Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Syringocystadenoma Papilliferum and Basal Cell Carcinoma Arising in Nevus Sebaceous
Authors Jiang J, Chen Y , He Q, Yang J, Zhang Z, Yang H, Zhang H, Yang C
Received 16 June 2022
Accepted for publication 13 September 2022
Published 23 September 2022 Volume 2022:15 Pages 2021—2026
DOI https://doi.org/10.2147/CCID.S378746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jingjing Jiang *, Yujuan Chen *, Qi He, Jiao Yang, Zhengzhong Zhang, Hao Yang, Huan Zhang, Chuan Yang
Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, Nanchong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhengzhong Zhang, Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, No. 1 Maoyuan South Road, Shunqing District, Nanchong City, Sichuan Province, 637000, People’s Republic of China, Tel +8618080339898, Email [email protected]
Abstract: Nevus sebaceous (NS) is a benign congenital hamartoma with the potential to develop into a secondary benign or malignant skin tumors. Two or more skin tumors rarely arise simultaneously from a single NS lesion. Herein, we report a case of syringocystadenoma papilliferum and basal cell carcinoma arising in an NS, based on dermoscopic, reflectance confocal microscopic, and histopathological findings.
Keywords: nevus sebaceous, syringocystadenoma papilliferum, basal cell carcinoma, dermoscopy, reflectance confocal microscopy
Introduction
Nevus sebaceous (NS) is a benign congenital hamartoma with the potential to develop into a secondary benign or malignant skin tumors. The lesion may present at birth, develop in puberty, and develop secondary tumors in adulthood.1 Most secondary tumors associated with NS are benign, and a single NS rarely develops into more than one secondary skin tumor.2,3 The most common benign secondary tumor is trichoblastoma, followed by syringocystadenoma papilliferum (SCAP). Basal cell carcinoma (BCC) is the most common malignant neoplasm associated with NS.3 Because of its potential for malignant transformation, early diagnosis and appropriate treatment are important.
Here we describe the clinical, dermoscopic, reflectance confocal microscopic (RCM), and histopathological features of SCAP and BCC arising in NS.
Case Report
A 46-year-old man presented with a hairless, yellowish asymptomatic patch on the scalp that had been present since birth. The lesion had gradually increased in size presenting as a verrucous plaque during puberty. He developed gray-brown masses in the anterolateral side and posterior of the plaque, rapidly increasing in size over the last year. On physical examination, the lesion revealed a 4 × 2 cm yellowish verrucous swelling plaque on the top of the left side of the scalp. A gray-brown nodule measuring 1 × 0.5 cm was seen in the anterolateral side of the plaque, and there was a 3 × 2 cm gray-brown nodule behind the plaque with a small amount of bleeding and exudation, surrounded by papillary papules and nodules (Figure 1).
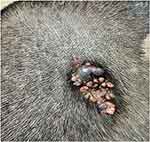 |
Figure 1 Clinical feature. The lesion on the top of the left side of the scalp. |
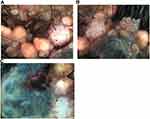
On dermoscopic examination, the lesion was found to comprise mixed structures, including solitary or aggregated yellow-white oval dots and polymorphic vessels with irregular linear and dotted vessels within papillary pink-white structures (Figure 2A and B). Further, large asymmetric blue-gray oval nests, multiple blue-gray globules, leaf-like and spoke-wheel structures, white structures, and arborizing telangiectasias were seen at the anterolateral side and posterior of the plaque (Figure 2B and C).
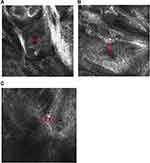
On RCM, a hyperkeratosis, roughly papillomatous epidermis, and sebaceous lobules were seen in the dermis (Figure 3A). Basal-like cell masses with peripheral palisading and focal retraction from the stroma were connected to the epidermis, mostly distributed in clumps or cords. The pigment was significantly increased (Figure 3B). Invaginations extended down from the epidermis to the dermis filled with papillary projections lined by an outer layer of tall columnar cells (Figure 3C). Infiltration of lymphocytic cells was seen in the superficial dermis.
A total excision was recommended for the patient based on the noninvasive findings, which strongly suggested that the lesion had already undergone malignant transformation. The patient subsequently underwent surgery.
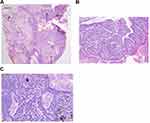
Histopathology showed epidermal hyperplasia, invaginations extending into the dermis with numerous papillary projections, and sebaceous gland hyperplasia in the dermis (Figure 4A). The papillary projections were covered by double layers of cuboidal and tall columnar cells with stroma rich in plasma cells (Figure 4B). There were basaloid cells in the dermis in the form of lump or cord, peripheral palisading, focal retraction from the stroma, and increased pigment with stroma rich in lymphocytic cells (Figure 4C). After correlation of the clinical, dermoscopic, RCM, and histopathological findings, the patient was diagnosed with SCAP and BCC associated with NS. The patient has just completed 5 months of follow-up by dermoscopy and RCM and has shown no signs of local recurrence.
Discussion
NS may present at birth and gradually develops with age.1 It can be divided into an infancy and childhood stage with undeveloped adnexal structures, a puberty stage with proliferative lesions transforming from a smooth into a verrucous plaque and involving adnexal and epidermal structures and an adulthood stage with the risk of developing secondary tumors.4 Two or more neoplasms rarely arise simultaneously from a single NS lesion.2,3 As our case showed the simultaneous presentation of SCAP and BCC arising in NS in a middle-aged man, we considered this worthy of discussion.
Over recent years, dermoscopy and RCM have improved the diagnostic accuracy in pigmented skin lesions and non-melanocytic skin lesions.5,6 These techniques improve the visibility of the skin microstructures from the epidermis to the superficial dermis.4 However, there are few reports of dermoscopy and RCM in relation to adnexal neoplasms, and only one report of RCM characteristics of SCAP.7 Thus, dermoscopic and RCM features of SCAP have not been well defined to date.
On dermoscopy, NS shows round-shaped structures of a yellowish-white color, grouped or presented singly.5 On RCM, the junction of the epidermis and the superficial dermis shows a grape cluster sebaceous gland structure, where the center is a tubular or stalk structure and the peripheral area consists of ovoid hyperplasia of sebaceous lobules. A verrucous or papillomatous hyperplasia of the upper epidermis can also be seen.8 In our case, the patient presented with solitary or aggregated yellow-white oval dots on dermoscopy, hyperkeratosis, a rough papillomatous epidermis, and sebaceous lobules in the dermis on RCM, which are typical features of NS. These findings were highly consistent with NS histology.
SCAP is a rare benign skin adnexal tumor with different clinical features but a characteristic histology, and usually arises from NS.5 There have only been four reports describing the dermoscopic features of SCAP arising from NS.4,5,9,10 The polymorphous vascular pattern on a pinkish-white background showing irregular linear, glomerular vessels, some of which were surrounded by a whitish halo and others grouped in a horseshoe arrangement was first described by Bruno et al.5 Zaballos and coworkers found the most frequent pattern associated with SCAP was a symmetric, erythematous lesion with exophytic papillary structures, ulceration, and vessels.4 Duman and colleagues also observed polymorphic vessels including irregular, dotted, hairpin-like, glomerular, and linear vessels on a central yellowish-white discoloration and a surrounding pinkish-white rim with peripheral hairpin-like vessels.9 On RCM, Karaarslan and colleagues observed “dark linear clefts” which were invaginations extending from the surface epithelium into the underlying dermis on histology and “grayish epithelial islands” within or around dark clefts which were the characteristic papillary structures on histology.7 In our case, we also observed polymorphic vessels with irregular linear and dotted vessels within papillary pink-white structures. However, we did not find the very representative RCM features of SCAP reported by Karaarslan. We also observed that the cystic invaginations extended down from the epidermis to the dermis and that the inner layer was composed of tall columnar cells. Lymphocytic cell infiltration was seen in the superficial dermis. SCAP arising from NS was therefore suggested, according to the findings, and was ultimately confirmed by histology. Thus, we inferred that polymorphous vascular patterns may be a common appearance in the dermoscopy of SCAP and that linear vessels may be the most common vascular type. More cases need to be evaluated through dermoscopy and RCM to establish the diagnostic significance of these findings.
BCC is a common malignant tumor worldwide.11 It is already known that dermoscopy and RCM achieve high accuracy in the diagnosis of BCC on the basis of its typical features with the two techniques.6,11 On dermoscopy, BCC usually presents as asymmetric large blue-gray ovoid nests, multiple blue-gray globules, leaf-like structures, white structures, spoke-wheel structures, arborizing telangiectasias and small fine telangiectasias.4,11 On RCM, epidermal disarray, basaloid tumor nests with peripheral palisading, and a surrounding cleft can be seen.6 Our case also showed the common imaging patterns. The observed dermoscopic and RCM features of BCC were consistent with the previous reports and correlated well with known histopathological characteristics.
In terms of the relationship among SCAP, BCC, and NS, SCAP is able to mimic BCC clinically and transform into BCC. While deletion at the PTCH gene can be detected in NS, SCAP, and BCC, it is speculated that SCAP may be the transitional step from NS to BCC.12,13 In this case, the lesion may be seen under the dermoscopy with linear and irregular blood vessels among the partially uniform light yellow structures, while the color of some structures changed to blue-gray, suggesting that NS may be transforming to the malignant tumor stage. Combined with the history and auxiliary examination results, we considered that SCAP and BCC in this patient were secondary to NS.
Conclusion
We presented an interesting case of SCAP and BCC simultaneously arising in NS. We used two noninvasive tools, dermoscopy and RCM to evaluate the lesion and proposed the initial diagnosis, then performed a total excision on the lesion without an initial biopsy. These findings indicated that dermoscopy and RCM are two effective techniques for evaluation of these lesions and may help early detection and diagnosis of diseases and also evaluate the conditions for recovery. We therefore advocate for dermatologists to make full use of the effective noninvasive tools in the clinical examination of a suspected skin lesion.
Ethics and Consent Statement
Written consent was obtained from the patient for the publication of the case details and accompanying images. Institutional approval was not required to publish the case details.
Acknowledgments
We would like to thank the patient and physicians for participating in our research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gozel S, Donmez M, Akdur NC, Yikilkan H. Development of six tumors in a sebaceus nevus of jadassohn: report of a case. Korean J Pathol. 2013;47(6):569–574. doi:10.4132/KoreanJPathol.2013.47.6.569
2. Fathaddin A, Almukhadeb E. A rare occurrence of sebaceous carcinoma, sebaceoma, syringocystadenoma papilliferum, and trichoblastoma in a single nevus sebaceous lesion. Case Rep Dermatol. 2021;13(2):271–277. doi:10.1159/000516351
3. Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70(2):332–337. doi:10.1016/j.jaad.2013.10.004
4. Zaballos P, Serrano P, Flores G, et al. Dermoscopy of tumours arising in naevus sebaceous: a morphological study of 58 cases. J Eur Acad Dermatol Venereol. 2015;29(11):2231–2237. doi:10.1111/jdv.13226
5. Bruno CB, Cordeiro FN, Soares Fdo E, Takano GH, Mendes LS. Dermoscopic aspects of syringocystadenoma papilliferum associated with nevus sebaceous. An Bras Dermatol. 2011;86(6):1213–1216. doi:10.1590/S0365-05962011000600027
6. Chuah SY, Tee SI, Tan WP, et al. Reflectance confocal microscopy is a useful non-invasive tool in the in vivo diagnosis of pigmented basal cell carcinoma in Asians. Australas J Dermatol. 2015;58(2):130–134. doi:10.1111/ajd.12401
7. Karaarslan I, Acar A, Yaman B, Ozdemir F. Reflectance confocal microscopic findings of a tiny syringocystadenoma papilliferum. JAAD Case Rep. 2019;5(4):323–325. doi:10.1016/j.jdcr.2019.02.023
8. Jiang Q, Chen HY, Ma L, Huang M, Xia Y, Chen LQ. Characteristic analysis of sebaceous nevus using dermoscopy and reflectance confocal microscopy. Chin J Dermatol. 2018;51(7):523–525.
9. Duman N, Ersoy-Evans S, Erkin Özaygen G, Gököz Ö. Syringocystadenoma papilliferum arising on naevus sebaceus: a 6-year-old child case described with dermoscopic features. Australas J Dermatol. 2015;56(2):e53–e54. doi:10.1111/ajd.12218
10. Lombardi M, Piana S, Longo C, et al. Dermoscopy of syringocystadenoma papilliferum. Australas J Dermatol. 2018;59(1):e59–e61. doi:10.1111/ajd.12654
11. Reiter O, Mimouni I, Gdalevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(5):1380–1388. doi:10.1016/j.jaad.2018.12.026
12. Askar S, Kilinc N, Aytekin S. Syringocystadenoma papilliferum mimicking basal cell carcinoma on the lower eyelid: a case report. Acta Chir Plast. 2002;44(4):117–119.
13. Böni R, Xin H, Hohl D, Panizzon R, Burg G. Syringocystadenoma papilliferum: a study of potential tumor suppressor genes. Am J Dermatopathol. 2001;23(2):87–89. doi:10.1097/00000372-200104000-00001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.