Back to Journals » International Journal of Nanomedicine » Volume 9 » Issue 1
Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin- resistant Staphylococcus aureus (MRSA)
Authors Paredes D, Ortiz C, Torres R
Received 7 November 2013
Accepted for publication 7 February 2014
Published 3 April 2014 Volume 2014:9(1) Pages 1717—1729
DOI https://doi.org/10.2147/IJN.S57156
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Daissy Paredes,1 Claudia Ortiz,2 Rodrigo Torres1
1Escuela de Química, Facultad de Ciencias, Universidad Industrial de Santander, Colombia; 2Escuela de Bacteriología y Laboratorio Clínico, Facultad de Salud, Universidad Industrial de Santander, Colombia
Abstract: Silver nanoparticles (AgNPs) have been shown great interest because of their potential antibacterial effect. Recently, this has been increased due to resistance in some pathogenic bacteria strains to conventional antibiotics, which has initiated new studies to search for more effective treatments against resistant microorganisms. For these reasons, AgNPs have become an important approach for applications in nanobiotechnology in the development of antibiotic treatment of different bacterial infections. This study was aimed at synthesizing AgNPs using cysteine as a reducer agent and cetyl-tri-methyl-ammonium bromide as a stabilizer in order to obtain more efficient treatment against the pathogen bacteria Escherichia coli O157:H7. These AgNPs were characterized through UV-Vis spectroscopy, transmission electron microscopy, and dynamic light scattering. From these analyses, formation of spherical nanoparticles with an average size of 55 nm was confirmed. Finally, minimal inhibitory concentration (MIC) and minimal bactericide concentration (MBC) of these AgNPs against pathogenic strains E. coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA) were determined in both solid and liquid media. MIC and MBC values were around 0.25 µg/mL and 1 µg/mL, respectively. These parameters were comparable to those reported in the literature and were even more effective than other synthesized AgNPs.
Keywords: nanomaterials, antibacterial activity, minimal inhibitory concentration, MIC, minimal bactericide concentration, MBC
Introduction
During the last decade, different studies on basic and applied nanobiotechnology have been conducted. These studies have been especially directed towards obtaining new materials with nanometer-scale applications.1,2
Among these nanomaterials, silver nanoparticles (AgNPs) have shown very unusual physical and chemical properties, as well as several biological activities.3 AgNPs possess both high electrical and thermal conductivity, have a large surface area for Raman dispersion, catalytic activity, non-linear behavior,4,5 and prominent antibacterial properties, caused mainly by their large surface area to volume ratio.6 Moreover, AgNPs can be produced using different synthetic methodologies, thus providing specific morphologies and unique characteristics.3
Consequently, AgNPs are arising as new bacteriostatic agents, because they are comparable in efficacy and even more potent antimicrobial compounds than conventional antibiotics.7 Infectious diseases, caused by pathogenic and opportunistic bacteria in hospitals, have instigated the development of both new pharmaceuticals and therapeutic targets. Few new antibiotics have been introduced by the pharmaceutical industry, and none of them have improved activity against multi-resistant bacteria.8 However, AgNPs,9 which are diverse compounds comprising silver, such as materials containing ionic silver (Ag+)10 or metallic silver (Ag0),11 have been recently synthesized and demonstrated antimicrobial activity against Gram-negative bacteria such as Escherichia coli.12 In recent years, various methods have been employed to obtain AgNPs, involving the use of toxic reducing and stabilizing reagents. Currently, new strategies for obtaining NPs are being tested, using less aggressive compounds to the environment; therefore, they are called green synthesis techniques. For example, the effectiveness of cysteine as a reducing agent and some polymers as stabilizing agents have already been demonstrated.13 Furthermore, the use of polymers, such as surfactant polyvinyl alcohol (PVA), influences the size, aggregation, and distribution of the NPs in the colloidal system, and therefore increases NP stability.14
Although most E. coli strains are non-pathogenic, some variants, such as E. coli O157:H7 produce verotoxins (toxins identical to those produced by Shigella dysenteriae)15 and cause diarrhea and abdominal cramps.16 This strain is considered to be the most pathogenic variant of E. coli. Outbreaks of E. coli O157:H7 have been associated with food consumption from animal sources (eg, meat that has not been properly cooked17 or contaminated water18). It has been estimated that these bacteria can cause more than 73,000 cases of disease per year in the US, and has been implicated in 250 deaths.19 In addition, E. coli can cause gastroenteritis, infections in the urinary tract, and neonatal meningitis. In rare cases, these pathogenic strains are also responsible for uremic hemolytic syndrome.20
Microbial infection by E. coli O157:H7 has become more common in children younger than 5 years, with high proportions of morbidity. It is estimated that between 0.6%–2.4% and specifically 15%–36% of all diarrhea with blood are caused by E. coli O157:H7.21 The prevalence rate of 4.7% for this strain in Colombia22 has been found in one study carried out in Spain, Argentina, Chile, Canada, and Belgium.23
Methicillin-resistant Staphylococcus aureus (MRSA) is another important pathogen that can be treated in order to eliminate hospital infections. In humans, MRSA can be caused by skin surface injuries and local abscesses, as well as infections in the central nervous system, or diseases such as osteomyelitis, invasive endocarditis, septic arthritis, septicemia, pneumonia, and infections in the urinary tract.24 For example, in 2004, more than 60% of all S. aureus isolated from Spanish hospitals were methicillin resistant.25 In the US, 46.3 per 1,000 of hospitalized patients were colonized or infected by MRSA.26 In Spain, the prevalence of MRSA increased from 1.5% in 1986 to 31.2% in 2002,27 with similar prevalence data for the rest of Europe.28 In the same way, bacterial infections caused by MRSA have resulted in 25%–63% mortality in some Latin American countries.29
For these reasons, it is very important to control the pathogen strains of E. coli and S. aureus, by avoiding their microbial growth by means of new non-conventional bacteriostatic agents. Among the range of compounds with bactericidal activity, AgNPs represent a very promising antibacterial agent that could be useful for the treatment of resistant bacteria.30 Other studies have shown that decreasing the size of AgNPs increases the antimicrobial activity of NPs, due to the increased surface area of AgNPs, which promotes higher interaction between NPs and the cell membrane.14 Furthermore, it has been shown that the use of cationic surfactants increases electrostatic interaction and therefore prevents aggregation of the NPs.
In this study, we present a simple route of AgNP synthesis, using cysteine as the reducing agent at mild conditions, and by stabilizing these synthesized NPs with cetyl-tri-methyl-ammonium bromide (CTAB). Synthesis of AgNPs employing amino acids as non-conventional reducing agents allows the acquisition of colloidal and stable AgNPs, of small sizes and displaying prominent antibacterial properties, without further purification steps.
Materials and methods
Materials and strains
Silver nitrate (AgNO3, 99.998%) and L-cysteine (C3H7NO2S, >99%) were purchased from Merck (Darmstadt, Germany). CTAB (99%) and PVA (99+%) were obtained from Sigma-Aldrich (St Louis, MO, USA). All reagents used in this study were of analytical grade and were used without further purification.
Pathogenic strain E. coli O157:H7 was obtained from Laboratorio de Biotecnología, Pontificia Universidad Javeriana, Bogotá, Colombia, and MRSA was donated by Hospital Universitario de Santander, Bucaramanga, Colombia. E. coli O157:H7 and MRSA were kept on brain heart infusion (BHI) agar slants (Oxoid, Basingstoke, UK).
Synthesis of AgNPs
The synthesis of AgNPs was carried out according to the method of Khan et al,31 with some modifications. A solution of 0.01 M cysteine and 0.01 M CTAB was mixed and gently shaken. Subsequently, deionized water was added in order to obtain a final volume of 50 mL. Finally, PVA was added at a final concentration of 0.02% (w/v). A red-wine color change indicates the formation of the AgNPs. Additionally, AgNP synthesis was standardized using different agitation rates (200, 400, and 800 rpm), agitation times (1, 2, and 4 hours), and by ultrasonic treatment of 40 Hz at different times (1, 2, and 4 hours). In this work, we indicate the best results in terms of time stability of AgNPs and higher antimicrobial activities.
Physical and chemical characterization of AgNPs
AgNPs were characterized using the following analytical techniques: a) UV-Vis (ultraviolet-visible) spectrophotometry with a Shimadzu UV-1601-1601 PC spectrophotometer (Kyoto, Japan), using a scan from 300 nm to 600 nm; b) transmission electron microscopy (TEM) using a FEI Tecnai F20 (Hillsboro, ON, USA), at the Center for Materials Research (Cornell University, Ithaca, NY, USA); c) light scattering in dynamic mode (DLS) using a Malvern Zetasizer Nano-ZS (Malvern Instruments, Malvern, UK), and d) zeta potential determination with a Malvern Zetasizer Nano-ZS, using a sample volume of the colloidal solution of NPs (1.5 mL) with a concentration range of 100–200 ppm; crystalline phase of the NPs was determined by X-ray diffraction (XRD) using a diffractometer (Philips, Eindhoven, the Netherlands) at a scan rate of 0.02 s−1 in a 2θ range of 20°–80° with CuKα radiation (λ=1.5418 Å).
The particle size and shape were observed by TEM using a Philips CM200 instrument operating at 200 kV accelerating voltage. The prepared silver suspensions were diluted at 20× with deionized water. A drop of the suspension was applied to a Formvar-coated copper grid.
Determination of the MIC of AgNPs
The MIC is defined as the lowest concentration of material that inhibits the growth of an organism, and was determined in multi-well culture plates of 96 wells according to standard protocols previously published.32,33
In general, E. coli O157:H7 and MRSA were grown in liquid Luria-Bertani (LB) medium at 37°C and 250 rpm over 9 hours, before they were diluted in fresh LB liquid medium using 5 × 108 colony forming units (CFU)/mL and 7 × 106 CFU/mL as the inoculum, respectively, without control and in the presence of different concentrations of AgNPs (0.5, 1, 5, 10, 20, and 50 μg/mL). Gradient concentrations of NPs (0.5, 1, 5, 10, 20, and 50 μg/mL) were then added to the culture medium. Bacteria/NP mixed cultures were tested using a microplate reader (Biorad, Hercules, CA, USA) at 37°C on a rotary shaker at 200 rpm.34,35 At intervals of 1 hour, and over a culture time of 9 hours, optical density (OD) at 595 nm was determined for bacterial cell growth.
The MIC was defined as the lowest concentration of AgNPs that produces inhibition of bacterial growth. Values of MIC99 and MIC90 corresponded to doses that inhibit 99% and 90% of bacterial growth, respectively.
Determination of AgNP antibacterial activity by disc diffusion test
We tested the antibacterial activity of AgNPs using a Mueller-Hinton (MH) agar culture medium (Merck KGaA, Darmstadt, Germany) as described in the diffusion disc method commonly used for antibiotics.36 The samples were inoculated (108 CFU/mL37) in petri dishes with MH medium agar and then paper disks of 5 mm in diameter were laid on the inoculated test organism, which was instilled with AgNPs at different concentrations (1, 5, 10, and 20–50 μg/mL). We incubated the petri dishes at 37°C over 24 hours and antimicrobial activity was determined by measuring the zone inhibition around the disk. The mean and standard deviation reported for each type of NP and each microbial strain was tested in triplicate.
Determination of MBC of AgNPs
The MBC is defined as the lowest concentration of NPs that kills 100% of the bacteria, and was also determined using the multi-well culture plate studies carried out for MIC determinations.38 For MIC90, the presence of viable microorganisms was tested, and the lowest concentration causing a bactericidal effect was reported as MBC. To test for the bactericidal effect, we transferred the contents of each well (200 mL) in an Eppendorf tube (Eppendorf, Hamburg, Germany) with 2 mL of BHI medium, which was then incubated at 37°C for 12 hours. Finally, from the cultures where no turbidity was observed, we withdrew 20 mL of this culture, which was inoculated on BHI agar and incubated at 37°C for 24 hours. The concentrations of AgNPs causing bactericidal effect were selected based on the absence of colonies on the agar plate. The MBC value was defined as the AgNP concentration where 100% of bacterial growth was inhibited when compared to a positive control (no treatment with AgNPs).30
Results
Characterization of AgNPs
A set of twelve different types of AgNP was synthesized at different reaction conditions (see Materials and methods section). We characterized AgNPs using TEM, DLS, UV-Vis, and zeta potential (Figure 1).
TEM images of AgNPs are shown in Figure 1A, which confirmed that AgNPs were in the nano-range and were mostly spherical in shape. In addition, the presence of long non-linear agglomerated chains was observed, which indicates interactions among AgNPs. DLS analyses showed that the mean size of AgNPs was <100 nm and zeta potential measurements revealed that AgNPs presented a surface charge near to zero (Figure 1B–D). UV-VIS showed an absorption shoulder at 420–450 nm. The Ag-NPs were observed with a particular plasmon resonance at 420 nm. The observation of this peak was measured at regular time intervals to determine their stability, displaying a characteristic surface plasmon resonant of AgNPs.39,40
Figure 2 shows the XRD pattern of Ag-NPs using cysteine as the reducer agent. These patterns were compared and interpreted with standard data from the International Centre of Diffraction Data. The peak characteristics for silver were consistent with a face-centered cubic system. Main peaks at 2¸ of 38.08°, 44.32°, and 64.5° correspond to cubic facet (111), (200), and (220), respectively. The XRD spectrum confirmed the crystalline structure of silver nanoparticles.41 All peaks in the X-ray diffractogram can be easily indexed to a cubic structure centered in the face of the silver according to available literature (Joint Committee on Powder Diffraction Standards, No 4-0783). This structural characterization allowed the confirmation of the silver composition of the NPs.
  | Figure 2 XRD spectra for the three samples of AgNPs. |
However, different peaks in the sample indicated the presence of impurities, probably AgBr, with its facet of bromargyrite with a hexoctahedral crystalline system. This impurity could be produced by electrostatic interactions between the bromide ion (Br−) from CTAB and non-reduced Ag+ in solution. The stability of the colloidal solution of AgNPs was evaluated at different times. One month after the formation of the AgNPs, these NPs aggregated and lost their physical, chemical, and antibacterial properties (Figure 3).
Summarized analyses of physical properties determined for the AgNPs synthesized with different agitation conditions at pH 4.0 can be found in Table 1. DLS analyses showed that AgNPs achieved mean hydrodynamic sizes of 20.49±1.00 nm (Type 1) when we employed agitation of 200 rpm over 1 hour.
  | Table 1 Characterization of AgNPs synthesized by cysteine reduction at different reaction conditions at pH =4.0 |
It has been described in the literature that a red-shift in the UV-visible spectrum indicates growth in NP size.42 In our case, type 1 and 3 AgNPs showed a red-shift with respect to maximum wavelength (with reference to 400 nm), which suggests an increase in NP size. On the other hand, a blue-shift was observed for type 2 AgNPs, which indicates a decrease in the particle size of the AgNPs.
The physical properties of AgNPs obtained at pH 7.0 are shown in Table 2. These AgNPs were synthesized at pH 4.0 and the pH was adjusted to 7.0 in order to carry out antimicrobial tests at the same pH of the bacterial cell cultures. Small changes in the mean sizes of the AgNPs were observed, while surface charge was dramatically affected by pH variation (from pH 4.0 to pH 7.0). It is possible that increasing the pH from 4.0 to 7.0 affected NP stability, causing agglomeration of the AgNPs over time.
  | Table 2 Characterization of AgNPs synthesized by cysteine reduction at different reaction conditions at pH =7.0 |
Antibacterial activity of AgNPs against E. coli O157:H7 and MRSA
Evaluation of bacteriostatic activity of AgNPs was carried out by determining the MIC of AgNPs for both E. coli O157:H7 and MRSA using two different methodologies.
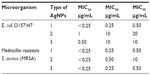
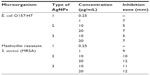
The results of bacterial growth kinetics in the presence of different concentrations of AgNPs are shown in Figures 4 and 5. Bacterial cultures of E. coli O157:H7 and MRSA showed typical kinetics of bacterial growth. However, AgNPs clearly affected growth curves, as a decrease of the absorbance was observed in the presence of low concentrations of AgNPs. This effect can be attributed to cell death in the stationary phase.35 In batch mode for multi-well culture microplates, a longer lag phase and lower maximum absorbance (at 595 nm) were observed as the concentration of AgNPs was increased. As the concentration of NPs increased to the MIC of the respective strains, no growth was observed. The bactericidal effect of the NPs was dependent on the concentration of NPs and the initial bacterial concentration. In this study, MIC50 of 0.25 μg/mL of AgNPs for MRSA and 0.50 μg/mL of AgNPs for E. coli O157:H7 were obtained (Table 3). The inhibitory activity of the AgNPs was also evaluated through diffusion disc methodology (Figure 6 and Table 4). The diameter values of the mean inhibition zones for paper discs soaked with different concentrations of AgNPs are shown in Table 4.
AgNP type 1 (particle mean size of 36 nm) exhibited higher antibacterial activity for both pathogen strains compared to data for commercial antibiotic, with MIC values from 0.25 μg/mL to 20 μg/mL. For instance, gentamicin shows a MIC of 64 μg/mL for MRSA and amikacin a MIC of 0.50 μg/mL for E. coli.30 We found bactericidal activity against MRSA and E. coli O157:H7 for all types of AgNPs used in this study (Figure 7). Both strains depicted high sensitivity for AgNP type, with MBCs of 0.50 μg/mL and 1 μg/mL for MRSA and E. coli O157:H7, respectively (Table 5).
  | Table 5 MBC of AgNPs against Escherichia coli O157:H7 and MRSA |
Discussion
AgNPs were synthesized following the methodology described by Khan et al,31 under different agitation modes. Synthesis of AgNPs by agitation with a magnetic stirrer at 200 rpm over 1 hour was more efficient for obtaining NPs with small sizes (Table 1). Ultrasonic agitation was not very effective for the synthesis of small AgNPs. On the other hand, AgNP type 1 displayed lower surface charge (zeta potential =−0.51 mV) at pH 7.0, suggesting that mechanical agitation and increasing pH could affect the zeta potential of NPs.
The absorption UV-VIS spectrum of Ag-NPs shows a non-defined shoulder with a maximum absorption at 410 nm, which is characteristic of surface resonant plasmons. It is possible that surface absorption of cysteine caused by the formation of a cysteine–AgNP complex produced plasmon signals of AgNPs observed in the absorption spectra (Figure 8).31
  | Figure 8 Proposed reaction mechanism for the formation of Ag–cysteine complex. |
On the other hand, Figure 3 shows sedimentation at different times of AgNPs obtained at high rates of agitation (800 rpm during 1 hour). Under these experimental conditions, aggregation of NPs was rapid, producing large “clusters” of AgNPs, even 30 days after their formation. When the stirring speed is too high, coalescence forces could increase NP aggregation. Therefore, we established that it is possible to maintain stable NPs up to 3 months using agitation of 200 rpm (Figure 1E and F).
Sandoval et al recently reported on the antibacterial activity of AgNPs in batch mode using multi-well culture microplates.44 In our study, the effect of AgNPs on E. coli O157:H7 and MRSA was also observed. Differences in the MIC were observed for the disc diffusion tests. The MRSA strain was more sensitive to AgNPs than E. coli O157:H7. Nevertheless, our results were more favorable than in other studies that reported a negligible inhibitory effect of AgNPs on E. coli.43,45 These differences could be attributed to differences in AgNP surface charge and size.31,46,47 Generally, NPs with smaller sizes have a large surface area available for interaction with the cell membrane, which could alter some primary functions of bacteria, such as permeability and cell respiration.12 According to our results, smaller NPs (Type 1) displayed high antibacterial activity against both pathogen strains. These NPs showed a mean hydrodynamic size of 36 nm, with low dispersion (Figure 1). Nevertheless, other authors have proposed that the antimicrobial activity of AgNPs is also dependent on the initial concentration of bacteria. In our case, MIC50 values for colloidal AgNPs synthesized using our method were in the range of 0.5–10 μg/mL, with mean sizes of 36 nm (Figures 4,5 and Table 3). These NP sizes were determined by DLS and were in the same range as those from TEM measurements. However, DLS measurements showed larger sizes in comparison to TEM values (around 10 nm), probably due to an agglomeration effect of AgNPs in water, which it is more dramatic at prolonged incubation times. However, this effect has been previously observed and may affect antibacterial activity.48
Interestingly, both E. coli O157:H7 and MRSA were very sensitive to the AgNPs. Using the same experimental conditions and AgNP concentrations used in the MIC experiments, it was observed that type 1 AgNPs were more active against E. coli O157:H7 and MRSA from AgNPs concentrations of 1 μg/mL (Figure 9). In these studies, a longer lag phase and lower maximum absorbance (at 595 nm) was observed as the concentration of the NPs was increased, with a greater effect for MRSA than E. coli O157:H7. The antimicrobial effect of AgNPs has been also reported.49
Nevertheless, Gram-positive MRSA bacteria were more sensitive for all AgNPs synthesized. Despite some reports that have shown that Gram-positive S. aureus are more resistant to AgNPs than Gram-negative E. coli,38 our results showed that MRSA was more sensitive than E. coli at the same AgNP concentrations. This could be attributed to the structural differences of the external cell wall between Gram-positive and Gram-negative bacteria that determine cell permeability. Gram-negative bacteria have a lipopolysaccharide (LPS) layer that is able to exclude macromolecules and hydrophilic compounds,50 and may be less susceptible to AgNPs and provide E. coli with intrinsic bacterial resistance to positive particles. However, Gram-positive bacteria (eg, MRSA) lack this LPS layer and are more sensitive to the bactericidal effects of AgNPs. Additionally, previous reports have confirmed greater antibacterial effects of AgNPs on Gram-positive bacteria than Gram-negative ones.45,51 Furthermore, Gram-positive bacteria have a cell wall constituted by multiple layers of peptidoglycan and teichoic acid. These acids are chains of negatively charged glycerin. On the contrary, cell walls of Gram-negative bacteria possess great amounts of LPS, with an internal network of peptidoglycan of one layer. LPS plays an important role in bacterial defense, providing negative charge and stabilization to the cell membrane.52,53,55 For this reason, E. coli O157:H7 and MRSA are susceptible to compounds positively charged by means of electrostatic interactions.
However, our results suggest that the bactericidal activity of AgNPs is not only dependent on the lipid composition and charge of the cell membrane of bacteria. An interesting inhibitory effect was observed using disc diffusion tests, with inhibition for E. coli O157:H7 and MRSA from AgNP concentrations of 10 μg/mL and inhibition halos of 5 and 10 mm, respectively (Table 4).
These results show a good correlation with the data obtained by the microdilution method. However, this last methodology is more accurate than disc diffusion tests (Figure 6), because the inhibition halos were measured on agar plates with a precision of 1 mm, which may increase the relative errors on these measurements. However, the method illustrates the potential antimicrobial effects of AgNPs to different bacterial strains. Higher sensitivity of microdilution assays compared to disc diffusion tests can also be attributed to better interaction of AgNPs with bacterial cells when we used NP suspension and agitation, while the use of agar plates decreases the interaction between AgNPs and bacteria. By using these two methodologies, similar results of antibacterial activity have been obtained by other authors.48
On the other hand, MBCs of 0.50 μg/mL and 1 μg/mL were achieved for E. coli O157:H7 and MRSA, respectively (Table 4). These MIC and MBC values are lower than reported in the literature for E. coli O157:H750 and MRSA.32 In this sense, AgNPs in suspension may release silver ions into the medium. These ions may interact with the negatively charged bacterial cell envelope, promoting its disruption. In addition, some studies have proposed the formation of “pores” in the bacterial cell membrane that alter both cell permeability and metabolic basic functions (eg, cell respiration), which causes bacterial cell death.38 Other studies have proposed that colloidal silver can react with sulfhydryl groups on the cell wall to generate disulfide bridges, which would block respiration and cause further cell death. Moreover, some studies have proposed that treating E. coli with AgNPs can cause significant damage to the cell walls and result in their rupture.41
Additionally, the interaction of AgNPs with the external membrane causes the accumulation of protein precursors, promoting dissipation of the driving force of protons in the cell membrane. Nevertheless, there is still controversy about whether AgNPs can break the cell membrane or can go into the bacterial cytoplasm and interact with the cytoplasmic DNA of bacteria, and therefore, avoid DNA replication.44,50,54 However, there is clear evidence about the interaction of AgNPs with the cell membrane, either by interaction with sulfhydryl groups or electrostatics or the formation of “pits” by AgNPs. This collapses the cell membrane, causing entry of AgNPs into the bacteria, and consequently, cell death.44 Although we did not study the mechanism of action of AgNPs on E. coli and MRSA, our results suggest that factors such as positive charge and mean size determine bactericidal activity obtained for the AgNPs synthesized in this study. This suggests that AgNPs may be acting by a mechanism that involves interaction with the cell membrane. In addition, we are performing toxicity tests in order to establish the effect of AgNPs on these strains. However, the literature has reported that from concentrations of 50 μg/mL and AgNP sizes of 10 nm, a decrease of cell viability >50% is produced in both skin and lung cell lines.56 As our MIC results were lower than 50 μg/mL, it is probable than the cytotoxic effect will be negligible.
In conclusion, the synthesis of AgNPs by chemical reduction with cysteine was carried out. AgNPs were stabilized with CTAB, obtaining NPs with mean sizes in the range of 36–110 nm. These AgNPs presented excellent antibacterial properties against the pathogen bacteria E. coli O157:H7. Finally, development and fabrication of these AgNPs could be useful for both reducing the colonization of pathogen microorganisms and nosocomial infections, by means of NP use in chirurgic and medical materials such as catheters, probes, tubes, dressings, etc.
Acknowledgments
The authors wish to thank the Cornell Center for Materials Research and the NanoBiotechnology Center Facilities of Cornell University for providing TEM images and some DLS measurements of nanoparticles. Authors also wish to thank the Vicerrectoría de Investigación y Extensión at Universidad Industrial de Santander and Colciencias in Colombia for financial support. Support from Universidad Industrial de Santander is also acknowledged. This work was funded by Colciencias (grant number: 1102-5453-1674).
Disclosure
The authors report no conflicts of interest in this work.
References
Liu WT. Nanoparticles and their biological and environmental applications. J Biosci Bioeng. 2006;102:1–7. | |
Albrecht MA, Evan CW, Raston CL. Green chemistry and the health implications of nanoparticles. Green Chem. 2006;8:417–432. | |
Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176:1–12. | |
Capek I. Preparation of metal nanoparticles in water in oil (w/o) microemulsions. Adv Colloid Interface Sci. 2004;110:49–74. | |
Frattini A, Pellegri N, Nicastro D, Sanctis OD. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater Chem Phys. 2005;94:148–152. | |
Gong P, Li H, He X, et al. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology. 2007;18:604–611. | |
Roy R, Hoover MR, Bhalla AS, et al. Ultradilute Ag-aquasols with extraordinary bactericidal properties: role of the system Ag-O-H2O. Mater Res Innov. 2008;11:3–18. | |
Conlon JM, Kolodziejek J, Nowotny N. Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim Biophys Acta. 2004;1696:1–14. | |
Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. | |
Jo SC, Rim AR, Park HJ, Yuk HG, Lee SC. Combined treatment silver ions and organic acid enhances growth-inhibition of Escherichia coli O157:H7. Food Control. 2009;46:296–299. | |
Lee D, Cohen RE, Rubner MF. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir. 2005;21:9651–9659. | |
Panacek A, Kvitek L, Prucek R, et al. Silver colloid nanoparticles: synthesis, characterization and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253. | |
Khan Z, Talib A. Growth of different morphologies (quantum dots to nanorod) of Ag-nanoparticles: role of cysteine concentrations. Colloids Surf B Biointerfaces. 2010;76:164–169. | |
Kvitek L, Panacek A, Soukupova J, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C. 2008;112:5825. | |
Nataro J, Kaper J. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. | |
Zhu Q, Li L, Guo Z, Yang R. Identification of Shiga-like Escherichia coli isolated from children with diarrhea by polymerase chain reaction. Chin Med J. 2002;115:815–818. | |
Ochoa ML, Harrington PB. Immunomagnetic isolation of enterohemorrhagic Escherichia coli O157:H7 from ground beef and identification by matrix assisted laser desorption/ionization time-of-flight mass spectrometry and databases searches. Anal Chem. 2005;77:5258–5268. | |
Miles SL, Gerba CP, Pepper IL, Reynolds KA. Point-of-use drinking water devices for assessing microbial contamination in finished water and distribution system. Environ Sci Technol. 2009;43:1425–1429. | |
Muniesa M, Jofre J, García-Aljaro C, Blanch AR. Occurrence of Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli in the environment. Environ Sci Technol. 2006;40:7141–7149. | |
Todak K. Pathogenic E. coli. Online Textbook of Bacteriology. Madison, WI: University of Wisconsin-Madison Department of Bacteriology; 2008:p11. | |
Isaäcson M, Cantor P, Effler P, Arntzen L, Bomans P, Heenan R. Hemorrhagic colitis epidemic in Africa. Lancet. 1993;341:961. | |
Mattar S. Prevalencia de E. coli O157:H7, serotipo-enterohemorrágico, en una población pediátrica de Bogotá, enfermedad diarreica aguda. Informe quincenal de casos y brotes de enfermedades. [Prevalence of E.coli O157:H7, entero hemorrhagic serotype in a pediatric population from Bogota. Acute Hemorrhagic Disease. Two-weekly Report pf Cases and Outbreaks]. INS. 1996;17:182–183. Spanish. | |
Mattar S, Visbal S, Arrieta G. E. coli O157:H7 enterohemorrágico: un agente etiológico de etermin en Colombia Subestimado. Parte II [Entero-hemorrhagic E. coli O157:H7: an etiological agent of diarrhea in Colombia Subestimado. Part II]. MVZ-Córdoba. 2001;6(2):81–86. Spanish. | |
Velazquez ME. [Staphylococcus aureus methicillin-resistant: emergence and dissemination]. Salud Publica Mex. 2005;47:381–387. Spanish. | |
Pujol M, Ariza J. Evaluación de la eficacia de las medidas de control en el manejo de las infecciones por Staphylococcus aureus resistente a meticilina [Evaluation of the efficacy on control measurements in the handle of infections caused by methicillin resistant Staphylococcus aureus]. Rev Clin Esp. 1997;197:2. Spanish. | |
Muto CA, Jeringan JA, Ostrowsky BE, et al. SHEA Guidelines for preventing nosocomial transmission of multi-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. | |
Department of Health. The patient’s charter: privacy and dignity and the provision of single sex accommodation. London: Department of Health; 1997. NHS Circular EL 97(3). | |
Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Available from: http://www.cdc.gov/hicpac/pdf/isolation/isolation2007.pdf. Accessed March 18, 2014. | |
Bustos-Martínez JA, Hamdan-Partida A, Gutiérrez-Cárdenas M. Staphylococcus aureus: la reemergencia de un patógeno en la comunidad [Staphylococcus aureus: the reemergence of a pathogen in the community]. Rev Biomed. 2006;17:287–305. Spanish. | |
Ayala NV, Lara HH, Ixtepan LC, Rodríguez C. Silver nanoparticles toxicity and bactericidal effect against methicillin-resistant Staphylococcus aureus: nanoscale does matter. Nanobiotechnol. 2009;5:2–9. | |
Khan Z, Al-Tnabaiti SA, El-Mossalamy EH, Obaid AY. Effect of macromolecule poly(vinyl alcohol) on the growth of cetyltrimethylammonium bromide stabilized Ag-nanoparticles. Colloids Surf A Physicochem Eng Asp. 2009;352:31–37. | |
Martinez F, Olive PL, Banuelos A, et al. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine. 2010;6(5):681–688. | |
Clinical and Laboratory Standards Institute. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes. Wayne, PA: CLSI; 2003. | |
Minelli EB, Benini A, Bassi C. Antimicrobial activity of human pancreatic juice and its interaction with antibiotics. Antimicrob Agents Chemother. 1996;40:2099–2105. | |
Holowachuk SA, Farid MB, Buddington RK. A kinetic microplate method for quantifying the antibacterial properties of biological fluids. J Microbiol Methods. 2003;55:441–446. | |
Case CL, Johnson TR. Laboratory Experiments in Microbiology. 5th ed. San Francisco, CA: Benjamin Cummings Pub Inc.; 1984:p126–p129. | |
McFarland J. The nephelometer: an instrument for estimating the numbers of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am Med Assoc. 1907;49:1176–1178. | |
Ruparelia J, Chatterjee A, Duttagupta S, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4:707–716. | |
Henglein A. Colloidal silver nanoparticles: photochemical preparation and interaction with O2, CCl4, and some metal ions. Chem Mater. 1998;10:444–450. | |
Link S, El-sayed M. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu Rev Phys Chem. 2003;54:331–366. | |
Raffi M, Hussain F, Bhatti L, Akhter J, Hameed A, Hasan M. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J Mater Sci Technol. 2008;24:192–196. | |
Jana N, Sau T, Pal T. Growing small silver particle as redox catalyst. J Phys Chem B. 1999;103(1):115–121. | |
Amin R, Mohamed M, Ramadan M, Verwanger T, Krammer B. Rapid and sensitive microplate assay for screening the effect of silver and gold nanoparticles on bacteria. Nanomedicine. 2009;4(6):637–643. | |
Jiang J, Oberdörster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res. 2009;11:77–89. | |
Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. | |
Song H, Ko K, Oh I, Lee B. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cells Mater. 2006;11:58–59. | |
Morones J, Elechiguerra J, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. | |
Lok CN, Ho CM, Chen R, et al. Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;12:527–534. | |
D’Souza L, Suchopar A, Richards R. In situ approaches to establish colloidal growth kinetics. J Colloid Interface Sci. 2004;279:458–463. | |
Kim J, Kuk E, Yu K, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. | |
Rastogi S, Rutledge V, Gibson C, Newcombe D, Branen J, Branen L. Ag colloids and Ag clusters over EDAPTMS-coated silica nanoparticles: synthesis, characterization, and antibacterial activity against Escherichia coli. Nanomedicine. 2011;7:305–314. | |
Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67(4):593–656. | |
Nanda A, Saravanan M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine. 2009;5(4):452–456. | |
Strominger J, Park J, Thompson R. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959;234(12):3263–3268. | |
Schleifer K, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36(4):407–477. | |
Park J, Yu N, Cheon J, Choi I. Cytotoxicity and inflammatory effect of silver nanoparticles in human cells. The Fifth US-Korea Forum on Nanotechnology: Nano-Biotechnology; April 17–18, 2008; Jeju, Korea. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.