Back to Journals » International Journal of Nanomedicine » Volume 13 » T-NANO 2014 Abstracts
Synthesis of biocompatible iron oxide nanoparticles as a drug delivery vehicle
Authors Kansara K, Patel P, Shukla RK, Pandya A, Shanker R , Kumar A, Dhawan A
Received 14 October 2016
Accepted for publication 21 November 2016
Published 15 March 2018 Volume 2018:13(T-NANO 2014 Abstracts) Pages 79—82
DOI https://doi.org/10.2147/IJN.S124708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Krupa Kansara, Pal Patel, Ritesh K Shukla, Alok Pandya, Rishi Shanker, Ashutosh Kumar, Alok Dhawan
Institute of Life Sciences, School of Science and Technology, Ahmedabad University, Ahmedabad, Gujarat, India
Abstract: Over the last decade, there has been growing interest in developing novel nanoparticles (NPs) for biomedical applications. A safe-by-design approach was used in this study to synthesize biocompatible iron oxide NPs. The size of the particles obtained was ~100 nm. Although these NPs were significantly (P<0.05) internalized in MCF-7 (human breast adenocarcinoma cell line) cells, no adverse effect was observed in the cells as assessed by cytotoxicity assays (neutral red uptake and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and cell cycle analysis. Our data demonstrate the potential of iron oxide NPs as a biocompatible carrier for targeted drug delivery.
Keywords: biocompatible iron oxide nanoparticles, human breast adenocarcinoma cells, coprecipitation
Corrigendum for this paper has been published.
Introduction
Iron-based magnetic nanoparticles (NPs) such as magnetite (Fe3O4) have been studied in detail due to their unique properties, such as stability over time, biocompatibility, and sensitivity to magnetic field.1–3 They can potentially be used as magnetic targeted drug delivery carriers and magnetic resonance imaging contrast agents due to their high saturation magnetization, low toxicity, and biocompatibility.4 The magnetic properties of Fe3O4 NPs are attributed to their size and size distribution, which, in turn, is dependent on the route of synthesis.
Therefore, in this study, an attempt was made to synthesize Fe3O4 NPs using a safe-by-design approach by the coprecipitation method. Polyethylene glycol (PEG) was used as surfactant to control the particle size and narrow size distribution. The biocompatibility of Fe3O4 NPs was evaluated by cytotoxicity assays and cell cycle analysis in the human breast adenocarcinoma cell line (MCF-7).
Materials and methods
Materials
Ferric chloride hexahydrate and ferrous sulfate were purchased from SD-Fine-Chem. Ltd, Mumbai, India. Ployetheleneglycol (PEG-6000), dimethylesulphoxide, sodium hydroxide (NaOH), minimum essential medium eagle, phosphate-buffered saline, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and antibiotic-antimycotic solution were purchased from HiMedia Laboratories Pvt. Ltd., (Mumbai, India). The MCF-7 cell line was purchased from the National Centre for Cell Sciences, Pune, India.
Fe3O4 NP synthesis
The preparation of Fe3O4 NPs was performed by a chemical coprecipitation method of Fe2+ and Fe3+ ions (1:2 molar ratios) by the addition of NaOH.5 A total volume of 15 mL of 0.25 M Fe2+ and 0.5 M Fe3+ solutions were prepared in deionized water. PEG was then added and the temperature slowly risen up to 80°C. During the initial 2 minutes of the reaction, NaOH was added to achieve a pH of 10. The reaction was allowed to continue on a magnetic stirrer for 2 hours. Thereafter, the suspension was centrifuged and washed several times with deionized water to lower the pH to 7. Finally, the particles were suspended in 10 mL of dimethylesulphoxide.
Characterization of Fe3O4 NPs
One milliliter of the stock suspension of Fe3O4 NPs was diluted in 10 mL complete minimum essential medium eagle. The hydrodynamic size and zeta potential were determined using Zetasizer Nano ZS.
Cytotoxicity assessment
The cytotoxic potential of Fe3O4 NPs was assessed in MCF-7 cells after 6 and 24 hours of treatment using MTT and neutral red uptake (NRU) assays as described by Mosmann6 and Borenfreund and Puerner, respectively.7
Cellular internalization of NPs
The internalization of Fe3O4 NPs in MCF-7 cells was assessed according to the method described in our earlier study.8
Cell cycle analysis
The effect of Fe3O4 NPs on cell cycle was assessed according to the method described in our earlier study.8
Results and discussion
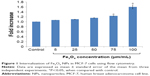
The mean hydrodynamic size and zeta potential of synthesized Fe3O4 NPs were 98.19±1.0 nm and 36±2 mV, respectively. Flow cytometric analysis revealed a significant (P<0.05) increase in the internalization of Fe3O4 NPs in MCF-7 cells after 24 hours exposure at the two higher concentrations, as evident by an increase in the side scatter intensity (Figure 1).
In the cytotoxicity assays, namely NRU and MTT, Fe3O4 NPs were found to be biocompatible as there was no significant increase in the NRU (88% at concentration 150 μM/mL) and a reduction (96%) in the mitochondrial succinate dehydrogenase activity was observed at the highest concentration after 24-hour exposure (Figures 2A and B).
Additionally, no change in cell cycle progression was observed in Fe3O4 NPs-treated MCF-7 cells, after 24-hour exposure (Figure 3).
Conclusion
Our results demonstrated that Fe3O4 NPs synthesized using the safe-by-design approach showed no adverse effect on cells, as assessed by cytotoxicity assays and cell cycle analysis in MCF-7 cells, even though they are significantly internalized. Therefore, these NPs have a potential to be used as a carrier for targeted drug delivery.
Acknowledgments
The authors acknowledge the funding from the Centre for Nanotechnology Research and Application (CENTRA) by The Gujarat Institute for Chemical Technology (GICT); and the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement number 263147 (NanoValid – Development of reference methods for hazard identification, risk assessment and LCA of engineered nanomaterials).
Disclosure
The authors report no conflicts of interest in this work.
References
Chin SF, Iyer, KS, Saunders M, et al. Encapsulation and sustained release of curcumin using superparamagnetic silica reservoirs. Chemistry. 2009;15(23):5661–5665. | ||
Liu G, Wang Z, Lu J, et al. Low molecular weight alkyl-polycation wrapped magnetite nanoparticle clusters as MRI probes for stem cell labelling and in vivo imaging. Biomaterials. 2011;32(2):528–537. | ||
Dandamudi S, Campbell RB. The drug loading, cytotoxicity and tumor vascular targeting characteristics of magnetite in magnetic drug targeting. Biomaterials. 2007;28(31):4673–4683. | ||
Pan BF, Gao F, Gu HC. Dendrimer modified magnetite nanoparticles for protein immobilization. J ColloidInterface Sci. 2005;284(1):1–6. | ||
Hariani PL, Faizal M, Ridwan, Marsi, Setiabudidaya D. Synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye. Int J Environ Sci Develop. 2013;4(3):336–340. | ||
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65(1–2):55–63. | ||
Borenfreund E, Puerner E. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24(2–3):119–124. | ||
Kansara K, Patel P, Shah D, et al. TiO2 nanoparticles induce DNA double strand breaks and cell cycle arrest in human alveolar cells. Environ Mol Mutagen. 2015;56(2):204–217. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.