified in the initial screening. The study selection was performed by one reviewer. Search results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.13
Results
A total of 28 studies were included, as shown in the PRISMA flow diagram in Figure 1.
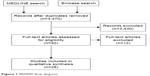  | Figure 1 PRISMA flow diagram. |
The main reasons for exclusion were (number of studies): study setting other than the USA (n=5); no data specific for BD (no results reported for BD or results of BD/schizophrenia/major depressive disorder data were pooled) (n=3); no data specific for MSs and/or antipsychotics (eg, studies reporting data on antidepressants and MS/antipsychotic together) (n=2); conference abstracts (detailed methodology not available) (n=1); and studies addressing “nonnatural” adherence (eg, studies addressing interventions/algorithms to improve adherence) (n=1).
Characteristics of the studies retrieved
Regarding the source of data reported, most studies analyzed administrative/claims data.14–31 Other sources of data used were population surveys32–36 and electronic health/medical records.37–41
Among included studies, most presented data specific for patients with BD-I15,16,18–20,23,24,28,35,36,39 or data for BD patients as a whole but in a sample of predominantly BD-I enrolled,14,33,37,40 while others described data for BD patients without detailing the proportions of patients with BD-I or BD-II within the sample.17,21,22,25–27,29–32,34,38,41
Nationally USA representative populations were included in all studies but four. These four included patients from one specific geographic area in the USA,22 or patients discharged from only one hospital,38 assessed care provided solely by academic medical centers,37 or did not report data allowing us to conclude about the generalizability of the results.17
Study methodology and results are described in Tables 2 and 3, and the main results are summarized in Table 4.
Studies reporting treatment prevalence
A national population-based survey conducted in the years 2001–2003 reported a 12-month treatment rate of 67.3% in patients with BD-I, while for patients with BD as a whole (BD-I, BD-II, or subthreshold BD) this rate was 50.7%.36 A contemporary registry of patients participant of the Veterans Affairs (VA) system found a similar 12-month treatment rate in patients with BD in the fiscal year 2003, with 44.6% receiving antipsychotic medication, but no data specific for BD-I were reported.41
More current data were provided by another population survey interviewing 36,309 adults in the years 2012–2013, and defining BD-I according to the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5). This study described a 12-month treatment rate of 46% and a lifetime treatment prevalence of 72.4%.35
Patterns of pharmacological treatment: polypharmacy rates
Pharmacological treatment for BD-I is usually started as monotherapy, according to the most recent studies addressing this issue (ranging from 88.7% in a VA claims database study16 to a 96.2% rate observed in a mix of commercially insured, Medicare, and Medicaid patients).20 However, following up to one year after the index treatment, most patients (up to 70%) were receiving polytherapy, both among commercially insured and among Medicare patients.14,15 Another study corroborating this finding reported that, among Medicaid patients with BD-I receiving antipsychotics, 68.6% received concomitant MSs or anticonvulsants.28 Thus, polytherapy is the most common pattern in prevalent patients with BD-I, with a mean of approximately 2.5 psychotropic medications per patient.33,39
Patterns of pharmacological treatment: medications most commonly used
All the included studies focused on treatment with oral medications, with no study addressing usage of long-acting injectable (LAI) for BD.
First intentional therapy for patients with BD-I has been studied with a focus on the use of SGA. A large study including commercially insured, Medicare, and Medicaid patients with BD-I initiating a new oral SGA between 2002 and 2008 found that MSs were already used by 35.7% of these patients in the baseline period, and the most commonly used SGAs were quetiapine (31.5%), olanzapine (28.7%), risperidone (20.5%), aripiprazole (9.7%), and ziprasidone (5.6%), with no relevant differences between the type of insurance.20 Another report, using data from VA claims of patients with BD-I receiving a first agent to treat mania, in a similar period of enrollment (2003–2010), reported that SGA and MS were the preferred first-line therapy (received by 43.2% and 42% of the patients, respectively), while 11.3% received SGA/traditional MS combination.16
As for prevalent patients with BD-I, SGAs have replaced the use of first-generation antipsychotics (FGAs) since the early 2000s: Among patients receiving antipsychotics, SGAs were used by 93.1% to virtually 100% of the patients, depending on the publication.14,22,28,32,39,41 As previously described, polytherapy is dominant in prevalent patients with BD-I, with SGAs used by a range from 45% to 61.1% of the patients33,34,39 and MSs used by 49.9%–83.3% of the patients receiving medication.14,33,34,39 One small study found that the proportions of patients receiving SGAs or MSs remained stable from the period between 2000–2005 and 2006–2011.39 Among children and adolescents with BD, one study showed that SGAs were the most used agent, followed by anticonvulsants (in this study, only 35% of BD sample comprised patients with BD-I).24
Regarding SGAs, specifically, some studies have reported the most frequently used agents within this class. Quetiapine has been the most commonly used SGA in nearly all studies, accounting for 35%–45% of patients receiving atypical antipsychotics.14,21,27,31 While olanzapine was the second most used SGA in the early 2000s,14,27 the two studies including patients during the most recent periods (up to 2011) reported that aripiprazole had taken that place, approaching quetiapine use rates.21,39 Indeed, the most recent research showed that aripiprazole was used by 37.2% of patients with BD.21 The only study addressing the use of SGA in children/adolescents with BD-I reported aripiprazole as the most commonly used agent (32.8%) followed by quetiapine (28.5%).24 There seems to be no apparent difference in the rate of use of different SGAs according to different types of insurance in the studies retrieved.
Patterns of pharmacological treatment: concordance with clinical practice guidelines
Concordance with clinical practice guidelines-recommended therapy has been studied and usually defined as the use of antipsychotics, MSs, and/or anticonvulsants. Moreover, in the absence of therapy for mania, use of antidepressants and other psychotropic medications has been classified as inappropriate treatment.18,23,26,36
Appropriate medication has been used by 41.6%–72% of patients with BD-I,18,26,36 and unopposed use of antidepressants was the major reason for non-concordance with clinical practice guidelines, occurring in up to 31% of the patients.26 Even among patients with BD depression discharged from hospital in one center, only 58% were medications prescribed based on scientific evidence.38
One study addressed patterns of recommended pharmacotherapy in patients with BD-I through the years 1991–1999 and concluded that rates of recommended medication treatment improved after the publication of the American Psychiatric Association Practice Guidelines in 1994.19 In other research, conducted in academic centers as part of the STEP-BD program, prescribing psychiatrists trained for participation in the study provided treatment concordant with recommendations for around 80% of patients with different patterns of BD.37
Two studies found that patients with BD-I treated by psychiatrists were at a higher chance of receiving appropriate treatment than those receiving care from nonpsychiatrists.23,36
Adherence to pharmacological therapy
Most published research addressed adherence specifically for oral treatments,14,17,18,20,25,27,29,30,33,34,40,41 while one study reported adherence figures for both oral and LAI drugs.28
Adherence to oral medications has been defined in different ways among the studies: using the Morisky Medication Adherence Scale,34 testing missed doses in the last days,33,40 and most usually, assessing the medication possession ratio (MPR), defined as the ratio between the number of days covered by filled prescription and the number of days in a given period of observation. But even MPR has been defined in two different ways: some studies use, as a denominator, the number of days between the index and the last day of medication dispensed,18,25,29 while others use the total period of patient observation within the study.14,17,20,27,28,30,41
Studies reporting adherence according to number of missed doses in the previous days or as adherence scales showed that only 45%–50% of patients could be considered adherent,34,40 with good adherence being an exception.33
In studies addressing MPR defined by the ratio between the days of medication supply and the total period of observation within the study (ranging from 6 months to 12 months in the studies included), the rates of MPR ≥80% ranged from 8.3% to 54.1%, with a median of 28%, while mean MPR ranged from 19% to 77%, with a median of 47.1%. In two studies evaluating the same registry of VA patients, similar rates of adherence were observed both for SGAs and for MSs.30,41
Regarding studies testing MPR defined by the ratio between the days of medication supply and the period between the first and the last day of medication supply, adherence rates were higher in these publications, with MPR ≥80% reported in 58%–62% of patients18,29 and mean MPR of 68%–71% for different SGAs.25
Persistence with pharmacological therapy and treatment modification
Time to modification in the treatment with SGAs (discontinuation, augmentation, or switching) has varied considerably in the studies,15,17,20,25 ranging from 66 days (in the larger sample of patients with BD-I)20 up to 220 days.25
Duration of treatment with SGAs has also varied substantially in the literature, from 96 days29 to 240 days,41 but figures reported range from treatment period shorter than 6 months in up to 42% of patients41 to a 12-month non-persistence rate of 82%–85%.20,29
Adherence and persistence with SGA LAIs
The only study retrieved and reporting persistence with SGA LAIs included patients from 2004 to 2006 and did not mention the LAI used. However, this suggested that LAI led to better treatment compliance, with a higher persistence rate (94% in a 12-month period vs the 84% rate observed for oral SGA) and a higher adherence rate (with MPR ≥80% in 52.9% of the patients vs the observed rate of 38.9% for oral SGAs).28
Factors related to nonadherence to pharmacological therapy
Some publications studied the association between clinical and demographic factors combined with adherence to antipsychotics (essentially oral SGA)20,25,28,29,33,34,40,41 and to MSs (Table 2).14,30
Younger age (definition varied among studies, but usually younger than 35–45 years) and comorbid substance use disorder were the factors most consistently associated with higher nonadherence to pharmacotherapy. When tested, minority ethnicity was another factor related to poor adherence, as well as higher disease complexity (eg, higher burden of symptoms) (Table 2).
Lower education level, marital status, and polytherapy had varying results among different studies, which limited the conclusion about their relation with adherence (Table 5).
Gender was not found to be associated with adherence, and another important observation was that no specific SGA was linked to higher nonadherence rates (Table 5).
The only study addressing the use of LAI reported that LAI formulations of SGA resulted in significantly lower nonadherence rates when compared with first-generation oral antipsychotics. This study observed that SGA and FGA appeared to have the same adherence rates, raising the possibility that LAI formulation, by itself, may be a factor for better adherence.28
Discussion
Treatment prevalence
Despite the chronic feature inherent to BD-I (demonstrated by a 12-month to lifetime treatment prevalence ratio of 71%), less than half of the patients (46%) sought treatment in 12 months as reported in the recent NESARC-III survey,35 suggesting there are barriers to overcome for increasing access to treatment and continuity of care. Notwithstanding the fact that this treatment prevalence rate was derived from a population survey, in which BD-I diagnosis was ascertained by self-reported structured interview, similar numbers were found in a registry of veterans with BD (44.6% receiving antipsychotics in 2003),41 giving support to NESARC-III findings.
Considering the prevalence of BD-I found in NESARC-III (1.5% 12-month prevalence), the estimated prevalence of treatment for BD-I in the USA general adult population would be approximately 0.69% (46%, SD 1.5%), which was close to the estimates reported by studies conducted in the 1990s,42 and raises concerns that eventual barriers to access to treatments have not been successfully addressed in the last decades.
Despite being lower than expected for a chronic condition, treatment prevalence for BD-I in the USA seems higher than observed for other chronic psychiatric illness. For instance, 12-month treatment prevalence for major depressive disorder in the USA has been reported as 33.9%.43
Patterns of treatment: polypharmacy rates
Monotherapy was the prevailing approach of index pharmacological treatment for BD-I,16,20 but polytherapy was the most common pattern in prevalent patients, with an average of 2.5 psychotropic medications per patient with BD.33,39 No specific clinical or demographic characteristics of BD patients have been correlated with the prescription of polypharmacy, but the fact that most patients start with monotherapy suggests that the lack of adequate response to treatment is a key driver for subsequent use of polytherapy. These findings show the difficulty in maintaining symptomatic control in patients with BD with a single agent and were in concordance with clinical practice guidelines, which recommend starting pharmacological treatment as monotherapy for most patients, but with consideration to add therapies in those who do not respond to monotherapy.7–9,44
Patterns of treatment: medications most commonly used
The choice of initial therapy has been investigated in only two studies including patients with BD-I, and these showed that SGA and MS monotherapy were used as first intentional therapy in similar proportions of patients.16 Among SGAs, quetiapine was the preferred initial drug, followed by olanzapine, risperidone, and aripiprazole.20 However, these studies included patients treated in the period from 2002 to 2010 and may not reflect the current standard of care for treatment-naive patients, since many SGAs only reached their peak of prescription later in that interval.32 This is an important issue to be addressed to improve our understanding and to provide better estimates on the use of maintenance treatment, since clinical practice guidelines suggest that patients with BD-I should receive long-term treatment with the same agent that proved to be effective as the initial therapy. It is relevant to remember that these guidelines do not recommend the use of a preferential SGA in BD-I, leaving this choice at the discretion of the prescriber.7–9 A key limitation of the available literature about patterns of treatment is the lack of studies addressing the use of LAI.
In prevalent patients, SGAs replaced FGAs since the early period of their approval for BD-I by the FDA, accounting for nearly all antipsychotics currently prescribed.14,41 SGA and MS were each used by most of the patients with BD-I receiving pharmacotherapy.39 Regarding SGA use, specifically, quetiapine has been the most commonly used agent in this class, while the use of aripiprazole has increased since mid-2000s, now being the second most used agent21,31,39 – in children/adolescents, aripiprazole was the most commonly used agent.24 It is worth to mention that olanzapine was the first SGA approved by the FDA for BD treatment, in March 2000, while risperidone, quetiapine, and aripiprazole approval occurred afterwards, between December 2003 and September 2004.4 Insurance type does not seem to impact the patterns of use of SGAs significantly,20 but this conclusion is fragile since it is based only on few studies, many of them old. There is a lack of studies assessing patterns of medication in most recent years (after 2010). In addition, there is a need for more studies describing specifically patients with BD-I, since many of them, despite including mostly BD-I and addressing agents that treat mania, report results for BD as a whole.
Patterns of pharmacological treatment: concordance with clinical practice guidelines
Among patients with BD-I receiving pharmacological therapy, appropriate medication has been used by only 40%–70% of patients. The use of unopposed antidepressant, not recommended due to the risk in precipitating mania,9,44 is the major reason for non-appropriateness.18,26,36 This low rate of appropriate treatment may be partially explained by the fact that it is common that nonpsychiatrists provide the care for these patients.18,26,36
However, there is a need for improvement in these figures, and disseminating clinical practice guidelines may be an opportunity for improvement. One survey conducted in 2005 reported that 64.1% of psychiatrists make routine use of clinical guidelines when deciding on treatment,45 and there is research showing improved appropriateness of therapy after implementation of clinical practice guidelines.19 Still, one study conducted in academic centers with patients receiving care by trained psychiatrists reported higher rates of recommended pharmacological therapy for BD as a whole and for BD-I specifically.37 These findings, in collection, strongly suggest that psychiatrists trained according to the recommendations from clinical practice guidelines may provide a more appropriate pharmacotherapy for patients with BD.
Adherence and persistence to pharmacological therapy
Despite the fact that randomized controlled trials testing SGA reported adherence rates up to 94%,46 it is not appropriate to extrapolate these figures to the real-world setting, as randomized trials tend to artificially inflate adherence to treatment due to reasons such as the close monitoring of patients47 and the so-called “Hawthorne effect”. Furthermore, about half of the patients with mania enrolled in clinical trials present clinical and demographic characteristics that differ from those observed in patients in clinical practice, limiting their representativeness.48 Thus, observational studies are of paramount importance to appropriately assess adherence and persistence to drug therapy in the real world.
MPR has been the most commonly used tool for measurement of adherence to pharmacotherapy in BD; however, there remains a need for standardizing this assessment regarding its denominator (time from first to last day of medication dispensed vs the total period of observation of patients). Studies using the former strategy18,25,29 report higher rates of adherence (SGA mean MPR of 68%–71%) when compared with studies using the latter one,14,17,20,27,28,30,41 with SGA mean MPR around 47%. Measuring MPR according to the total period of follow-up brings a limitation that refers to the reason why a medication was not used: nonadherence or simply the fact that the treating physician stopped prescribing that therapy during that period. Nevertheless, adherence rates, no matter the method used, are considered to be low, with MPR <80% in at least 40% of the patients.
Persistence rates also vary significantly in the literature, but usual figures suggest that a large proportion of patients do not receive long-term therapy for the period suggested by clinical guidelines, since it has been reported a treatment period shorter than 6 months in up to 42% of the patients41 and a 12-month non-persistence rate of 82%–85%.20,29
There is a lack of studies addressing compliance with therapy involving LAIs. In fact, only one study retrieved reported that data and showed a better persistence rate than observed for oral SGA.28
Since nonadherence has been related to worse clinical outcomes and higher costs in patients with BD-I,25,27,28,40,49,50 it is evident that there is a need to improve adherence rates in these patients.
Factors associated with adherence to pharmacological therapy
Younger age14,28–30,33,40,41 and comorbid substance use disorder28–30,33,34,40,41 were the factors most consistently associated with higher nonadherence to pharmacotherapy with SGAs and MSs. Minority ethnicity and higher disease complexity (eg, higher burden of symptoms) have also been related to lower adherence.14,29,30,34,40,41 This suggests that these groups should be the main focus when developing and testing interventions to improve adherence rates in BD. It is interesting to note that polypharmacy has not been consistently reported as a driver for adherence to BD treatment.
These findings are similar to those observed in a recent systematic review about adherence to antipsychotic medication in BD and patients with schizophrenia including international studies.11 One factor described in other studies investigating barriers to adherence,11,51 but that has not been addressed in a study of the USA patients, is a poor insight into having an illness or needing medication, which may be particularly common in patients during acute episodes of BD-I.
Conclusion
There is a need for more studies addressing the patterns of therapy and patterns of adherence to pharmacological treatment in patients with BD-I in the USA, especially to better understand current standards.
Treatment prevalence in patients with BD-I in the USA is lower than expected for a chronic condition, despite being higher than for other chronic psychiatric illness, such as major depressive disorder. Also, patients receiving treatment are commonly managed with the use of inappropriate medication choices. Low adherence and low persistence rates seem to be important factors deriving from this finding, but the literature also suggests that there is a need for improving dissemination of clinical practice guidelines among general practitioners as well as psychiatrists.
There is an urgent need to improve the rates of adherence and persistence to pharmacotherapy in BD-I, especially in younger patients and those with psychiatric comorbidities. There is some suggestion in the literature that the use of SGA LAI may help address this issue, but more studies are needed to confirm this conclusion.
Acknowledgments
The study was contracted to Evidências – Kantar Health and funded by Otsuka Pharmaceutical Development & Commercialization, Inc. and Lundbeck Inc.
Disclosure
The authors Luciano Paladini, Teresa Lemmer, Alexandra Piedade, and Otavio Clark are employees of Evidências – Kantar Health. Mallik Greene is an employee of Otsuka Pharmaceuticals, and Maelys Touya is an employee of Lundbeck Inc. The authors report no other conflicts of interest in this work.
References
Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387(10027):1561–1572. | ||
Forte A, Baldessarini RJ, Tondo L, Vazquez GH, Pompili M, Girardi P. Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J Affect Disord. 2015;178:71–78. | ||
Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. | ||
Demland JA, Jing Y, Kelton CM, Guo JJ, Li H, Wigle PR. Use pattern and off-label use of atypical antipsychotics in bipolar disorder 1998–2002. Am Health Drug Benefits. 2009;2(4):184–191. | ||
Dols A, Kupka RW, van Lammeren A, Beekman AT, Sajatovic M, Stek ML. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16(2):113–118. | ||
Grunze H, Vieta E, Goodwin GM, et al; WFSBP Task Force on Treatment Guidelines for Bipolar Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2012 on the long-term treatment of bipolar disorder. World J Biol Psychiatry. 2013;14(3):154–219. | ||
Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49(12):1087–1206. | ||
Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1–44. | ||
Department of Veterans Affairs (VA), Department of Defense (DOD). Clinical practice guideline for clinical management of bipolar disorder in adults. Washington: VA/DoD; 2010. Available from: http://www.healthquality.va.gov/guidelines/MH/bd/bd_306_sum.pdf. Accessed October 04, 2016. | ||
Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102(2A):43–49. | ||
Garcia S, Martinez-Cengotitabengoa M, Lopez-Zurbano S, et al. Adherence to antipsychotic medication in bipolar disorder and schizophrenic patients: a systematic review. J Clin Psychopharmacol. 2016;36(4):355–371. | ||
Velligan DI, Sajatovic M, Hatch A, Kramata P, Docherty JP. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–468. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. | ||
Baldessarini R, Henk H, Sklar A, Chang J, Leahy L. Psychotropic medications for patients with bipolar disorder in the United States: polytherapy and adherence. Psychiatr Serv. 2008;59(10):1175–1183. | ||
Baldessarini RJ, Leahy L, Arcona S, Gause D, Zhang W, Hennen J. Patterns of psychotropic drug prescription for U.S. patients with diagnoses of bipolar disorders. Psychiatr Serv. 2007;58(1):85–91. | ||
Bauer MS, Miller CJ, Li M, Bajor LA, Lee A. A population-based study of the comparative effectiveness of second-generation antipsychotics vs older antimanic agents in bipolar disorder. Bipolar Disord. 2016;18(6):481–489. | ||
Berger A, Edelsberg J, Sanders KN, Alvir JM, Mychaskiw MA, Oster G. Medication adherence and utilization in patients with schizophrenia or bipolar disorder receiving aripiprazole, quetiapine, or ziprasidone at hospital discharge: a retrospective cohort study. BMC Psychiatry. 2012;12:99. | ||
Burns ME, Busch AB, Madden JM, et al. Effects of Medicare Part D on guideline-concordant pharmacotherapy for bipolar I disorder among dual beneficiaries. Psychiatr Serv. 2014;65(3):323–329. | ||
Busch AB, Ling D, Frank RG, Greenfield SF. Changes in the quality of care for bipolar I disorder during the 1990s. Psychiatr Serv. 2007;58(1):27–33. | ||
Chen W, Deveaugh-Geiss AM, Palmer L, Princic N, Chen YT. Patterns of atypical antipsychotic therapy use in adults with bipolar 1disorder in the USA. Hum Psychopharmacol. 2013;28(5):428–437. | ||
Citrome L, Kalsekar I, Guo Z, Laubmeier K, Hebden T. Diagnoses associated with use of atypical antipsychotics in a commercial health plan: a claims database analysis. Clin Ther. 2013;35(12):1867–1875. | ||
Depp C, Ojeda VD, Mastin W, Unutzer J, Gilmer TP. Trends in use of antipsychotics and mood stabilizers among Medicaid beneficiaries with bipolar disorder, 2001–2004. Psychiatr Serv. 2008;59(10):1169–1174. | ||
Dusetzina SB, Gaynes BN, Weinberger M, Farley JF, Sleath B, Hansen RA. Receipt of guideline-concordant pharmacotherapy among children with new diagnoses of bipolar disorder. Psychiatr Serv. 2011;62(12):1443–1449. | ||
Dusetzina SB, Weinberger M, Gaynes BN, Farley JF, Sleath B, Hansen RA. Prevalence of bipolar disorder diagnoses and psychotropic drug therapy among privately insured children and adolescents. Pharmacotherapy. 2012;32(12):1085–1094. | ||
Hassan M, Madhavan SS, Kalsekar ID, et al. Comparing adherence to and persistence with antipsychotic therapy among patients with bipolar disorder. Ann Pharmacother. 2007;41(11):1812–1818. | ||
Huang H, Goren JL, Chan YF, et al. Pharmacologic management of bipolar disorder in a Medicare Advantage population. Psychosomatics. 2014;55(6):572–577. | ||
Lage MJ, Hassan MK. The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: a retrospective study. Ann Gen Psychiatry. 2009;8:7. | ||
Lang K, Korn J, Muser E, Choi JC, Abouzaid S, Menzin J. Predictors of medication nonadherence and hospitalization in Medicaid patients with bipolar I disorder given long-acting or oral antipsychotics. J Med Econ. 2011;14(2):217–226. | ||
Rascati KL, Richards KM, Ott CA, et al. Adherence, persistence of use, and costs associated with second-generation antipsychotics for bipolar disorder. Psychiatr Serv. 2011;62(9):1032–1040. | ||
Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R. Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder. Psychiatr Serv. 2007;58(6):855–863. | ||
Seabury SA, Goldman DP, Kalsekar I, Sheehan JJ, Laubmeier K, Lakdawalla DN. Formulary restrictions on atypical antipsychotics: impact on costs for patients with schizophrenia and bipolar disorder in Medicaid. Am J Manag Care. 2014;20(2):e52–e60. | ||
Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177–184. | ||
Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol. 2008;23(2):95–105. | ||
Bates JA, Whitehead R, Bolge SC, Kim E. Correlates of medication adherence among patients with bipolar disorder: results of the bipolar evaluation of satisfaction and tolerability (BEST) study: a nationwide cross-sectional survey. Prim Care Companion J Clin Psychiatry. 2010;12(5). | ||
Blanco C, Compton WM, Saha TD, et al. Epidemiology of DSM-5 bipolar I disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions – III. J Psychiatr Res. 2016;84:310–317. | ||
Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. | ||
Dennehy EB, Bauer MS, Perlis RH, Kogan JN, Sachs GS. Concordance with treatment guidelines for bipolar disorder: data from the systematic treatment enhancement program for bipolar disorder. Psychopharmacol Bull. 2007;40(3):72–84. | ||
Freeland KN, Cogdill BR, Ross CA, et al. Adherence to evidence-based treatment guidelines for bipolar depression in an inpatient setting. Am J Health Syst Pharm. 2015;72(23 Suppl 3):S156–S161. | ||
Hooshmand F, Miller S, Dore J, et al. Trends in pharmacotherapy in patients referred to a bipolar specialty clinic, 2000–2011. J Affect Disord. 2014;155:283–287. | ||
Perlis RH, Ostacher MJ, Miklowitz DJ, et al. Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry. 2010;71(3):296–303. | ||
Sajatovic M, Valenstein M, Blow FC, Ganoczy D, Ignacio RV. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8(3):232–241. | ||
Unutzer J, Simon G, Pabiniak C, Bond K, Katon W. The treated prevalence of bipolar disorder in a large staff-model HMO. Psychiatr Serv. 1998;49(8):1072–1078. | ||
Gonzalez HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry. 2010;67(1):37–46. | ||
National Institute for Health and Clinical Excellence (NICE). Bipolar disorder (update): the management of bipolar disorder in adults, children and adolescents in primary and secondary care. Clinical Guideline 185. 2014. Available from: https://www.nice.org.uk/guidance/cg185. Accessed October 04, 2016. | ||
Perlis RH. Use of treatment guidelines in clinical decision making in bipolar disorder: a pilot survey of clinicians. Curr Med Res Opin. 2007;23(3):467–475. | ||
Tohen M, Calabrese JR, Sachs GS, et al. Randomized, placebo-controlled trial of olanzapine as maintenance therapy in patients with bipolar I disorder responding to acute treatment with olanzapine. Am J Psychiatry. 2006;163(2):247–256. | ||
Whalley Buono E, Vrijens B, Bosworth HB, Liu LZ, Zullig LL, Granger BB. Coming full circle in the measurement of medication adherence: opportunities and implications for health care. Patient Prefer Adherence. 2017;11:1009–1017. | ||
Hoertel N, Le Strat Y, Lavaud P, Dubertret C, Limosin F. Generalizability of clinical trial results for bipolar disorder to community samples: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2013;74(3):265–270. | ||
Busch AB, Neelon B, Zelevinsky K, He Y, Normand SL. Accurately predicting bipolar disorder mood outcomes: implications for the use of electronic databases. Med Care. 2012;50(4):311–319. | ||
Jiang Y, Ni W. Estimating the impact of adherence to and persistence with atypical antipsychotic therapy on health care costs and risk of hospitalization. Pharmacotherapy. 2015;35(9):813–822. | ||
Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70 Suppl 4:1–46; quiz 47–48. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




