Back to Journals » Infection and Drug Resistance » Volume 15
Systematic Characterization of Epidemiology, Antifungal Susceptibility, Risk Factors and Outcomes of Candidaemia: A Six-Year Chinese Study
Authors Ye N, Liu Z, Tang W , Li X, Chu W , Zhou Q
Received 17 June 2022
Accepted for publication 17 August 2022
Published 26 August 2022 Volume 2022:15 Pages 4887—4898
DOI https://doi.org/10.2147/IDR.S378629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Naifang Ye, Zhou Liu, Wei Tang, Xin Li, Wenwen Chu, Qiang Zhou
Department of Clinical Laboratory Medicine, The Second Hospital of Anhui Medical University, Anhui Medical University, Hefei, People’s Republic of China
Correspondence: Qiang Zhou, Department of Clinical Laboratory Medicine, The Second Hospital of Anhui Medical University, Anhui Medical University, Hefei, Anhui, People’s Republic of China, Tel +86-0551-63866024, Email [email protected]
Background: Candida bloodstream infection (BSI), the fourth most common nosocomial BSI, is an urgent global health challenge with the tremendous growth in antifungal resistance rate and mortality rate.
Purpose: To establish the epidemiology, species distribution, risk factors, and 30-day mortality of candidaemia among 115 patients in this 6-year surveillance study.
Materials and Methods: We retrospectively analyzed the clinical characteristics, epidemiology, antifungal susceptibility patterns, and risk factors for morbidity and mortality of 115 candidaemia cases diagnosed in one tertiary care hospital from January 2016 through December 2021.
Results: Of the 115 candidaemia cases, the most prevalent species were Candida tropicalis (33.0%), followed by Candida albicans (27.8%), Candida parapsilosis complex (19.1%), and others. The overall incidence was 0.21 cases/1000 admissions. The overall crude resistance rate of Candida spp. against azoles was 20.0% (23/115), while Candida tropicalis showed a significant increase in the resistance rate to azoles (from 1/6, 16.7% in 2017 to 6/10, 60.0% in 2021). Multivariate analyses demonstrated that hematological malignancy and neutropenia were significantly associated with Candida tropicalis BSI than Candida non-tropicalis BSI. Candida albicans BSI had a significantly higher rate of previous surgery than Candida non-albicans BSI. Candida parapsilosis BSI had a significantly higher rate of receiving total parenteral nutrition (TPN). The overall 30-day mortality rate was 27.0% (31/115). The presence of high age-adjusted Charlson comorbidity index (aCCI), neutropenia, and septic shock were factors independently associated with increased 30-day mortality.
Conclusion: Candida tropicalis are emerging as the predominant isolate in candidaemia. Of note, the unexpectedly increased resistance rate to azoles in Candida tropicalis BSI was observed. The aCCI scores, neutropenia, and septic shock were independently associated with 30-day mortality. Prompt, adequate antifungal treatment among high-risk patients may lead to a reduction in mortality.
Keywords: candidaemia, epidemiology, resistance, risk factor, mortality
Introduction
Bloodstream infections (BSI) due to Candida spp. have become the most common fungal bloodstream infection with significant morbidity and mortality.1–3 Candida albicans was the most prevalent pathogen causing candidaemia in hospitals for the last two decades, based on previous studies from the United States and Europe.4–7 However, discordant results in recent studies reveal that the distribution of candidaemia has shifted toward Candida non-albicans in Asia.8,9 Similar conclusions were drawn from Shanghai,10 Shenyang,11 and the multicenter CHIF-NET study in China.12 Candida non-albicans species accounted for over two-thirds of isolates in the candidaemia. Resistance to antifungal agents varies among Candida species in different regions.13,14 Reduced in vitro susceptibility to azoles has been observed among Candida non-albicans species.15 More than 95% of Candida albicans and Candida parapsilosis complex isolates were susceptible to all antifungal agents and fluconazole resistance rate in Candida tropicalis tripled over three years among 4010 Candida species which were reported in the CHIF-NET study in China.12 The spatiotemporal heterogeneity of candidaemia worldwide prompts us to explore the epidemiology trends in China.
Recent literature has shown that the underlying risk factors contributing to candidaemia have changed over time. Known risk factors include complicated surgery, immunosuppressive therapies (eg chemotherapy, corticosteroids), hemodialysis, exposure to broad-spectrum antibacterial agents, prolonged use of central venous catheters (CVC), undergoing mechanical ventilation and administration of total parenteral nutrition (TPN).16,17 Early diagnosis and treatment are usually absent due to the lack of specific clinical manifestations. The attributable mortality caused by candidaemia is reported to be 5%~58%.10,13,18 Therefore, investigating the local epidemiology, monitoring the antifungal drug susceptibility, and identifying the potential risk factors of morbidity and mortality are essential for selecting the first-line antifungal agents and treatment measures to improve patient’s clinical outcomes.
In this study, we retrospectively analyzed data from 115 patients with candidaemia at our hospital between 2016 and 2021. We aimed to describe their local epidemiology, clinical characteristics, species distribution of infection, and antifungal drug susceptibility and determine the risk factors for Candida tropicalis/Candida non-tropicalis, Candida albicans/Candida non-albicans, Candida parapsilosis/Candida non-parapsilosis candidaemia and 30-day mortality. It is the first report in China to explore the risk factors of the dominant species causing infection (Candida tropicalis, Candida albicans, and Candida parapsilosis complex) in the Chinese population.
Materials and Methods
Patient Identification and Surveillance
Between January 2016 and December 2021, 115 candidaemia cases were enrolled at the Second Hospital of Anhui Medical University, a 2600-bed tertiary care hospital. An episode of candidaemia was defined as systemic manifestations and positive culture for Candida species in one or more sets of blood cultures. For patient with two or more episodes of candidaemia, the next episode was counted separately if the interval between infections was more than 30 days. Previous treatments were defined as: (1) Previous surgery: surgery in the last three months; (2) Exposure to broad-spectrum antibacterial agents: the use of broad-spectrum antibacterial agents for at least five days within 30 days of one episode; (3) Prior use of antifungals: the use of antifungals within three months from diagnosing; (4) Prior use of steroids: the use of steroids for three weeks within two months from diagnosing; (5) Chemotherapy: the use of chemotherapy drug within three months from diagnosing; (6) Neutropenia: an absolute neutrophil count less than 500 cells/mm3. The study was approved by the Medical Ethics Committee in the Second Hospital of Anhui Medical University (reference number: SZR 2021047). The research was conducted in accordance with the Declaration of Helsinki.
Clinical and Epidemiological Data
For each case, we recorded the demographics (age, sex, inpatient department), baseline treatments (prior hospital stay, prior ICU stay, and prior treatments), disease history, and immunocompromisation (neutropenia, chemoradiotherapy, septic shock, etc.). We explored the epidemiology of this cohort, including species distribution of candidaemia and antifungal susceptibility patterns. We recorded several risk factors of candidaemia, including the application of CVC, mechanical ventilation, carrying urinary catheters, receiving total parenteral nutrition (TPN), insertion of body cavity drainage tube, use of broad-spectrum antibacterial agents, prior use of antifungals and steroids, previous surgery, chemotherapy, and hemodialysis. Next, patients’ comorbidities and 30-day outcomes were evaluated. Of the comorbidities, we mainly examined the presence of hematological malignancy, solid-organ cancer, coronary heart disease, and diabetes mellitus. Comorbidity was measured according to the age-adjusted Charlson comorbidity index (aCCI).19
Microbiological Test
Blood samples were isolated under sterile conditions and incubated in a BD BACTEC FX400 (Becton Dickinson Diagnostic Instrument Systems) blood culture system for up to 5 days. Positive bottles were subjected to Gram-staining and sampled onto Columbia blood and Sabouraud dextrose agar plates (Tianda Co., Ltd.). After incubation at 37 °C for up to 48.00 h, Candida species were identified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (IVD MALDI Biotyper 2.3, Bruker Daltonics).20 We performed antifungal susceptibility testing to detect in vitro resistance to fluconazole, voriconazole, itraconazole, and amphotericin B using the ATB FUNGUS 3 (BioMérieux) strip.21 The quality control strain of Candida parapsilosis ATCC22019 and Candida krusei ATCC6258 was used. Candida isolates were classified as susceptible (S), intermediate (I), and resistant (R) according to the minimal inhibitory concentration (MIC) using the clinical breakpoints (CBPs) of antifungals. For fluconazole and voriconazole, the CBPs were defined based on the recommendation from the Clinical and Laboratory Standards Institute (CLSI M60).22 The CBPs for susceptibility of Candida spp. against itraconazole were from the European Committee on Antimicrobial Susceptibility Testing (EUCAST 10.0) and epidemiological cutoff values (ECVs).23 For amphotericin B, the CBPs were defined based on the recommendation from the EUCAST 10.0.23 5-fluorocytosine was not reported in the current study because of lacking CBPs in the CLSI or EUCAST.
Statistical Analysis
Continuous variables were expressed as the mean value SD or medians with interquartile ranges (IQR) according to the normality of data distribution. Categorical variables were compared by χ2 tests. The univariate logistic regression was performed on the variables to estimate odds ratios (ORs) and their 95% CIs. Further multivariate logistic regression was performed to estimate the effects on different risk factors when controlling for other factors. P less than 0.05 was considered statistically significant. All statistical analyses were made in SPSS (Version 16.0).
Results
Patient Characteristics and Incidence Rates
Among the 115 patients, 57 (49.6%) were men, and the median age was 63 (IQR, 51–71 yrs). The median aCCI of the initial admission was 5 points (IQR, 3–6). More details regarding baseline clinical characteristics are described in Table 1. In general, the total candidaemia incidence rate was 0.21 cases/1000 admissions. A rise in candidaemia incidence was seen from 2016 to 2021, with an incidence rate increasing gradually from 0.18 to 0.27 episodes/1000 admissions (Figure 1). Among the study patients, 39.1% (45/115) were staying in the ICU when diagnosed and 25.2% (29/115) were residents in the department of hematology. Regarding frequent risk factors for candidaemia, prior exposure to broad-spectrum antibiotics (114 patients, 98.2%) and the presence of central venous catheters (69 patients, 60.0%) were the most common in this study.
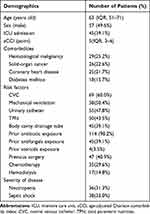 |
Table 1 Demographics and Clinical Characteristics of the Patients with Candidaemia |
Species Distribution, Drug Susceptibility, and Antifungal Therapy
Taxonomic analyses of species isolated from the 115 candidaemia cases indicated that the most prevalent infection species was Candida tropicalis (38/115, 33.0%), followed by Candida albicans (32/115, 27.8%), Candida parapsilosis complex (22/115, 19.1%), Candida glabrata (17/115, 14.8%), Candida krusei (4/115, 3.5%) and other Candida spp. (2/115, 1.8%) in Table 2. Candida non-albicans species comprised more than half of the isolates, mainly attributed to Candida tropicalis accounting for 45.8% of non-albicans isolates. The trend in the species distribution of candidaemia is shown in Figure 2.
 |
Table 2 Azole Resistance Among Candida Isolates from 2016–2021 |
All 115 isolates were tested for in vitro susceptibility to antifungal agents. The crude resistance rate of Candida spp. strains against azoles was 20.0% (23/115). The resistance rates of Candida spp. strains against fluconazole, itraconazole and voriconazole were 19.1% (22/115), 13.9% (16/115) and 8.7% (10/115), respectively. Except for Candida krusei strains, which were naturally resistant to fluconazole, Candida tropicalis strains were the most resistant to azoles (15/38, 39.5%) and an increased resistance rate for Candida tropicalis to azoles was observed (from 1/6, 16.7% in 2017 to 6/10, 60.0% in 2021) in Table 2. Candida albicans and Candida parapsilosis strains showed the most susceptible to azoles, and only one Candida albicans isolate and one Candida parapsilosis isolate showed fluconazole resistance. Candida glabrata strains showed significantly high intermediate rate to azoles (6/17, 35.3%) and only one Candida glabrata isolate was resistant to azoles. Together, 78.3% (18/23) of isolates were cross-resistant to azoles. No isolate was found to be resistant to amphotericin B.
Of the 115 patients, 34.8% (40 of 115) received voriconazole, 23.5% (27 of 115) received fluconazole, 1.7% (2 of 115) received itraconazole, 29.6% (34 of 115) received echinocandin, and 3.5% (4 of 115) received a combination of an azole and echinocandin; 8 patients never had any antifungals. In addition, more than one-third of patients (39.1%, 45 of 115) were given azoles as the initial antifungal agents.
Risk Factors Associated with Candida tropicalis BSI and Candida non-tropicalis BSI
Table 3 shows the risk factors between Candida tropicalis and Candida non-tropicalis BSI. Univariate analyses showed that Candida tropicalis BSI patients had significantly higher rate in patients with hematological malignancy (OR, 13.750; 95% CI, 5.012–37.720; P < 0.001), prior antifungal exposure (OR, 5.481; 95% CI, 2.362–12.718; P < 0.001), chemotherapy (OR, 10.286; 95% CI, 4.109–25.746; P < 0.001), and neutropenia (OR, 10.417; 95% CI, 4.192–25.882; P < 0.001) and a lower rate of ICU admission (OR, 0.232; 95% CI, 0.091–0.590; P = 0.002), mechanical ventilation (OR, 0.311; 95% CI, 0.137–0.707; P = 0.005), urinary catheter (OR, 0.254; 95% CI, 0.108–0.596; P = 0.002) and previous surgery (OR, 0.198; 95% CI, 0.078–0.505; P = 0.001) than Candida non-tropicalis BSI patients. Multivariate analyses demonstrated that Candida tropicalis BSI patients had significantly higher rates of hematological malignancy (OR, 5.424; 95% CI, 1.507–19.520; P = 0.010) and neutropenia (OR, 3.800; 95% CI, 1.160–12.453; P = 0.027) than Candida non-tropicalis BSI patients.
 |
Table 3 Comparison Between Candida tropicalis BSI and Candida non-tropicalis BSI |
Risk Factors Associated with Candida albicans BSI and Candida non-albicans BSI
When Candida albicans compared with Candida non-albicans BSI, univariate analyses showed that Candida albicans BSI patients had significantly higher rate of solid-organ cancer (OR, 3.372; 95% CI, 1.358–8.376; P = 0.009), mechanical ventilation (OR, 2.373; 95% CI, 1.016–5.543; P = 0.046), urinary catheter (OR, 2.751; 95% CI, 1.175–6.441; P = 0.020) and previous surgery (OR, 7.375; 95% CI, 2.909–18.697; P < 0.001) and a lower rate of hematological malignancy (OR, 0.063; 95% CI, 0.008–0.489; P = 0.008), prior use of antifungals (OR, 0.260; 95% CI, 0.097–0.698; P = 0.008), chemotherapy (OR, 0.101; 95% CI, 0.023–0.451; P = 0.003), and neutropenia (OR, 0.091; 95% CI, 0.020–0.408; P = 0.002) in Table 4. Multivariate analyses revealed that Candida albicans BSI patients had significantly higher rates of previous surgery (OR, 4.915; 95% CI, 1.853–13.034; P = 0.001) than Candida non-albicans BSI patients.
 |
Table 4 Comparison Between Candida albicans BSI and Candida non-albicans BSI |
Risk Factors Associated with Candida parapsilosis Complex BSI and Candida non-parapsilosis Complex BSI
We then analyzed and compared the factors that may be affiliated with Candida parapsilosis complex BSI and Candida non-parapsilosis complex BSI in Table 5. Univariate analyses showed that Candida parapsilosis complex BSI patients had significantly higher rate of ICU admission (OR, 2.753; 95% CI, 1.063–7.130; P = 0.037), mechanical ventilation (OR, 3.238; 95% CI, 1.164–9.009; P = 0.024) and TPN (OR, 6.182; 95% CI, 2.091–18.273; P = 0.001) and a lower rate of neutropenia (OR, 0.274; 95% CI, 0.076–0.994; P = 0.049) than Candida non-parapsilosis complex BSI patients. Multivariate analyses demonstrated that Candida parapsilosis complex BSI patients had significantly higher rates of TPN (OR, 6.182; 95% CI, 2.091–18.273; P = 0.001) than Candida non-parapsilosis complex BSI patients.
 |
Table 5 Comparison Between Candida parapsilosis BSI and Candida non-parapsilosis BSI |
Outcome Predictors in Candidaemia
During our follow-ups, candidaemia’s overall crude 30-day mortality was 27.0% (31/115). Candida tropicalis BSI showed the highest 30-day mortality as 31.6% (12/38), followed by Candida glabrata BSI (29.4%, 5/17), Candida albicans BSI (25.0%, 8/32), Candida krusei BSI (25.0%, 1/4), and Candida parapsilosis BSI (22.7%, 5/22). There was no difference in mortality found between Candida tropicalis and other Candida spp. (P = 0.433). Logistic regression models then estimated the patients’ outcomes (Table 6). By performing univariate analysis, the aCCI scores (OR, 1.233; 95% CI, 1.028–1.477; P = 0.024), neutropenia (OR, 2.642; 95% CI, 1.122–6.219; P = 0.026) and septic shock (OR, 5.417; 95% CI, 2.235–13.127; P < 0.001) were risk factors for candidaemia related 30-day mortality, while the use of body cavity drainage tube (OR, 0.353; 95% CI, 0.137–0.909; P = 0.031) was a protective factor against the infection. Further multivariate analysis, the aCCI scores (OR, 1.303; 95% CI, 1.052–1.614; P = 0.015), neutropenia (OR, 5.558; 95% CI, 1.847–16.722; P = 0.002) and septic shock (OR, 8.679; 95% CI, 2.983–25.246; P < 0.001) were independent risk factors for candidaemia related 30-day mortality. There was no correlation between the high resistance rate to azoles and the outcome (P = 0.916).
 |
Table 6 Logistic Regression Analysis of Risk Factors Associated with 30-Day Crude Mortality in Patients with Candidaemia |
Discussion
Candidaemia is currently the leading cause of invasive fungal infections in hospitals. It accounts for 98% of fungemia, high often leads to septic shock.2,6,24 The changing trend in the epidemiology of candidaemia is observed globally. At our research site, the incidence of candidaemia has increased dramatically in the past six years. In addition, Candida species distribution, antifungal susceptibility, and risk factors varied widely across different geographic regions.25–28 A United States multicenter retrospective study demonstrated Candida albicans accounted for 39% of cases, followed by Candida glabrata (28%) and Candida parapsilosis (15%).5 Another Greece study showed similar results that the species distribution with Candida albicans (41.0%) was the most common species in Candidaemia.14 However, the incidence of Candida tropicalis (33.0%) rather than Candida albicans (27.8%) ranked first in leading candidaemia according to our observation. Similar results were proved in several reports that Candida tropicalis strains were the most prevalent non-albicans candidaemia in Saudi, Latin America, and Hong Kong, China.9,27,29
The change in the species distribution of candidaemia and the reduced sensitivity of Candida spp. to azole drugs make the infection a more severe challenge for medical management. In our study, the crude resistance rate of Candida spp. against azole were 20.0%. Specifically, Candida non-albicans were more resistant to azoles than Candida albicans, consistent with the recent study.10 Candida albicans and Candida parapsilosis complex to azoles exhibited excellent susceptibility (more than 99.0%). Candida glabrata exhibited a high intermediate rate to azoles (35.3%), and Candida krusei exhibited intrinsic resistance. Of note, Candida tropicalis accounted for the highest proportion of azole resistance (39.5%). Moreover, an increased resistance rate from Candida tropicalis to azoles was observed (from 16.7%, 2017 to 60.0%, 2021), significantly higher than those reported in other studies.8,15 Whether the discrepancy could be attributed to the infectious species or just the geographical distribution of pathogens needs further clarified.
Different risk factors associated with the infection of different Candida isolates, along with clinical manifestation, guide the medical administration of prophylactic antifungal therapy and empirical therapy. Thus, it is significant to have a comprehensive view of the risk factors for different infections. The most common candidaemia predisposing factors include complicated surgery, immunosuppressive therapies, hemodialysis, exposure to broad-spectrum antibacterial agents, and prolonged use of CVCs.16,17 Furthermore, our data showed that Candida tropicalis BSI patients had significantly higher hematological malignancy and neutropenia rates than Candida non-tropicalis BSI patients. In line with our findings, several studies reported that Candida tropicalis BSI was the most common Candida bloodstream infection in patients with hematological malignancy.30–32 A recent study in Algeria revealed that neutropenia and leukemia were the most prevalent underlying conditions for Candida tropicalis BSI.33
The possible mechanism of increased rates of Candida tropicalis BSI in hematological malignancy had not yet been established. One possible explanation is related to the disruption of normal cellular immunity and mucosal barriers. The virulence of Candida spp. is related to its ability to form biofilms and invade host cells with the effect of secretory aspartyl proteinases and phospholipases. During hospitalization in the department of hematology, overgrowth and translocation of pathogens in the bloodstream can occur under the neutropenia status due to immunocompromisation arising from the use of chemotherapy and steroids. Disruption of the normal mucosal barriers provided by the respiratory tract or gastrointestinal tract may lead to the breakthrough of candidaemia in immunocompromised patients.34 It is noteworthy that the Candida tropicalis BSI had significantly higher rates of azole resistance than Candida non-tropicalis BSI, which may be mainly due to commonly azoles prior used in hematological malignancy patients with neutropenia. Together, clinicians should pay more attention to potential Candida tropicalis BSI in immunocompromised patients of hematological malignancy.
Consistent with some previous studies,35,36 we found that a higher rate of solid tumor, postoperative status, use of mechanical ventilation, and urinary catheter tended to be presented in patients with Candida albicans BSI compared with non-albicans BSI in univariate analysis. Further multivariate analysis showed that postoperative status was an independent factor significantly associated with the breakthrough of Candida albicans BSI. Candida albicans is part of the normal flora of the gastrointestinal tract. Xia et al37 revealed that multifocal and high-grade Candida colonization was the precondition of candidaemia, which was caused by the invasion of locally colonized Candida into the blood system after the destruction of the integrity of gastrointestinal mucosa during gastrointestinal surgery. Unfortunately, the pathophysiologic mechanism of Candida colonization and the breakthrough of candidaemia is not completely clear recently.
On the other hand, the presence of CVCs, TPN, and mechanical ventilation are common invasive medical procedures in ICU patients, which are associated with an increased risk of nosocomial infection, especially bloodstream infections.38,39 Of note, our study demonstrated that Candida parapsilosis complex BSI was associated with ICU admission, mechanical ventilation, and TPN. Multivariate analysis showed that TPN was an independent risk factor of Candida parapsilosis BSI. Candida parapsilosis is the most common cause of catheter-associated candidaemia, probably due to the particular affinity for synthetic material.40 In a study by Herek et al, it was reported that Candida parapsilosis might contribute to TPN catheter-related fungemia.41 Thus, physicians should pay attention to prevent life-threatening complications, such as catheter-related bloodstream infections, during medical management by removing the catheter and applying antifungal treatment.42
When it comes to patient outcomes of candidaemia, the main influencing factor for prognosis is disease severity, multiorgan dysfunction, and non-adequate antifungal management, not what kind of Candida species the patient is infected with, or the patient’s drug-resistant status. This idea comes from a German study on candidaemia with a mortality rate of 47.3%.6 In our study, we found a 30-day mortality rate of 27.0%. Our results are consistent with a previous study43 and lower than the mortality rate in a Spanish study of 47.9%.44 Compared with infection of other species of Candida, patients with Candida tropicalis BSI tended to present a higher 30-day mortality rate of 31.6%. No significant difference in mortality was found between Candida tropicalis and other Candida spp. In addition, there was no correlation between the high resistance rate to azoles and the patient outcome. These results are consistent with a Taiwan study conducted on five tertiary hospitals.15 Prior studies have identified increasing age, mechanical ventilation, underlying comorbidities, and disease severity are associated with an increased risk of mortality.17,45 Similar to previous studies,8,15 our study showed that high aCCI scores, neutropenia, and septic shock were independent risk factors for candidaemia-related 30-day mortality. Thus, our results confirm the importance of comorbid conditions, immunocompromisation, and multiorgan dysfunction for the outcome in candidaemia patients.
This analysis takes advantage of complete data from one single center from 2016 to 2021 and assesses the incidence of candidaemia in the retrospective data, as well as provides the risk factors for dominant species causing BSI incidence and outcomes in the study center. As with other retrospective studies, our study is limited in some points. First, our study was a single-center retrospective study that lacked the ability to produce cause–effect relationship data with control variables. In addition, a larger validation dataset to confirm our results is ideal. Second, as azole resistance has become a major concern worldwide, echinocandins have been recommended as first-line therapy for candidaemia by the Infectious Diseases Society of America and the European Society of Clinical Microbiology and Infectious Diseases Guidelines. This study detected relatively few choices of antifungal agents without using echinocandins for candidaemia due to the limitation of the drug sensitivity kit.
Conclusion
The incidence of non-albicans candidaemia seems to have increased over the last years. Candida tropicalis BSI showed the highest crude incidence but the worst patient outcome. Of note, the unexpectedly high resistance rate to azoles in Candida tropicalis BSI was observed. Additionally, the aCCI scores, neutropenia, and septic shock were independently associated with BSI patients’ 30-day mortality. Based on our results, we urge physicians to adjust empirical antifungal therapy timely, relying on local epidemiology and antifungal susceptibility data for high-risk patients, which are essential to improve clinical outcomes.
Ethical Approval
In this study, strains isolated from patient samples were used for research, and there were no adverse reactions and risks to subjects. The research data shall be confidential to the personal information of the subjects, including medical records and biological samples. The consent had been waived by the ethics committee in this study, and this experiment was approved by The Medical Ethics Committee of the Second Hospital of Anhui Medical University. The research was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
This work is supported by the Natural Science Foundation of Anhui Medical University (No. 2020xkj031 to Naifang Ye), the National Natural Science Foundation of China (No. 82102460 to Wei Tang), Anhui Natural Science Foundation (No. 1908085QH366 to Zhou Liu), and Clinical Research Cultivation Project of the Second Hospital of Anhui Medical University(No. 2021LCZD13 to Qiang Zhou).
Disclosure
The authors declared that there are no conflicts of interest in this work.
References
1. Barac A, Cevik M, Colovic N, et al. Investigation of a healthcare-associated Candida tropicalis candidiasis cluster in a haematology unit and a systematic review of nosocomial outbreaks. Mycoses. 2020;63:326–333. doi:10.1111/myc.13048
2. Li Y, Du M, Chen LA, et al. Nosocomial bloodstream infection due to Candida spp. in China: species distribution, clinical features, and outcomes. Mycopathologia. 2016;181:485–495. doi:10.1007/s11046-016-9997-3
3. Posteraro B, De Carolis E, Criscuolo M, et al. Candidaemia in haematological malignancy patients from a SEIFEM study: epidemiological patterns according to antifungal prophylaxis. Mycoses. 2020;63:900–910. doi:10.1111/myc.13130
4. Samonis G, Kofteridis DP, Saloustros E, et al. Candida albicans versus non-albicans bloodstream infection in patients in a tertiary hospital: an analysis of microbiological data. Scand J Infect Dis. 2008;40:414–419. doi:10.1080/00365540701765657
5. Toda M, Williams SR, Berkow EL, et al. Population-based active surveillance for culture-confirmed Candidemia - four sites, United States, 2012–2016. MMWR Surveill Summ. 2019;68:1–15. doi:10.15585/mmwr.ss6808a1
6. Schroeder M, Weber T, Denker T, et al. Epidemiology, clinical characteristics, and outcome of candidemia in critically ill patients in Germany: a single-center retrospective 10-year analysis. Ann Intensive Care. 2020;10:142. doi:10.1186/s13613-020-00755-8
7. Fortún J, Gioia F. Invasive candidiasis in the neutropenic patient. Rev Esp Quimioter. 2017;30(Suppl 1):22–25.
8. Wu PF, Liu WL, Hsieh MH, et al. Epidemiology and antifungal susceptibility of candidemia isolates of non-albicans Candida species from cancer patients. Emerg Microbes Infect. 2017;6:e87. doi:10.1038/emi.2017.74
9. Alkharashi N, Aljohani S, Layqah L, et al. Candida bloodstream infection: changing pattern of occurrence and antifungal susceptibility over 10 years in a tertiary care Saudi hospital. Can J Infect Dis Med Microbiol. 2019;2019:2015692. doi:10.1155/2019/2015692
10. Zheng YJ, Xie T, Wu L, et al. Epidemiology, species distribution, and outcome of nosocomial Candida spp. bloodstream infection in Shanghai: an 11-year retrospective analysis in a tertiary care hospital. Ann Clin Microbiol Antimicrob. 2021;20:34. doi:10.1186/s12941-021-00441-y
11. Zhang W, Song X, Wu H, et al. Epidemiology, risk factors and outcomes of Candida albicans vs. non- albicans candidaemia in adult patients in Northeast China. Epidemiol Infect. 2019;147:e277. doi:10.1017/S0950268819001638
12. Xiao M, Chen SC, Kong F, et al. Distribution and antifungal susceptibility of Candida species causing candidemia in China: an Update from the CHIF-NET study. J Infect Dis. 2020;221:S139–S147. doi:10.1093/infdis/jiz573
13. Matos T, Lejko Zupanc T, Skofljanec A, et al. Candidaemia in Central Slovenia: a 12-year retrospective survey. Mycoses. 2021;64:753–762. doi:10.1111/myc.13278
14. Siopi M, Tarpatzi A, Kalogeropoulou E, et al. Epidemiological trends of fungemia in Greece with a focus on candidemia during the recent financial crisis: a 10-year survey in a tertiary care academic hospital and review of literature. Antimicrob Agents Chemother. 2020;64:01516–01519. doi:10.1128/AAC.01516-19
15. Liu WL, Huang YT, Hsieh MH, et al. Clinical characteristics of Candida tropicalis fungaemia with reduced triazole susceptibility in Taiwan: a multicentre study. Int J Antimicrob Agents. 2019;53:185–189. doi:10.1016/j.ijantimicag.2018.10.015
16. Ko JH, Jung DS, Lee JY, et al. Poor prognosis of Candida tropicalis among non-albicans candidemia: a retrospective multicenter cohort study, Korea. Diagn Microbiol Infect Dis. 2019;95:195–200. doi:10.1016/j.diagmicrobio.2019.05.017
17. Doğan Ö, Yeşilkaya A, Menekşe Ş, et al. Effect of initial antifungal therapy on mortality among patients with bloodstream infections with different Candida species and resistance to antifungal agents: a multicentre observational study by the Turkish Fungal Infections Study Group. Int J Antimicrob Agents. 2020;56:105992. doi:10.1016/j.ijantimicag.2020.105992
18. Al-Musawi TS, Alkhalifa WA, Alasaker NA, et al. A seven-year surveillance of Candida bloodstream infection at a university hospital in KSA. J Taibah Univ Med Sci. 2021;16:184–190. doi:10.1016/j.jtumed.2020.12.002
19. Yang CC, Fong Y, Lin LC, et al. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg. 2018;53:235–240. doi:10.1093/ejcts/ezx215
20. Oviaño M, Rodríguez-Sánchez B. MALDI-TOF mass spectrometry in the 21st century clinical microbiology laboratory. Enferm Infecc Microbiol Clin. 2021;39:192–200. doi:10.1016/j.eimc.2020.02.027
21. Lei J, Xu J, Wang T. In vitro susceptibility of Candida spp. to fluconazole, itraconazole and voriconazole and the correlation between triazoles susceptibility: results from a five-year study. J Mycol Med. 2018;28:310–313. doi:10.1016/j.mycmed.2018.03.005
22. Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts, M60.
23. Arendrup MC, Friberg N, Mares M, et al. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin Microbiol Infect. 2020;26:1464–1472. doi:10.1016/j.cmi.2020.06.007
24. Prigitano A, Cavanna C, Passera M, et al. Evolution of fungemia in an Italian region. J Mycol Med. 2020;30:100906. doi:10.1016/j.mycmed.2019.100906
25. Israel S, Amit S, Israel A, et al. The epidemiology and susceptibility of candidemia in Jerusalem, Israel. Front Cell Infect Microbiol. 2019;9:352. doi:10.3389/fcimb.2019.00352
26. Pfaller MA, Diekema DJ, Turnidge JD, et al. Twenty years of the SENTRY antifungal surveillance program: results for candida species from 1997–2016. Open Forum Infect Dis. 2019;6:S79–S94. doi:10.1093/ofid/ofy358
27. Treviño-Rangel RJ, Peña-López CD, Hernández-Rodríguez PA, et al. Association between Candida biofilm-forming bloodstream isolates and the clinical evolution in patients with candidemia: an observational nine-year single center study in Mexico. Rev Iberoam Micol. 2018;35:11–16. doi:10.1016/j.riam.2017.01.005
28. Yamin D, Husin A, Harun A. Distribution of candidemia in Malaysian tertiary care hospital revealed predominance of Candida parapsilosis. Trop Biomed. 2020;37:903–910.
29. Seneviratne CJ, Rajan S, Wong SS, et al. Antifungal susceptibility in serum and virulence determinants of Candida bloodstream isolates from Hong Kong. Front Microbiol. 2016;7:216. doi:10.3389/fmicb.2016.00216
30. Chen CY, Tien FM, Sheng WH, et al. Clinical and microbiological characteristics of bloodstream infections among patients with haematological malignancies with and without neutropenia at a medical centre in northern Taiwan, 2008–2013. Int J Antimicrob Agents. 2017;49:272–281. doi:10.1016/j.ijantimicag.2016.11.009
31. You L, Yao C, Yang F, et al. Echinocandins versus amphotericin B against Candida tropicalis fungemia in adult hematological patients with neutropenia: a multicenter retrospective cohort study. Infect Drug Resist. 2020;13:2229–2235. doi:10.2147/IDR.S258744
32. Kim SH, Choi JK, Cho SY, et al. Risk factors and clinical outcomes of breakthrough yeast bloodstream infections in patients with hematological malignancies in the era of newer antifungal agents. Med Mycol. 2018;56:197–206. doi:10.1093/mmy/myx038
33. Megri Y, Arastehfar A, Boekhout T, et al. Candida tropicalis is the most prevalent yeast species causing candidemia in Algeria: the urgent need for antifungal stewardship and infection control measures. Antimicrob Resist Infect Control. 2020;9:50. doi:10.1186/s13756-020-00710-z
34. Puig-Asensio M, Ruiz-Camps I, Fernández-Ruiz M, et al. Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: results from a population-based surveillance in Spain. Clin Microbiol Infect. 2015;21:491 e491–410. doi:10.1016/j.cmi.2014.12.027
35. Wallis LS, Stull CM, Rakita U, et al. Postoperative Candida infection following complex periocular reconstruction. Plast Reconstr Surg Glob Open. 2021;9:e3891. doi:10.1097/GOX.0000000000003891
36. Adámková V, Vaňková A, Ulrych J, et al. Analýza záchytu kvasinek u pacientů po břišním chirurgickém výkonu. Rozhl Chir. 2017;96:426–431. Czech.
37. Xia R, Wang D. Risk factors of invasive candidiasis in critical cancer patients after various gastrointestinal surgeries: a 4-year retrospective study. Medicine. 2019;98:e17704. doi:10.1097/MD.0000000000017704
38. Altintop YA, Ergul AB, Koc AN, et al. Evaluation of Candida colonization and use of the Candida colonization index in a paediatric intensive care unit: a prospective observational study. Infez Med. 2019;27:159–167.
39. Yamin DH, Husin A, Harun A. Risk factors of Candida parapsilosis catheter-related bloodstream infection. Front Public Health. 2021;9:631865. doi:10.3389/fpubh.2021.631865
40. Boyce JM, Dumigan DG, Havill NL, et al. A multi-center outbreak of Candida tropicalis bloodstream infections associated with contaminated hemodialysis machine prime buckets. Am J Infect Control. 2021;49:1008–1013. doi:10.1016/j.ajic.2021.02.014
41. Herek TC, Menegazzo VR, Ogaki MB, et al. Biofilm formation by blood isolates of Candida parapsilosis sensu stricto in the presence of a hyperglycidic solution at comparable concentrations of total parenteral nutrition. Rev Soc Bras Med Trop. 2019;52:e20180182. doi:10.1590/0037-8682-0182-2018
42. Phua AI, Hon KY, Holt A, et al. Candida catheter-related bloodstream infection in patients on home parenteral nutrition - Rates, risk factors, outcomes, and management. Clin Nutr ESPEN. 2019;31:1–9. doi:10.1016/j.clnesp.2019.03.007
43. Santolaya ME, Thompson L, Benadof D, et al. A prospective, multi-center study of Candida bloodstream infections in Chile. PLoS One. 2019;14:e0212924. doi:10.1371/journal.pone.0212924
44. Tiraboschi IN, Pozzi NC, Farías L, et al. Epidemiología, especies, resistencia antifúngicay evolución de las candidemias en un hospital universitario de Buenos Aires, Argentina, durante 16 años. Rev Chilena Infectol. 2017;34:431–440. Spanish. doi:10.4067/S0716-10182017000500431
45. Fernández-Ruiz M, Puig-Asensio M, Guinea J, et al. Candida tropicalis bloodstream infection: incidence, risk factors and outcome in a population-based surveillance. J Infect. 2015;71:385–394. doi:10.1016/j.jinf.2015.05.009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


