Back to Journals » Drug Design, Development and Therapy » Volume 15
Synthesis, Radiolabeling, and Preliminary in vivo Evaluation of [68Ga] IPCAT-NOTA as an Imaging Agent for Dopamine Transporter
Authors Farn SS , Chang KW, Lin WC, Yu HM , Lin KL, Tseng YC, Chang Y, Yu CS, Lin WJ
Received 24 October 2020
Accepted for publication 11 May 2021
Published 17 June 2021 Volume 2021:15 Pages 2577—2591
DOI https://doi.org/10.2147/DDDT.S288600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Shiou-Shiow Farn,1,2 Kang-Wei Chang,3 Wan-Chi Lin,1 Hung-Man Yu,1 Kun-Liang Lin,1 Yu-Chin Tseng,1 Yu Chang,1 Chung-Shan Yu,2,4 Wuu-Jyh Lin1
1Isotope Application Division, Institute of Nuclear Energy Research, Taoyuan, 32546, Taiwan; 2Department of Biomedical Engineering and Environmental Sciences, National Tsing-Hua University, Hsinchu, 300, Taiwan; 3Laboratory Animal Center, Office of Research and Development, Taipei Medical University, Taipei, 11031, Taiwan; 4Institute of Nuclear Engineering and Science, College of Nuclear Science, National Tsing-Hua University, Hsinchu, 300, Taiwan
Correspondence: Wuu-Jyh Lin; Chung-Shan Yu Email [email protected]; [email protected]
Introduction: Novel radiotracer development for imaging dopamine transporters is a subject of interest because although [99mTc]TRODAT-1, [123I]β-CIT, and [123I]FP-CIT are commercially available; 99Mo/99mTc generator is in short supply and 123I production is highly dependent on compact cyclotron. Therefore, we designed a novel positron emission tomography (PET) tracer based on a tropane derivative through C-2 modification to conjugate NOTA for chelating 68Ga, a radioisotope derived from a 68Ge/68Ga generator.
Methods: IPCAT-NOTA 22 was synthesized and labeled with [68Ga]GaCl4− at room temperature. Biological studies on serum stability, LogP, and in vitro autoradiography (binding assay and competitive assay) were performed. Furthermore, ex vivo autoradiography, biodistribution, and dynamic PET imaging studies were performed in Sprague Dawley rats.
Results: [68Ga]IPCAT-NOTA 24 obtained had a radiochemical yield of ≥ 90% and a specific activity of 4.25 MBq/nmol. [68Ga]IPCAT-NOTA 24 of 85% radiochemical purity (RCP%) was stable at 37°C for up to 60 minutes in serum with a lipophilicity of 0.88. The specific binding ratio (SBR%) reached 15.8 ± 6.7 at 60 minutes, and the 85% specific uptake could be blocked through co-injection at 100- and 1000-fold of the cold precursor in in vitro binding studies. Tissue regional distribution studies in rats with [68Ga]IPCAT-NOTA 24 showed striatal uptake (0.02% at 5 minutes and 0.007% at 60 minutes) with SBR% of 6%, 25%, and 62% at 5– 15, 30– 40, and 60– 70 minutes, respectively, in NanoPET studies. The RCP% of [68Ga]IPCAT-NOTA 24 at 30 minutes in vivo remained 67.65%.
Conclusion: Data described here provide new information on the design of PET probe of conjugate/pendent approach for DAT imaging. Another chelator or another direct method of intracranial injection must be used to prove the relation between [68Ga]IPCAT-NOTA 24 uptake and transporter localization.
Keywords: Parkinson disease, dopamine transporter, Ga-68
Introduction
Dopamine transporter (DAT), a protein complex located in presynaptic dopaminergic nerve terminals, serves as the primary means for removing dopamine from the synaptic cleft.1,2 DAT provides an excellent neurochemical marker for the density and structural integrity of the dopaminergic system in inactivating and recycling dopamine.1 The regional brain distribution of DATs was largely concentrated in the striatum, with much lower levels in the amygdala, hippocampus, substantia nigra, and ventral tegmental area.2,3 Therefore, measuring the decrease in DAT may be a useful indicator of dopamine neuronal loss.4
Most DAT imaging agents for positron emission tomography (PET) and single photon emission computed tomography (SPECT) are based on tropane derivatives,5 such as [11C]CFT, [11C]WIN-35428, [18F]FE-PE2I, [123I]β-CIT, [123I]FP-CIT, [123I]Altropane, and [99mTc]TRODAT-1. Of these, [99mTc]TRODAT-1, [123I]β-CIT, and [123I]FP-CIT were commercialized for clinical application.6 However, the disadvantage of [123I]β-CIT is its lack of specificity, and adequate imaging should be performed 20–30 hours after injection. [99mTc]TRODAT-1, one of the conjugate/pendent approaches for cocaine-based SPECT imaging agents through N-8 or C-2 modification, was first published by Prof. Kung at the University of Pennsylvania, USA.6–10 [99mTc]TRODAT-1, which is technetium based, is relatively inexpensive and available in kit form. However, its specific signal is lower than that of 123I-based SPECT tracers.11
In recent years, 68Ga has become a superior PET radionuclide due to increased photon flux per unit of radiation dose delivered to a patient and broad clinical usage.12,13 It has the following advantages: (a) a short 68-minute physical half-life with 89% β+ emission; (b) an alternative to well-established radiopharmaceuticals based on 99mTc, 111In, 123/124I, 18F, and 11C; (c) less expensive compared with 123I; and (d) high detection sensitivity and resolution, fast data acquisition, dynamic scanning, and low radiation effective dose.13
Several 68Ga-labeled imaging agents have been clinically applied, particularly in oncology, such as [68Ga]DOTANOC, [68Ga]DOTATOC, [68Ga]DOTATATE, and [68Ga]HA-DOTATATE for imaging somatostatin receptors,14 [68Ga]DOTA-PSMA-617 for imaging prostate-specific membrane antigen,15 and [68Ga]DOTA-Pentax for imaging chemokine receptor CXCR4 based on DOTA as chelator. However, for brain research, only one report of [68Ga]TC3-OGDOTA for Alzheimer disease imaging was published.16 Another chelator, NOTA (1,4,7-triazacyclononane-N,N′,N″-triacetic acid), and its derivatives are promising stable chelators for 68Ga for targeting proteins and peptides.17
To date, although [99mTc]TRODAT-1, [123I]β-CIT, and [123I]FP-CIT are commercially supplied, novel radiotracer development for imaging dopamine transporters is still a subject of interest because of the shortage in the supply of 99Mo/99mTc generator and high dependence on compact cyclotron for [123I] production. Therefore, we designed a novel PET tracer based on tropane derivative through C-2 modification to conjugate NOTA for chelating 68Ga, a radioisotope derived from a 68Ge/68Ga generator.
Materials and Methods
Chemistry
Reagents used in the syntheses were purchased from Sigma-Aldrich (Sweden), MACRON (USA), J.T.Baker (USA) or Alfa Aesar (USA) and used without further purification unless otherwise indicated. Anhydrous Na2SO4 was used as a drying agent. 68GaCl3 was obtained from a 68Ge/68Ga generator (IGG100, Eckert & Ziegler, Berlin, Germany) and eluted with 0.1 N ultrapure HCl (J.T. Baker, Center Valley, PA, USA). Reaction yields are reported without attempts at optimization. Melting points were determined in open capillary tubes with a Buchi apparatus and are uncorrected. IR spectra were obtained on a Perkin-Elmer 283 spectrophotometer (KBr pellets for solid or nujol for liquid). 1H NMR spectra were determined on a Varian 390 or Bruker 300 MHz instrument. Chemical shifts are given in ä values downfield from Me4Si as an internal standard. Mass spectra were recorded on a Hewlett-Packard 5995c GC-MS low-resolution spectrometer. All compounds showed appropriate IR, 1H NMR, and mass spectra. Elemental analyses were performed on a Hewlett-Packard 185 C, H, N analyzer and agreed with theoretical values within (0.40%. Silica gel 60 (Merck 70–230 mesh)) was used for column chromatography.
Preparation of Ecgonine Hydrochloride 2
Cocaine hydrochloride (1) (30.0 g, 99.6 mmol) was dissolved in 0.8N HCl (35% w/w, 150 mL) and heated at 105°C for 24 hrs. The resulting mixture was cooled to room temperature and left to stand for 30 minutes to 1 hr at 4°C. Precipitated benzoic acid was removed by washing with 200 mL of ether for three times. The water layer was collected, evaporated to dryness, then redissolved in CHCl3 (500 mL) and stirred for 1 hr and evaporated under reduced pressure, and the resulting residue gave the corresponding compounds (2) with 100% yield (18.4 g). 1H-NMR (CD3OD):δ 4.35 (m, 1 H, H3), 4.10 (d, 1 H, H1), 3.88 (m, 1 H, H5), 3.15 (dd, 1 H, H4), 2.82 (s, 3 H, NCH3), 2.36 (m, 2 H, H6 and H7), 2.10 (m, 3 H, H4 and H6 and H7)
Preparation of (R)-(-)-Anhydroecgonine Methyl Ester 3
Ecgonine hydrochloride (2) (18.4 g, 99.5 mmol) was dissolved in 100 mL of POCl3 and heated at 110°C to reflux for 3 hrs, the resulting mixture was cooled to room temperature and concentrated in vacuum under nitrogen gas, 200 mL of anhydrous methanol was added to sticky compounds and stirred until completely dissolved under ice water bath, then remove the water bath and stirred for overnight. Water (150 mL) and NaOH were then added and the reaction mixture was extracted into CH2Cl2 (3 x 100 mL). The organic layers were combined, dried over Na2SO4, and concentrated in vacuum under nitrogen gas. The resulting mixture gave the corresponding compound (4) with 15.6 g, 86.1 mmol and 87% yield. 1H-NMR (CD3OD):δ 6.81 (m, 1 H, H3), 3.98 (d, J = 5.6 Hz, 1 H, H1), 3.94 (s, 3 H, OCH3), 3.45 (m, 1 H, H5), 2.83 (d, br, J = 19.8 Hz, 1 H, H4), 2.54 (s, 3 H, NCH3), 2.36 (m, 2 H, H6 and H7), 2.04 (m, 2 H, H4 and H7), 1.72 (m, 1 H, H6).
Preparation of 2β-Carbomethoxy-3β-(4-Chlorophenyl)Tropane 4
Compound (3) (24.2 g, 133 mmol) dissolved in anhydrous CH2Cl2 (50 mL) was added dropwise of a stirred solution of the 1.0 M 4-chlorophenyl magnesium bromide (155 mL, 155.8 mmol) in solution of anhydrous CH2Cl2 (250 mL) at −40°C~-45°C under nitrogen gas for 3 hours. The mixture solution was cooled to −78°C, and a solution of CF3COOH (12 mL, 155.8 mol) in anhydrous CH2Cl2 (10 mL) was added under nitrogen gas. The mixture was allowed to warm to room temperature and stirred for 30 min. H2O (300 mL) was added, then pH was adjusted to 1 followed by acidifying with 12N HCl, it was neutralized by NaOH, after partition, water layers were combined, then was extracted into ether (3 x 200 mL). The ether layers were combined, dried over Na2SO4, and concentrated in vacuum under nitrogen gas. The resulting mixture was purified by chromatography to obtain colorless solid corresponding compounds (4) with 45.7% yield (17.8 g). 1H-NMR(CDCl3):δ 7.20 (AB, J = 8.8 Hz, 4 H, C6, H4), 3.56 (m, 1 H, H1), 3.50 (s, 3 H, OCH3), 3.35 (m, 1 H, H5), 2.96 (td, 1 H, H3), 2.87 (m, 1 H, H2), 2.55 (td, 1 H, H4), 2.22 (s, 3 H, NCH3), 2.02–2.28 (m, 2 H, H6 and H7), 1.48–1.78 (m, 3 H, H4, H6 and H7).
Preparation of 3β-(4-Chlorophenyl)-Nortropane-2β-Carboxylic Methyl Ester 5
To a stirred solution of the ecgonine hydrochloride (4) (2.0g, 6.8 mmol) in CH2Cl2 (100 mL) were added 1-chloroethyl-chloroformate (2.9g, 20.5 mmol). Stirring was prolonged at 50°C for 24 hours under nitrogen gas. The mixture was concentrated under reduced pressure followed by addition of 100 mL of CH3OH and stirred at room temperature for 4 hours. The organic layer was separated, washed with water and saturated brine, and dried over Na2SO4. The filtrate was concentrated under reduced pressure to give the corresponding compound (5) with 80% yield (1.6 g). LTQ-MS: m/z calculated for C15H18ClNO2 [M+H]+ = 280.11, found 280.17.
Preparation of (E) 3-Iodoacrylic Acid 7
To propiolic acid (6) (6.8g, 97.3 mmol) in a double necked glass bottle under oil bath and nitrogen gas was added HI and stirred gently. The resulting mixture was stirred for 12 hours until precipitation of white crystal solid powder. The white crystal solid powder was filtered, washed with water and n-hexane, and was concentrated under reduced pressure to give the corresponding compounds (7) with 79% yield (15.4 g). 1H NMR (300 MHz, CDCl3) δ 8.09 (d, J = 14.8 Hz, 1H), 6.90 (d, J = 14.8 Hz, 1H).
Preparation of (E) Ethyl 3-Iodoprop-2-Enoic Acid 8
To a stirred solution of the (E) 3-Iodoacrylic acid (7) (5 g, 25.3 mmol) in absolute C2H5OH (30 mL) in a double necked glass bottle was added H2SO4 (1.7 mL, 30.3 mmol). Stirring was prolonged at stirring temperature of 80°C and reflux for 24 hours under nitrogen gas. Saturated NaHCO3 was added dropwise to the orange resulting mixture until pH 7.4. The organic layer was separated, washed with EtOAc (20 mL) and saturated brine, and dried over MgSO4, and solvent was distilled under reduced pressure. The resulting orange viscostic residue was dissolved in ether (1 mL) and that was chromatographed on silica gel (Et2O/n-hexane = 15:85, Rf = 0.62) to obtain the corresponding compound (8) with 40% yield (2.3 g). 1H NMR (300 MHz, CDCl3) δ 7.87 (d, J = 14.8 Hz, 1H), 6.87 (d, J = 14.9 Hz, 1H), 4.20 (q, J = 7.1 Hz, 2H), 1.29 (t, J = 7.1 Hz, 3H).
Preparation of (E) 3-Iodoprop-2-En-1-Ol 9
To a stirred solution of the (E) Ethyl 3-Iodoprop-2-enoic acid (8) (2.3g, 10.3 mmol) in CH2Cl2 (40 mL) in a double necked glass bottle was added (i-Bu2AlH)2 (2.8g, 20.0 mmol). Stirring was prolonged at 600 rpm and temperature of −78°C for 45 minutes under nitrogen gas; the reaction mixture was allowed to warn to 0°C. Methanol (20 mL), 70% CH3OH (20 mL) and water (20 mL) were sequentially added to the resulting solution and stirred at room temperature for 5 min. The white resulting mixture was extracted with 10% Potassium sodium tartrate (30 mL) and CH2Cl2 (10 mL) for three times. The organic layer was collected and extracted with 10% KNaC4H4O6·4H2O (30 mL) for three times, washed with saturated brine, and dried over Na2SO4. Solvent was distilled at 35°C under reduced pressure, the mixture residues was chromatographed on silica gel (60% Ether, Rf = 0.45) and stained with 15% H2SO4 to obtain the corresponding pale compound (9) with 38% yield (710 mg). 1H NMR (300 MHz, CDCl3) δ 6.70 (dt, J = 14.5 Hz, 1H), 6.40 (dd, J = 14.5 Hz, 1H), 4.10 (dd, J = 5.0 Hz, 2H), 1.73 (s, 1H, -OH).
Preparation of (E)-3-Iodo-2-Propenyl-1-Methylbenzenesulfonate 10
To a stirred solution of the (E) 3-iodoprop-2-en-1-ol (9) (710 mg, 3.9 mmol) in CH2Cl2 (10 mL) in a two necked glass bottle were added C14H14O5S2 (1.3 g, 3.9 mmol) and C10H21N (1.0 mL, 5.8 mmol). Stirring was prolonged at temperature of 50°C for 12 hours under nitrogen gas. The reaction mixture was concentrated under reduced pressure. The resulting residue was diluted with CH2Cl2 (3 mL) and extracted with n-hexane (20 mL), The organic layer was collected and chromatographed on silica gel (10% EtOAc, Rf = 0.45) and stained with KMnO4(eq) to obtain the corresponding compound (10) with 81% yield (1.06 g). 1H NMR (300 MHz, CDCl3) δ 7.77 (dd, 2H), 7.35 (dd, 2H), 6.52 (m, 2H), 4.43 (dd, 2H), 2.45 (s, 3H).
Preparation of (E) N-3-Iodo-2-Propen-1-Yl-2β-Carbomethoxy-3β-(4-Chlorophenyl)nortropane 11
To a stirred solution of the (E)-3-iodo-2-propenyl-1-methylbenzenesulfonate (10) (0.5 g, 1.6 mmol) in CH2Cl2 (10 mL) in a two necked glass bottle were added 3β-(4-chlorophenyl)-nortropane-2β-carboxylic methyl ester (5) (0.4 g, 1.6 mmol) and K2CO3 (0.2 g, 1.6 mmol) as a catalytic agent. Stirring was prolonged at temperature of 50°C for 20 hours under nitrogen gas, the appearance of the reaction mixture was changed from orange to turbidity and p-toluenesulfonic salt was salt out. The resulting mixture was chromatographed on silica gel (EtOAc/n-hexane = 1:4, Rf = 0.48) and stained with KMnO4(eq) to obtain the corresponding compound (11) with 76% yield (534 mg). LTQ-MS: m/z calculated for C18H21ClNO2 [M+H]+ = 446.04, found 446.13. 1H NMR (300 MHz, CDCl3) δ 7.24–7.16 (m, 4H), 6.52–6.45 (m, 1H), 6.20 (d, J = 14.4 Hz, 1H), 3.65 (s, 1H), 3.53 (s, 3H), 3.39 (s, 1H), 2.99–2.94 (m, J = 5.3 Hz, 1H), 2.85–2.75 (m, J = 6.5 Hz, 1H), 2.57 (td, J = 2.8 Hz, 1H), 2.15–1.91 (m, J = 4.5 Hz, 3H), 1.72–1.61 (m, 3H).
Preparation of (E) N-3-Iodo-2-Propen-1-Yl-2β-Carboxyl-3β-(4-Chlorophenyl)Nortropane 12
To a stirred solution of the (E) N-3-iodo-2-propen-1-yl-2β-carbomethoxy-3β- (4-chlorophenyl)nortropane (11) (115.7 mg, 0.3 mmol) in solution of 1,4-dioxane:H2O = 1:1 (10 mL) at 110°C for 7 days. The reddish resulting mixture was concentrated under reduced pressure and dried in vacuum to remove solvent. Then purified by silica gel (CH2Cl2:CH3OH = 9:1) to obtain white solid corresponding compound (12) with 31% yield (35 mg) and chemical purity of 99%. LTQ-MS: m/z calculated for C17H19ClNO2 [M + H]+ = 432.02, found 432.17. 1H NMR (300 MHz, CDCl3) δ 1.83–1.72 (m, 2H), 1.90 (m, J = 6.3 Hz, 2H), 2.17 (m, J = 9.3, 5.3 Hz, 2H), 2.36 (to, J = 13.5 Hz, 1H, CH, H-2), 2.67 (d, J = 6.0 Hz, 1H, CH, H-1), 3.10 (t, J = 5.6 Hz, 2H, CH2, H-1ʹ), 3.11 (q, J = 6.0Hz, 1H, CH), 3.6 (s, 2H), 6.47 (d, J = 15.0 Hz, 1H, CH, H-3ʹ), 6.62 (t, 1H, CH, H-2ʹ), 7.14 (d, J = 9.0 Hz, 2H, Harom., meta), 7.27 (d, J = 9.0 Hz, 2H, Harom., ortho).
Preparation of 1,4-Di-Tert-Butyl 2,2ʹ-(7-(2-Ethoxyl)-1,4,7-Triazonan-1,4-Diyl) Diacetate 16
To a stirred solution of the NOTA(t-Bu)2 (14) (200 mg, 0.6 mmol) and 2-Bromoethanol (15) (70 mg, 0.6 mmol) in CH2Cl2 (10 mL) were added Et3N (41.8 ul, 0.3 mmol) as a catalytic agent. Stirring was prolonged at temperature of 50°C for 12 hours, the resulting mixture was purified by MPLC (RP-18 column, 250×4.1 mm, flow rate 1 mL/min, gradient program of 10% CH3OH to 90% CH3OH in 15 minutes). The residue was concentrated under reduced pressure to give the 96.4 mg yellow pale oil corresponding compounds (16) with 85% yield (96.4 mg). LTQ-MS: m/z calculated for C20H39N3O5 [M + H]+ = 402.30, found 402.39.
Preparation of 2,2ʹ-(7-(2-Ethoxyl)-1,4,7-Triazonan-1,4-Diyl)Diacetic Acid 17
To a stirred solution of the 1,4-di-tert-butyl 2,2ʹ-(7-(2-ethoxyl)-1,4,7-triazonan- 1,4-diyl)diacetate (16) (96.4 mg, 0.2 mmol) in solution of CH2Cl2:TFA = 1:1 (10 mL). Stirring was prolonged at room temperature for 4 hours, ice-cooled ether was added dropwise to stirred solution, water (5 mL) was added and evaporated under reduced pressure, and the white solid corresponding compound (17) was obtained in 99% yield (69 mg). LTQ-MS: m/z calculated for C12H23N3O5 [M + H]+ = 290.17, found 290.4.
Preparation of N-t-Boc-(O-p-Tosyl)-2-Aminoethanol 18
To a stirred solution of the N-t-Boc-ethanolamine (1 g, 6.2 mmol) in CH2Cl2 (10 mL) and Et3N (1.9 mL, 13.6 mmol) above ice bath, p-TsCl (1.4 g, 7.4 mmol) was dissolved in CH2Cl2 (10 mL) that was added dropwise to stirred solution of the N-t-Boc-ethanolamine. Stirring was prolonged at room temperature for 48 hours under nitrogen gas, the resulting mixture was extracted with 10% NH4Cl (5 mL) twice and washed with saturated brine (10 mL) and dried over MgSO4, and organic layer was concentrated under reduced pressure to provide the white solid corresponding compound (18) with 84% yield (1.6 g). 1H NMR (300 MHz, cdcl3) δ 7.79 (d, J = 8.3 Hz, 1H), 7.39–7.33 (d, 1H), 4.10–4.03 (t, 1H), 3.38 (s, 1H), 2.45 (s, 2H), 1.41 (s, 5H).
Preparation of 1,4-Di-Tert-Butyl 2,2ʹ-(7-(2-Aminoethyl)-1,4,7-Triazonan-1,4-Diyl)Diacetate 19
To a stirred solution of the NOTA(t-Bu)2 (14) (200 mg, 0.6 mmol) and N-t-Boc-(O-p-tosyl)-2-aminoethanol (18) (264.6 mg, 0.8 mmol) in CH2Cl2 (5 mL) were added Et3N (0.1 mL, 0.7 mmol) as a catalytic agent. Stirring was prolonged at room temperature for 12 hours, the resulting mixture was purified by MPLC (PRP-1 column, 250×4.1 mm, flow rate 1 mL/min, gradient program of 10% CH3OH to 90% CH3OH in 15 minutes). The residue of LTQ-M responded data were collected and concentrated under reduced pressure to afford the pale oil corresponding compounds (19) with 52% yield (148 mg). LTQ-MS: m/z calculated for C25H48N4O6 [M+H]+ = 501.37, found 501.47.
Preparation of 2,2ʹ-(7-(2-Aminoethyl)-1,4,7-Triazonan-1,4-Diyl)Diacetic Acid 20
To a stirred solution of the 1.4-di-tert-butyl 2,2ʹ-(7-(2-aminoethyl)- 1,4,7-triazonan-1,4-diyl)diacetate (19) (148 mg, 0.3 mmol) in solution of CH2Cl2:TFA = 1:1 (10 mL). Stirring was prolonged at room temperature for 4 hours, ice-cooled ether was added dropwise to stirred solution, H2O (5 mL) was added to dilute, and solvent was distilled under reduced pressure, the resulting residue (20) was obtained in 93% yield (80 mg). LTQ-MS: m/z calculated for C12H24N4O4 [M+H]+ = 289.19, found 289.23.
Preparation of 8-[(2E)-3-Iodo-2-Propenyl]-2β-[(2-(4,7-Bis(2-Ethylcarboxyl) −1,4,7-Triazonan-1-Yl)Ethyl)Oxy]Carbonyl-3β-(p-Chlorophenyl)Nortropane (IPCET-NOTA) 21
To a stirred solution of the (E) N-3-iodo-2-propen-1-yl-2β-carboxyl- 3β-(4-chlorophenyl)nortropane (12) (20.5 mg, 0.04 mmol) in anhydrous CH2Cl2 (1 mL) in reaction vial under nitrogen gas was added oxalyl chloride (12.0 mg, 0.09 mmol) immediately. Stirring was prolonged at room temperature for 3 hours. The resulting mixture (13) was concentrated under reduced pressure and dried in vacuum for 6 hours to remove oxalyl chloride. 2,2ʹ-(7-(2-ethoxy) −1,4,7-triazonan-1,4-diyl)diacetic acid (17) (83.3 mg, 0.3 mmol) was dissolved in anhydrous DMF and added TEA (199 μL, 1.4 mmol), the solution was added to the reaction vial under reduced pressure for 2 hours. The resulting mixture was purified by HPLC (PRP-1 column, 250×4.1 mm, flow rate 1 mL/min, gradient program of 10% CH3OH to 90% CH3OH in 15 minutes) to obtain the white solid corresponding compounds (21) with less than 2% yield (0.2 mg) and less than 5% of chemical purity. LTQ-MS: m/z calculated for C29H41ClIN4O6 [M+H]+ = 703.18, found 703.08.
Preparation of 8-[(2E)-3-Iodo-2-Propenyl]-2β-[2-(4,7-Bis(2-Ethylcarboxyl) −1,4,7-Triazonan-1-Yl)Ethyl]Carboxamido-3β-(p-Chlorophenyl)Nortropane (IPCAT-NOTA) 22
To a stirred solution of the (E) N-3-iodo-2-propen-1-yl-2β-carboxyl- 3β-(4-chlorophenyl) nortropane (12) (17.6 mg, 0.04 mmol) in anhydrous CH2Cl2 (1 mL) in reaction vial under nitrogen gas was added oxalyl chloride (10.4 mg, 0.08 mmol) immediately. Stirring was prolonged at room temperature for 3 hours. The resulting mixture (13) was concentrated under reduced pressure and dried in vacuum for 6 hours to remove oxalyl chloride. 2,2ʹ-(7-(2-aminoethyl)-1,4,7-triazonan-1,4-diyl)diacetic acid (20) (23.5 mg, 0.08 mmol) was dissolved in anhydrous DMF and added TEA (22.7 μL, 0.16 mmol) as a catalytic agent, the solution was added to the reaction vial under reduced pressure for 2 hours. The resulting mixture was purified by HPLC (PRP-1 column, 250×4.1 mm, flow rate 1 mL/min, gradient program of 10% CH3OH to 90% CH3OH in 15 minutes) to obtain the white solid corresponding compounds (22) with 2% yield (0.6 mg). LTQ-MS: m/z calculated for C29H41ClIN5O5 [M+H]+ = 702.19, found 702.15.
Synthesis of [68Ga]IPCET-NOTA 23
For radiolabeling, 0.1 mL [68Ga]GaCl3 (~74 MBq) and 10 μg (14.2 nmol) of IPCAT-NOTA 21 were added to 30 μL 1M HEPES buffer. The final pH of the reaction mixture was 4.1 ~ 4.3, which incubated at room temperature for 15 minutes. For quality control, the product was analyzed by radio-TLC, silica gel plate TLC-SG (1.5 × 6 cm) with 0.1 M EDTA as the mobile phase was used. In this system, radiolabeled ligand remain at the origin (Rf = 0–0.1) while free gallium-68 migrates with the solvent front (Rf = 0.8–1).
Synthesis of [68Ga]IPCAT-NOTA 24
For radiolabeling, 0.1 mL [68Ga]GaCl3 (~74 MBq) and 10 μg (14.2 nmol) of IPCAT-NOTA 22 were added to 30 μL 1M HEPES buffer. The final pH of the reaction mixture was 4.1 ~ 4.3, which incubated at room temperature for 15 minutes. For quality control, the product was analyzed by radio-TLC, silica gel plate TLC-SG (1.5 × 6 cm) with 0.1 M EDTA as the mobile phase was used. In this system, radiolabeled ligand remained at the origin (Rf = 0–0.1) while free gallium-68 migrates with the solvent front (Rf = 0.8–1).
Biological
Adult male SD rats (BioLASCO Taiwan Co., Taipei, Taiwan), approximately six weeks old and 193–224 g at the beginning of the experiments were maintained under an artificial 12-h light/dark cycle (light on from 8:00 a.m. to 8:00 p.m.) at a constant temperature, and maintained at 21 ± 2°C with 50 ± 20% relative humidity. Food and water were freely available, and the animals were acclimated for >7 days before use. Experiments were performed between 8:00 a.m. and 2:00 p.m. All the animal experiments were conducted in accordance with “A Guidebook for the care and use of laboratory animals (3rd edition, 2010)”. The animal experiment application was filed to the Ethical Animal Use Committee of Institute of Nuclear Energy Research (INER) and approved in 2015 with the IACUC number of 104132. The application indicated the personnel participated in animal experiments (including the experience for performing animal experiments, the education and training procedures), the number of animals required, the methods and procedures of animal experiments (including methods and procedures for reducing animal suffering and humane treatment process), animal disposal methods after the end of the experiment, etc. The experience, education, and training for the personnel participated in animal experiments also indicated on the application form. All the experiments were carried out after approval by the Laboratory Animal Care and Use Committee of INER.
Determination of Partition Coefficients (LogP)
The partition coefficients (log P) of [68Ga]IPCAT-NOTA 24 was determined by adding 3.7 MBq of labeled complex to a solution containing 2.5 mL of 1-octanol and 2.5 mL of phosphate buffer saline (PBS; pH 7.4). The resulting solutions were then vigorously vortexed for 10 minutes at room temperature and centrifuged for 2 minutes at 1000 rpm. Octanol (0.1 mL) was removed and back extracted with 0.5 mL of 1-octanol and 2.5 mL of PBS, repeat steps sixfold. Finally, octanol (2 mL) was removed and counted. Aliquots samples were taken from each phase and assayed the radioactivity in a gamma counter (1470 WIZARD Gamma Counter, Wallac, Finland). The partition coefficient was calculated as a ratio of counts in the octanol fraction to counts in the water fraction per extraction. The experiment was performed in triplicate.
In vitro Serum Stability
Serum preparation: Health SD rat was deeply anesthetized with isoflurane gas (3% isoflurane in 50% oxygen, 1 mL/min). 1 mL whole blood of health SD rat was withdrawn through tail vein at room temperature and centrifuged at 3000 rpm/min for 10 minutes to achieve a prepared serum for stability test.
Stability in serum. The stability was studied by incubation of 3.7MBq of [68Ga]IPCAT-NOTA 24 in 200uL of prepared serum at 37°C. At different time points (5, 10, 30, and 60 minutes), each sample was treated with 1 mL of acetonitrile for precipitating the serum proteins. After mixing and centrifuging for 2 minutes at 5000 rpm, the radiochemical purity of [68Ga]IPCAT-NOTA 24 was determined using radio-TLC methods. Radio-TLC was performed on a silica gel plate TLC-SG (1.5 × 6 cm), using 0.1M EDTA as the developing agent.
In vitro Autoradiography (Binding and Competitive Assay)
The binding and competitive studies were carried out in 20 µm thick cryostat-cut sagittal section slices of brain tissue of SD rat. After unfreezing of the section slices, the slices were immediately rinse with water for recovery. 500 μCi of [68Ga]IPCAT-NOTA 24 (binding assay) was incubated with 1:1, 1:100 and 1:1000 ratio of precursor (competitive assay) in 100% C2H5OH solution, respectively. Incubation of reacted slices at room temperature for 1 h with gentle and constant shaking and then transferred to a glass tank of 40% C2H5OH for 1 minute as well as water for 1 minute, repeat steps twice. The slices were dried on a warm dryer and placing in the image of the Imaging plate cassette (2040, Fuji Photo Film Co., Tokyo, Japan) for automatic exposure for at least 7 days. The image was counted and analyzed by FLA-5000 (Fuji Photo Film Co., Tokyo, Japan) and software Science Lab V4.0 (Fuji Photo Film Co., Tokyo, Japan). The specific binding ratio (SBR%) can be calculated by the formula as follows.
Ex vitro Autoradiography
Health SD rat was injected with 55.5 MBq/0.1 mL of [68Ga]IPCAT-NOTA 23 through lateral tail veins. The rat was sacrificed by CO2 euthanasia at 60 minutes and 120 minutes postinjection, and immediately dipped into isopentane (Nacalai Tesque Inc., Japan) which will be pre-chilled with liquid nitrogen. The whole brain was frozen for 1–2 minutes and placed on a cryostat holder (7 x 5 cm) and embedded with 4% carboxymethylcellulose (CMC). The specimens were placed in a cryostat (Bright Instrument Company Ltd., UK) and sliced (thickness 20 μm) at a constant temperature of −40°C. The sections were placed on an imaging plate cassette (2040, Fuji Photo Film Co., Tokyo, Japan) for automatic exposure for at least 7 days. The image was counted and analyzed by FLA-5000 (Fuji Photo Film Co., Tokyo, Japan) and software Science Lab V4.0 (Fuji Photo Film Co., Tokyo, Japan).
In vitro Metabolite Assay
Ault male Health SD rat was injected with 55.5 MBq/0.1 mL of [68Ga]IPCAT-NOTA 24 through lateral tail veins and distributed for 5, 10, 30 minutes, Health SD rat was deeply anesthetized with isoflurane gas (3% isoflurane in 50% oxygen, 1 mL/min). 1 mL whole blood of rat was withdrawn through tail vein at room temperature and centrifuged at 3000 rpm for 10 min to achieve supernatant. The radiochemical purity of [68Ga]IPCAT-NOTA 24 was determined using radio-TLC methods. Radio-TLC was performed on a silica gel plate TLC-SG (1.5 × 6 cm), using 0.1 M EDTA as the developing agent.
In vivo NanoPET/CT Image
Health SD rat was intravenously injected with 37 MBq/0.1 mL of [68Ga]IPCAT-NOTA 24 through lateral tail veins and anesthetized with isoflurane gas (3% isoflurane in 50% oxygen, 1 mL/min). The imaging of [68Ga]IPCAT-NOTA 24 was acquired for 60 minutes using nanoPET/CT (BioScan, Inc., Washington, DC, USA) with dynamic image and semi-quantification was processed by PMOD software (PMOD Technologies Ltd., Zürich, Switzerland). We set up a PMOD template for brain including striatum and cerebellum, respectively. Cerebellum served as the reference region, and region of interest (ROI) was calculated from the average radioactivity with the following equation: (target - reference)/reference.
In vivo Regional Brain Distribution
Regional brain distribution in SD rats was measured after an i.v. injection of the 37 MBq/0.1 mL of [68Ga]IPCAT-NOTA 24. Samples from different brain regions (cortex, striatum, hippocampus, and cerebellum) were dissected, weighed and counted (WIZARD2 2480, PerkinElmer Instruments Inc.). The percentage dose/g of each sample was calculated by comparing sample counts with the counts of the diluted initial dose described above. The %ID/g of specific uptake in each region was obtained by dividing the difference between the biodistribution of striatum and cerebellum image data.
Results and Discussion
Chemical Preparation
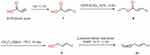
Starting from cocaine, compound 12 was synthesized (Scheme 1) through the substitution reaction of compound 10 (Scheme 2) at the N-8 position. Compounds 17 and 20 were prepared (Scheme 3) from ethylene glycol and diethylenetriamine as starting precursors, and then compound 13 reacted with compound 17 and 20 through substitution reaction to produce IPCET-NOTA 21 and IPCET-NOTA 22 with <5% yield, and only IPCAT-NOTA 22 had chemical purity of >99%. The yields of IPCAT-NOTA 21 and IPCAT-NOTA 22 were <5% because of the steric hindrance of NOTA-OH and NOTA-NH2. The structures of 2,2ʹ-(7-(2-ethoxyl)-1,4,7-triazonan-1,4-diyl)diacetic acid 21 and 2,2ʹ-(7-(2-aminoethyl)-1,4,7-triazonan-1,4-diyl)diacetic acid 22 were bulky to react with the C-2 position of tropane derivatives.
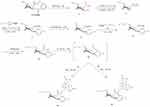 |
Scheme 1 Synthesis of IPCET-NOTA 21 and IPCAT-NOTA 22. |
 |
Scheme 2 Synthesis of (E)-3-iodo-2-propenyl-1-methylbenzenesulfonate 10. |
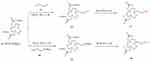 |
Scheme 3 Synthesis of 2,2ʹ-(7-(2-ethoxyl)-1,4,7-triazonan-1,4-diyl)diacetic acid 17 and 2,2ʹ-(7-(2-aminoethyl)-1,4,7-triazonan-1,4-diyl) diacetic acid 20. |
Radiochemistry and Serum Stability
First, IPCET-NOTA 21 and IPCAT-NOTA 22 were chelated with [68Ga]GaCl3 to produce [68Ga]IPCET-NOTA 23 and [68Ga]IPCAT-NOTA 24 in HEPES buffer at pH 4.1–4.3 for 15 minutes (Scheme 4). The specific activity of labeled ligand was poor if the amount of NOTA was <10 μg (data not shown). Both [68Ga]IPCET-NOTA 23 and [68Ga]IPCAT-NOTA 24 were obtained with a high radiochemical yield of ≥90% and specific activity of 4.25 MBq/nmol (Figure 1). Regarding biological experiments, [68Ga]IPCAT-NOTA 24 was selected as the follow-up biological test item because the precursor IPCAT-NOTA 22 had >99% chemical purity. In general, Ga(III) could form stable complexes with 4-, 5-, or 6-coordinate ligands, with 6-coordinate ligands being the most stable.18 NOTA was accepted as the “gold standard” chelator for gallium-67 ([67Ga]) or gallium-68 ([68Ga]), and Ga-NOTA has a high formulation constant and in vivo kinetic stability; although NOTA was used to conjugate with the molecule, one of the carboxyl groups becomes unavailable for the coordination,13 but it remains stable. Moreover, 2 MBq of [68Ga]IPCAT-NOTA 24 was incubated at 37°C with the serum isolated from healthy Sprague Dawley (SD) rat (Table 1); the radiochemical purity (RCP%) values of [68Ga]IPCAT-NOTA 24 were 90.69%, 86.09%, 88.64%, 90.39%, and 89.12% at 0, 5, 10, 30, and 60 minutes, respectively. Our stability result shows that intact [68Ga]IPCAT-NOTA 24 is >85% up to 1 hour under physiological conditions, and the precursor IPCAT-NOTA 22 of [68Ga]IPCAT-NOTA 24 could stably chelate with 68Ga even when it has 5-coordinate ligands.
 |
Scheme 4 Preparation of radiolabeled probe of [68Ga]IPCAT-NOTA 23 and [68Ga]IPCET-NOTA 24 (X = O, for IPCET and X = N, for IPCAT). |
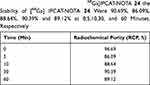 |
Table 1 In vitro Serum Stability of [68Ga]IPCAT-NOTA 24 the Stability of [68Ga] IPCAT-NOTA 24 Were 90.69%, 86.09%, 88.64%, 90.39% and 89.12% at 0,5,10,30, and 60 Minutes, Respectively |
 |
Figure 1 Radiochemical purity (RCP) analysis of [68Ga]IPCET-NOTA 23 (I) and [68Ga]IPCAT-NOTA 24 (II). |
Partition Coefficient (LogP)
The octanol-water partition coefficient (LogP) of [68Ga]IPCAT-NOTA 24 was determined to be 0.88 ± 0.30. In addition, the cLogP values of precursors IPCAT-NOTA 22, IPCET-NOTA 21, and TRODAT-1 were estimated as 2.39, 3.07, and 3.34, respectively, by using ChemDraw (data not shown). An ideal radiotracer for brain imaging should be relatively small, neutral, and lipophilic to cross the intact blood–brain barrier (BBB). According to Dischino et al,19 an ideal radiopharmaceutical design to pass the BBB should have a LogP value of 0.9–2.5. Our result shows that the LogP value of [68Ga]IPCAT-NOTA 24 was 0.7, which is on the borderline of the range suggested by Dischino; it was assumed that it has partial ability to cross the BBB. However, [68Ga]IPCAT-NOTA 24 may fail to reach the target due to the slightly positively charged 68Ga/chelator-complex, which cannot pass the intact BBB, which is consistent with the result from the estimation of quantitative structure–activity relationship computer models of Discovery Studio (data not shown).
Autoradiography and Competition Assay
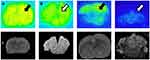
In vitro Autoradiography
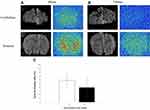
The specific binding ratio (SBR%) of [68Ga]IPCAT-NOTA 24 was 18% in healthy rats (Figure 2). In a competition study, SBR% of [68Ga]IPCAT-NOTA 24 was significantly inhibited (up to 85% and 100%) through coincubation with a nonradioactive precursor at 100- and 1000-fold concentration in the DAT-rich regions of the brain (striatum; Figure 3 and Table 2). Furthermore, we analyzed and compared the amount of DAT in cerebellum, cortex, and striatum of younger (5-week-old) and older (21-week-old) healthy SD rats by using ELISA. The results showed that the DAT concentration in older rats was more than that in younger rats (data not shown). In addition, the binding affinity of [68Ga]IPCAT-NOTA 24 exhibits less potency than that of [99Tc]TRODAT-1. However, the ST to CB ratio was low when a higher carrier amount was added, which indicates that the excess free ligand would result in the specific binding of [68Ga]IPCAT-NOTA 24.
 |
Table 2 In vitro Autoradiography (Include Competition Assay) Analysis |
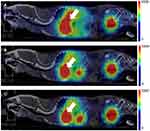
Ex vivo Autoradiography
The SBR% values of [68Ga]IPCAT-NOTA 24 were estimated through intravenous injection of 30 MBq/100μL of [68Ga]IPCAT-NOTA 24 in healthy SD rats followed by their sacrifice to observe brain coronal sections 60 and 120 minutes after injection. Our result revealed that the SBR% of [68Ga]IPCAT-NOTA 24 in healthy SD rats was 15.8 ± 6.7% and at 60 and 120 minutes after [99mTc]TRODAT-1 injection, they were 82.3 ± 0.7% and 214.7 ± 1.6% (data not shown), respectively (Figure 4). Compared with [99mTc]TRODAT-1, [68Ga]IPCAT-NOTA 24 exhibited less binding affinity to DAT. Notably, the stability of [68Ga]IPCAT-NOTA 24 during the ex vivo procedure remains unknown. Furthermore, the 68Ga complexing moiety (ie, NOTA) is inherently bulky and hydrophilic, which most likely does not pass the BBB and leads to less binding affinity to DAT. Moreover, the limitation of this study is that 68Ga has a half-life of 68 minutes, which requires rapid use of the tracer after its generation and high radiolabel-specific activity of [68Ga]IPCAT-NOTA 24 to achieve high imaging quality.
NanoPET/CT Image and Regional Brain Distribution
The NanoPET/CT imaging data of SD rats showed that the SBR% values of the striatum of [68Ga]IPCAT-NOTA 24 were 6%, 25%, and 62% at 5–15, 30–40, and 60–70 minutes, respectively (Figure 5). Furthermore, the striatum/cerebellum ratios of [68Ga]IPCAT-NOTA 24 were 1.05, 1.25, and 1.62 at 5–15, 30–40, and 60–70 minutes, respectively. For other radiotracers, the striatum/cerebellum ratios at 60 minutes after injection of [99mTc]TRODAT-13 and [18F]FECNT20 were 2.8 and 1.52, respectively. The striatal uptake at 45 min postinjection of [18F]LBT-999 and [11C]LBT-999 were 2.5 ± 0.4 and 4.2 ± 0.4%ID/g, respectively.21 In a head-to-head comparison of dopamine transporter SPECT radioligands [123I]FP-CIT and [123I]PE2I in health subjects, both of them had approximately about 3 folds ratios.22 At 60 minutes after injection of [68Ga]IPCAT-NOTA 24, this ratio reached 1.62, the result was similar with [18F]FECNT but significantly lower than other radiotracer. This indicates that [68Ga]IPCAT-NOTA 24 could still strongly bind to DAT. However, most of [68Ga]IPCAT-NOTA 24 were in the outer nonspecific binding regions due to low lipophilicity and tendency to be washed out quickly from the brain, with appearance in the liver and bladder at 5–15 and 30–40 minutes (Figure 6). Moreover, compared with the uptake data of [99mTc]TRODAT-1 of 0.4% at 2 minutes and 0.12% at 1 hour after injection,3 the overall brain uptake was calculated from all brain regions (cortex, striatum, hippocampus, and cerebellum). The results showed that the overall brain uptake was 0.07% ID/g at 30 hours after injection due to the less penetration.
Metabolite Assay
The in vitro metabolite assay of [68Ga]IPCAT-NOTA 24 showed that the RCP% values at 0, 5, 10, and 30 minutes were 92.58%, 84.03%, 79.01%, and 67.65%, respectively (Table 3). Thus, [68Ga]IPCAT-NOTA 24 was unstable in blood for a long duration. One possible reason is the most prominent gallium interaction in plasma: gallium transchelation to serum proteins, such as iron transport protein transferrin (a monomeric glycoprotein). Only one-third of the iron-binding sites of transferrin was typical occupied, leaving the other free binding sites to coordinate with gallium. Thus, free [68Ga] would easily detach from NOTA and conjugate with transferrin. We concluded that free gallium in serum is primarily bound to transferrin in equilibrium with gallate species [Ga(OH)4].23 The prolonged circulating time allows increased radioactive metabolite formation, and most of the less lipophilic metabolites observed at 30 minutes would form [68Ga]IPCAT-NOTA 24, which could not pass through the BBB. This study’s limitation is the unknown stability and rapid in vivo kinetics of [68Ga]IPCAT-NOTA 24 during the in vivo metabolite procedure. Future studies evaluating radiotracer stability under various pH conditions and analyzing the amount of labeled transferrin and metabolites are warranted.
 |
Table 3 In vivo Metabolite Study of [68Ga]IPCAT-NOTA 24 Showing the Radiochemical Purity (RCP%) at 0, 5, 10, and 30 Minutes Were 92.58%, 84.03%, 79.01%, and 67.65%, Respectively |
Conclusion
Precursor IPCAT-NOTA 22 was efficaciously labeled with 68Ga. The [68Ga] complex was easily chelated with a RCP% of >95%. Because of the presence of LogP and carboxyl group of NOTA, [68Ga]IPCAT-NOTA 24 may not be a potential imaging agent for DAT. However, the in vitro binding affinity of [68Ga]IPCAT-NOTA 24 is noteworthy because of its structural features, which leads to the development of PET probe in conjugate/pendent approach for DAT imaging. To evaluate the relationship between [68Ga]IPCAT-NOTA 24 uptake and transporter localization, a different chelator must be used or direct intracranial injection must be administered.
Acknowledgments
Authors would like to thank Cheng-Li, Chou Ph.D. for providing the LogP estimation of QSAR computer models by Discovery Studio software and the financial support of MOST (NSC 104-3111-Y-042A-065).
Disclosure
Shiou-Shiow Farn and Kang-Wei Chang are co-first authors. The authors declare that they have no conflicts of interest.
References
1. Goodman MM, Keil R, Shoup TM, et al. Fluorine-18-FPCT: a PET radiotracer for imaging dopamine transporters. J Nucl Med. 1997;38(1):119–126.
2. Koivula T, Marjamaki P, Haaparanta M, et al. Ex vivo evaluation of N-(3-[18F]fluoropropyl)-2 beta-carbomethoxy-3 beta-(4-fluorophenyl)nortropane in rats. Nucl Med Biol. 2008;35(2):177–183. doi:10.1016/j.nucmedbio.2007.09.006
3. Meegalla SK, Plossl K, Kung MP, et al. Specificity of diastereomers of [99mTc]TRODAT-1 as dopamine transporter imaging agents. J Med Chem. 1998;41(4):428–436. doi:10.1021/jm970742b
4. Abi-Dargham A, Kegeles LS, Zea-Ponce Y, et al. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry. 2004;55(10):1001–1006. doi:10.1016/j.biopsych.2004.01.018
5. Abbasi Gharibkandi N, Hosseinimehr SJ. Radiotracers for imaging of Parkinson’s disease. Eur J Med Chem. 2019;166:75–89. doi:10.1016/j.ejmech.2019.01.029
6. Ma KH, Huang WS, Chen CH, et al. Dual SPECT of dopamine system using [99mTc]TRODAT-1 and [123I]IBZM in normal and 6-OHDA-lesioned Formosan rock monkeys. Nucl Med Biol. 2002;29(5):561–567. doi:10.1016/S0969-8051(02)00303-7
7. Kung HF. Development of Tc-99m labeled tropanes: TRODAT-1, as a dopamine transporter imaging agent. Nucl Med Biol. 2001;28(5):505–508. doi:10.1016/S0969-8051(01)00220-7
8. Kung HF, Kim HJ, Kung MP, Meegalla SK, Plossl K, Lee HK. Imaging of dopamine transporters in humans with technetium-99m TRODAT-1. Eur J Nucl Med. 1996;23(11):1527–1530. doi:10.1007/BF01254479
9. Kung HF, Meegalla S, Kung MP, Plössl K. Development of 99mTc-labelled complexes for imaging dopamine transporters in the brain. Transit Met Chem. 1998;23(4):531–536. doi:10.1023/A:1006921811871
10. Johannsen B, Pietzsch HJ. Development of technetium-99m-based CNS receptor ligands: have there been any advances? Eur J Nucl Med Mol Imaging. 2002;29(2):263–275. doi:10.1007/s002590100652
11. Stepanov V, Krasikova R, Raus L, Loog O, Hiltunen J, Halldina C. An efficient one-step radiosynthesis of [18F]FE-PE2I, a PET radioligand for imaging of dopamine transporters. J Label Compd Radiopharm. 2012;55(6):206–210. doi:10.1002/jlcr.2927
12. Kilian K. 68)Ga-DOTA and analogs: current status and future perspectives. Rep Pract Oncol Radiother. 2014;19(Suppl):S13–S21. doi:10.1016/j.rpor.2014.04.016
13. Velikyan I. Continued rapid growth in (68) Ga applications: update 2013 to June 2014. J Labelled Comp Radiopharm. 2015;58(3):99–121.
14. Kaemmerer D, Peter L, Lupp A, et al. Molecular imaging with (6)(8)Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38(9):1659–1668. doi:10.1007/s00259-011-1846-5
15. Petersen LJ, Zacho HD. PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: an expedited systematic review. Cancer Imaging. 2020;20(1):10. doi:10.1186/s40644-020-0290-9
16. Ostapchenko VG, Snir J, Suchy M. Detection of active caspase-3 in mouse models of stroke and alzheimer’s disease with a novel dual positron emission tomography/fluorescent tracer [68Ga]Ga-TC3-OGDOTA. Contrast Media Mol Imaging. 2019;2019:17. doi:10.1155/2019/6403274
17. Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43(1):260–290.
18. Cutler CS, Giron MC, Reichert DE, et al. Evaluation of gallium-68 tris(2-mercaptobenzyl)amine: a complex with brain and myocardial uptake. Nucl Med Biol. 1999;26(3):305–316. doi:10.1016/S0969-8051(98)00108-5
19. Dishino DD, Welch MJ, Kilbourn MR, Raichle ME. Relationship between lipophilicity and brain extraction of C-11-labeled radiopharmaceuticals. J Nucl Med. 1983;24(11):1030–1038.
20. Zoghbi SS, Shetty HU, Ichise M, et al. PET imaging of the dopamine transporter with 18F-FECNT: a polar radiometabolite confounds brain radioligand measurements. J Nucl Med. 2006;47(3):520–527.
21. Saba W, Peyronneau MA, Dolle F, Goutal S, Bottlaender M, Valette H. Difficulties in dopamine transporter radioligand PET analysis: the example of LBT-999 using [18F] and [11C] labelling Part I: PET studies. Nucl Med Biol. 2012;39(2):227–233. doi:10.1016/j.nucmedbio.2011.08.003
22. Ziebell M, Holm-Hansen S, Thomsen G, et al. Serotonin transporters in dopamine transporter imaging: a head-to-head comparison of dopamine transporter SPECT radioligands 123I-FP-CIT and 123I-PE2I. J Nucl Med. 2010;51(12):1885–1891. doi:10.2967/jnumed.110.078337
23. Harris WR, Pecoraro VL. Thermodynamic binding constants for gallium transferrin. Biochemistry. 1983;22(2):292–299. doi:10.1021/bi00271a010
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.